Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.12 no.2 Texcoco feb./mar. 2021 Epub 25-Abr-2022
https://doi.org/10.29312/remexca.v12i2.2687
Articles
Biosorption and tolerance of Pb, Cr and Cd by the biomass of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm
1Posgrado en Ciencias Ambientales-Benemérita Universidad Autónoma de Puebla. CU Edificio IC6, Puebla, México. CP. 72570. (maria.vallejo@viep.com.mx; sonia.silvagomez@viep.buap.mx; diego.ibarrac@alumno.buap.mx). Tel. 222 2295500, ext. 7356.
2Departamento de Investigación en Ciencias Agrícolas-Instituto de Ciencias-Benemérita Universidad Autónoma de Puebla. CU Edificio IC1, Laboratorio de Biotecnología 10314 sur 6301. Tel. 222 2295500, ext. 7356. (marco.marin@correo.buap.mx).
3Facultad de Ingeniería Química-Benemérita Universidad Autónoma de Puebla. Avenida San Claudio y 18 Sur, Puebla, México. Tel. 222 2295500, ext. 7356. CP. 72570. (elena.ramos@correo.buap.mx).
Globally, the indiscriminate use of metal products discharged into water used in agriculture has led to severe contamination, therefore it is important to establish a methodology for decontamination through biological processes, with basidiomycete fungi because it has been shown to degrade a number of persistent organic pollutants using extracellular enzymes, in addition to removing dissolved metals from the water by biosorption, acting, as a natural exchanger. The objectives of this work were to determine between three metals (Pb, Cr and Cd) which was most efficiently removed from the aqueous solution by the fungus mycelium of Pleurotus ostreatus, as well as to evaluate its tolerance and describe the effects caused by each on its cellular structure. The adsorption of metals by mycelium in liquid medium was evaluated using modified Kirk medium, the concentrations retained for 8 days in solution of 20 mg L-1 of each element were quantified, the results indicated that the adsorption of Pb (75%) was the most efficient, followed by Cr (42%) Cd (2.25%). In the results in solid culture medium (PDA) no concentration of Pb and Cr inhibited mycelium growth, however, with Cd was inhibited from 60 mg L-1; however, the three elements caused structural alterations in mycelium. In conclusion, the P. ostreatus strain had tolerance and significant biosorption (p≤ 0.05) for Pb and Cr, so it could be a specific bioremediation biomaterial for Pb and Cr.
Keywords: Pleurotus ostreatus; biomass; biosorption; heavy metals
A nivel mundial, el uso indiscriminado de productos metálicos vertidos en agua utilizada en la agricultura ha generado una grave contaminación, por lo tanto, es importante establecer una metodología para su descontaminación a través de procesos biológicos, con hongos basidiomicetos ya que se ha demostrado que pueden degradar una serie de contaminantes orgánicos persistentes utilizando enzimas extracelulares, además de eliminar metales disueltos del agua mediante biosorción, actuando, como un intercambiador natural. Los objetivos de este trabajo fueron determinar entre tres metales (Pb, Cr y Cd) cuál fue removido más eficientemente de la solución acuosa por el micelio del hongo Pleurotus ostreatus, así como evaluar su tolerancia y describir los efectos causados por cada uno sobre su estructura celular. La adsorción de metales por el micelio en medio líquido se evaluó utilizando medio Kirk modificado, se cuantificaron las concentraciones retenidas durante 8 días en solución de 20 mg L-1 de cada elemento, los resultados indicaron que la adsorción de Pb (75%) fue la más eficiente, seguida de Cr (42%) y Cd (2.25%). En los resultados en medio de cultivo sólido (PDA) ninguna concentración de Pb y Cr inhibió el crecimiento del micelio, sin embargo, con Cd se inhibió a partir de 60 mg L-1; no obstante, los tres elementos causaron en el micelio alteraciones a nivel estructural. En conclusión, la cepa de P. ostreatus presento tolerancia y biosorción significativa (p≤ 0.05) para Pb y Cr, por lo que podría ser un biomaterial de biorremediación específica para Pb y Cr.
Palabras clave: Pleurotus ostreatus; biomasa; biosorción; metales pesados
Introduction
Indiscriminate use of chemicals and increased industrial activities have led to contamination of wastewater with high concentrations of heavy metals, the World Health Organization (WHO) established that the maximum concentration of these ions in water should be in a range of 0.01-1 ppm (Tejada, 2015). Heavy metals are persistent substances prone to accumulation and magnification at different trophic levels (Deng, 2005; Singh et al., 2008), which causes severe health damage such as weakening of the immune system, liver damage, kidneys and cancer because they hinder different cellular processes.
Therefore, the increase in alternatives for its removal in polluted waters (Morales et al., 2010). In Mexico, 85% of wastewater is discharged without pre-treatment to rivers and seas (Sánchez, 2017). According to the Environmental Protection Agency (EPA), several heavy metals are listed as pollutants that pose a public health risk. Lead, copper and cadmium come from numerous activities, above permissible limits, causing adverse effects and disorders in humans when they accumulate in living tissues (Ramos, 2018).
Lead is found in batteries, plumbing, paints, oils (Pawar et al., 2016). Chromium comes from the manufacture of chemicals, wood preservation, pesticides among others (Awual et al., 2013). Cadmium is found in dyes, nuclear plants, electroplating, nickel-cadmium batteries, paints etc. (Ismadji et al., 2015).
Biosorption is a physical-chemical phenomenon that is based on the ability of so-called living or dead biological materials (biomass) to accumulate heavy metals mainly from wastewater, involving microbial metabolism, highlighting the physicochemical processes of adsorption, desorption or absorption of such metal elements (Bou et al., 2018). Environmental factors such as temperature, pH and the presence of nutrients affect the biosorption process (Prakash, 2017).
The potential of fungal biomass as a biosorbent has been accepted for the removal of heavy metals from contaminated water due to its excellent binding properties and tolerance to metals and adverse environments, such as various pH and temperature conditions (Qazilbash, 2004; Anand et al., 2006; Yazdani et al., 2010; Abbas et al., 2014). It has been studied that fungi have the ability to modify or affect the bioavailability of metals (Prakash, 2017), therefore, bioremediation is an option, as it uses biomass through biodegradation, bioaccumulation, and bioconversion processes that operate in different ways (Kulhreshtha et al., 2014; Mosa et al., 2016).
Studies conducted by Vaseem et al. (2017) suggest that Pleurotus ostreatus can be used as a promising alternative to the removal of heavy metals from effluents, as efficiency has been observed for its remediation and has been the highest up to 50% diluted effluents. It has been revealed that this species of fungus has a variety of mechanisms to respond to the presence of heavy metals in solution, can tolerate and remove them from water (Yang et al., 2017)
Consequently, assess the specific adsorption potential of Pb, Cr and Cd for its mycelium in liquid medium, determine the inhibitory metal concentrations of its growth in solid medium and know the effect caused on its cell morphology can allow to design the appropriate methodology for the proposal of a model of bioremediation of polluted sewage with specific heavy metals.
Materials and methods
Biological material
Strain of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm (Fungi: Agaricomycetes: Pleurotaceae) obtained from the mycology laboratory of the Agricultural Sciences Research Department (DICA).
Reagents
Vegetative mycelium activated mycelium and biomass of P. ostreatus, culture medium agar potato-dextrose (PDA) BD Bioxon®, lead nitrate standard solutions [Pb (NO3)2] CAT. 7731 Accu standard Inc batch: A8035019, chromium nitrate standard [Cr (NO3)3•9H2O] CAT. 7731 Hycel of Mexico, SA of CV batch: 298404 and cadmium nitrate standard [Cd (NO3)2•4H2O)] CAT. 7801 Hycel of Mexico SA of CV batch: 298399. The methodologies used in this work are part of the bioremediation processes proposed by Beyer (2005); Noble (2005); Stamets (2005).
Obtaining the vegetative mycelium
The methodologies proposed by Flegg et al (1985); Smith et al. (1994); Chang et al. (2013) were used, modified according to equipment conditions and budget of the institution. In Petri dishes with potato-dextrose agar culture medium, vegetative tissue from fresh basidiocarps of P. ostreatus was inoculated at 25 °C for 7 days (Staments, 1983; Mumpuni et al., 2017).
Preparation of activated mycelium
Based on the methodology proposed by Chalmers (1993) and Stamets et al. (1983) it was placed in a bioreactor with a capacity of 1 L, 1 kg of sorghum seed (Sorghum sp.), previously hydrated at 40% (p/v) was sterilized to 121 °C and 15 psi per 1 h (Sánchez and Roice 2004), subsequently the seed was inoculated with 3 circles 1 cm in diameter of agar with vegetative mycelium of P. ostreatus (0.01 mg in dry weight) and was incubated at a temperature of 25 °C ±2 for 5 days (Staments, 1983).
Obtaining biomass (adsorbent) in liquid medium
The methodology used was the methodology proposed by Chalmers (1993). 12 Erlenmeyer flasks of 500 ml with 250 ml of Kirk medium modified to pH 5.5 with 1 g of natural fiber from wheat bran and 0.01 g of CuSO4 were sterilized in an autoclave at 121°C and 15 psi for 15 min. 3 discs of mycelium of 1 cm diameter of agar with mycelium of P. ostreatus (0.01 mg in dry weight) were inoculated, were incubated for 10 days at 25 °C ±2 (Díaz et al., 2013).
Solutions for evaluating metal adsorption in liquid medium (adsorbate)
Heavy metals were determined in an EAA (atomic absorption spectrophotometer, Varian 55B), using methodologies proposed by the EPA (2002), APHA (2018), as well as CONAGUA recommendations (2018) in its statistics and the Atlas of water in Mexico, using NOM-003-SEMARNAT-1997. From AccuStandard Inc. standard solutions of 1 000 mg L-1, four repetitions of metal solutions were prepared at concentrations of 20 mg L-1 of Pb (NO3)2, Cr (NO3)3•9H2O, and Cd (NO3)2•4H2O, which were placed in 500 ml bioreactors, 1 g of glucose was added, pH was adjusted to 5.5 and they were sterilized at 121°C for 15 minutes 15 psi.
Subsequently, the adsorbent biomass generated in the liquid medium was deposited and was incubated for 10 days at 25 °C. Aliquots of 2 ml were extracted every 24 h for 10 days to analyze the metal concentration of each solution in an atomic adsorption spectrophotometer model VARIAN 55B, wavelength for Pb 217.0 nm, Cr 425.4 nm and Cd 228.8 nm (Kumar et al., 2011; Fleites, 2015).
Culture medium to assess the tolerance and inhibition of micellar growth
From standard solutions of 1000 mg L-1 of Pb(NO3)2, Cr(NO3)3•9H2O, and Cd(NO3)2•4H2O different concentrations (20, 40, 60, 80, and 100 mg L-1) of solutions were prepared for use in the preparation of PDA (dextrose potato agar), it was adjusted pH to 5.5, they were sterilized to 121 °C by 15 min, 15 psi, they were poured into Petri boxes (Fleites, 2015) each inoculated the activated mycelium, it was incubated at 25 °C ±2 and quantified daily radial growth in millimeters for 8 days (Morales et al., 2010).
Description of the effect of metal concentrations on the mycelium of P. ostreatus by scanning electron microscopy (SEM)
Scanning electron microscopy analysis was performed whit a JECL microscope model JSM-6610LV, complemented by an EDX Inca Energy-350 microanalysis equipment, Oxford Instruments, with X-ray detector model X-Max 50, of 50 mm2, with theoretical resolution of 127 eV at the peak energy K of Mn. Each sample was dehydrated and covered with gold dust in a Balzers coating equipment model SCD 004 (Lozano et al., 2014). Also, the analysis of X-ray energy dispersive spectrometry (EDX) was performed to the mycelium of P. ostreatus, to describe the presence of metals inside the cell.
Statistical analysis
A completely random design (DCA) was used with three repetitions to evaluate the effect of independent variables: concentration of Pb, Cr and Cd metals, and mycelium growth days on response variables: retention percentage or adsorption. The experimental data set was analyzed through a variance test (Anova) of 2-way (factors were experimental metals and days of contact with mycelium to assess its retention or adsorption) and a Tukey mean test (p≤ 0.05) with R-commander software (Fox, 2005).
Results and discussion
Evaluation of metal adsorption in liquid medium
Figure 1 describes the behavior of Pb, Cr and Cd metals in solution at a concentration of 20 mg L-1 depending on the time of contact with the biomass of P. ostreatus.

Figure 1 Quantification of concentrations retained by P. ostreatus biomass in contact with solutions of (a) lead; (b) chromium; and (c) cadmium based on time at a concentration of 20 mg L-1.
The results in Figure 1 indicated that Pb adsorption (75%) was the most efficient, followed by Cr (42%) and Cd (2.25%). This demonstrates that the mycelium of P. ostreatus has the retention capacity of Pb and Cr, according to the assessments from day one (Figure 1a) the Pb recorded a drastic decrease in the initial concentration from 20 mg L-1 to 5.065 mg L-1, with day 5 being the highest adsorption of said metal (75%), the other days desorption and adsorption processes were observed with a significant difference with respect to from time zero (Table 1), something similar happens in the work of Marín et al. (2015), who describe an adsorption process of 57.9% in the first 48 hours, with an initial concentration of 100 mg L-1.
Table 1 Effect of time on the absorption of heavy metals in a liquid medium.
Day |
Witness (mg L-1) |
Pb (mg L-1) |
Cr (mg L-1) |
Cd (mg L-1) |
|
0 1 2 3 4 5 6 7 8 9 10 |
0.02 ± 0.027 a 0.017 ±0.009 a 0.02 ±0.016 a 0.017 ±0.017 a 0.022 ±0.018 a 0.015 ±0.012 a 0.04 ±0.008 a 0.02 ±0.008 a 0.057 ±0.026 a 0.037 ±0.026 a 0.027 ±0.009 a |
20 ±0 a 5.065 ±0.84 c 4.987 ±0.798 c 5.592 ±0.519 bc 7.197 ±1.271 b 2.897 ±0.589 d 3.9 ±0.787 cd 4.68 ±1.213 cd 5.047 ±0.903 c 4.297 ±0.878 cd 4 ±0.081 cd |
20 ±0 a 13.15 ±1.643 c 11.565 ±0.867 c 13.265 ±1.173 c 11.632 ±0.938 c 17.267 ±1.751 ab 16.562 ±1.778 b 17.167 ±0.805 ab 17.945 ±1.925 ab 17.292 ±1.104 ab 17 ±1.061 ab |
20 ±0 a 19.545 ±0.247 abc 19.717 ±0.12 ab 19.365 ±0.365 bcd 19.4 ±0.249 bcd 19.202 ±0.077 cd 19.025 ±0.17 d 19.1 ±0.081 cd 18.925 ±0.095 d 18.975 ±0.25 d 18.972 ±0.251 d |
DMS |
0.0614 |
1.9841 |
3.178 |
0.492 |
Values mean ± standard deviation. The significant statistical difference according to Tukey’s test is (p≥ 0.05).
In the same way, Yang et al. (2017) observed the elimination of Pb from 99.9-100% and that this was transported to the fungal cell wall from 68.2 to 91.2%. This indicates that the metal in addition to being adsorbed on the surface of the myceliar biomass, entered inside the cells interfering with metabolism causing alterations in structure and morphology, this process was favored by the variation of the initial pH from 5.5 to pH of 3.5, since according to Javaid et al. (2011); Eliescu et al. (2020); Huang et al. (2009), this change led to the chemical dissociation of carboxylic functional groups, amino, methyl, phosphate and hydroxides constituents of the cell wall and metals in solution, increasing their solubility and favoring the availability of bioorption in biomass and intracellular bioaccumulation.
The behaviour of Cr in solution is shown in Figure 1b, the initial concentration (20 mg L-1) decreased to 13.15 mg L-1 (34.2%) at pH of 5.5, bio adsorption did not show significant difference until day 4 when it registered 11.6 mg L-1 (41.9%) corresponding to the maximum retention, the following days metal desorptions were observed without registering further bio adsorption at the final pH of 6.6. In this regard, Marín et al. (2015) observed that Cr at a concentration of 100 mg L-1 had an upward adsorption from the fourth day of sampling of 33.9 mg L-1, up to 59.8 mg L-1 at 10 days at pH 5.2, in addition in the works of Da Rocha et al. (2019) when using 25 mg L-1 Cr (VI) solution, the adsorption in 15 days reached 100%.
This allows to consider that Cr retention occurred in the first days of contact and subsequently the pH influenced the desorption of the metal making it insoluble for micelium retention. On the other hand, Prasenjit et al. (2005) indicate that working with Aspergillus foetidus with initial concentration of 5 mg L-1 of chromium, at pH 7.0 in 92 h of contact recorded metal retentions of 97%. Nasseri et al. (2002) report that experimenting with Aspergillus oryzae, at initial concentration of 240 mg L-1, at pH of 5 at 36 h of contact, they recorded 97% chromium retention and Bai and Abraham (2005) indicate that working with Rhizopus nigricans, at initial concentrations of 100 mg L-1 of chromium, at pH of 2, in 4 h of contact, they evaluated 80% metal retention.
This indicates that the pH of the medium and the contact time are important factors for the retention of the metal and also indicates that the nature of the strain can be decisive in the results obtained. Cd adsorption (Figure 1c) was 0.46 mg L-1 (2.25%) from the first day of contact at pH from 5.5, until day 7 of incubation.
Table 1 presents the statistical analysis of the effect of time on the absorption of heavy metals in the experimental liquid medium, in the case of cadmium on days 9 and 10 the metal retention reached 1.08 mg L-1 (5.5%), at pH 2.8, together with inhibition of miceliar growth, possibly due to the toxic characteristics of the metal and the drastic change of the initial pH from 5.5 to 2.8, this retention trend coincides with that described by Yang et al. (2017) who observed a 45.9-61.1% decrease from an initial concentration of 40 mg L-1 to pH of 4.5.
According to Frutos et al. (2016); Deng et al. (2009), the pH values of a solution should be considered as an important factor influencing the biosorption process, also influencing the toxicity and chemistry of the metal solution, as well as in the hydrolysis and complexation properties by causing changes in the ionic form.
Therefore, the ionic charge of the functional groups and the speciation of the metal at different pH values can affect the biosorption. Another important aspect is the species of the fungus used because according to Javaid et al. (2011); Ogbo and Okhuoya (2011) in each species biosorption can vary with the type of metal, its concentration and composition of the culture medium or substrate, and the behavior of an element depends on the particular species in which it is present because the highest reactivity of a species will not always coincide with the higher initial concentration of the metal in that chemical form. Therefore, the behavior of an element in the environment (bioavailability, toxicity, distribution, etc.) cannot be predicted based on its concentration.
In this analysis you can see in the column of each metal the significant differences between the zero time and the time of greatest metal retention by the biomass of P. ostreatus, in case of lead is day 5, with chromium is day 4 and with cadmium is day 7. It is also important to note that there is a significant difference between the retention of metals by biomass, regardless of the days indicated above, the difference is between Pb and Cr and between Pb and Cd.
Assessment of inhibitory metal concentrations of micellar growth
Radial growth of mycelium of P. ostreatus is presented in Figure 2, in mm per day at different metal concentrations (20, 40, 60, 80 and 100 mg L-1) of Pb, Cr and Cd. In this figure, it is described as the mycelium of P. ostreatus, with no concentration of lead and chromium present inhibition of its growth, however, cadmium was able to inhibit it from a concentration of 60 mg L-1. The results indicate that the mycelium of P. ostreatus has tolerance to certain metal solutions, which is supported by the analysis of Figure 2a, where the continuous development of mycelium in the 5 concentrations is observed without inhibition or significant difference (p≤ 0.05) on days 1 and 2, instead, it shows difference from day 3.

Figure 2 . Radial growth of the fungus mycelium P. ostreatus in mm per day at 5 different concentrations (20, 40, 60, 80 and 100 mg L-1) of: a) lead; b) chromium; and c) cadmium.
In addition, it is observed that on day 8 reaches the highest growth at the concentration of 100 mg L-1, a result that coincides with the work of Yang et al. (2017) who observed the same phenomenon of resistance. Similarly, Morales-Fonseca et al. (2010) quantified radial growth, at low concentrations between 0.02-1.5 mg L-1 and highs between 15-11 000 mg L-1, Pb acetate and observed a growth of mycelium of P. ostreatus of 50 mm at 10 days of incubation without alteration in macroscopic and microscopic morphology, when concentrations were high, alterations were presented but tolerance towards lead was recorded between 200 and 500 mg L-1, it should also be mentioned that Marín et al. (2015).
When examining the mycelial growth of P. ostreatus in concentration of 50 mg L-1 of Pb solution observed that it was not inhibited, remaining viable after 20 days of exposure in solid medium. It can be assumed that P. ostreatus has a great capacity to tolerate and eliminate Pb; through consecutive adsorption and bioaccumulation processes because this metal can be fixed in the functional groups amines (N) and hydroxyls (OH) of the chemical structure of the cell wall, managing to penetrate to the cell depositing in the cytosol of the cell structure of the mycelium (Huang et al., 2009; Wang et al., 2019; Eliescu et al., 2020).
The radial growth of the mycelium of the fungus P. ostreatus in mm per day at different concentrations of Cr (20, 40, 60, 80, and 100 mg L-1) solutions, is represented in Figure 2b, its development was continuous, without inhibition in the 5 concentrations of Cr, without significant difference. Also, on days 7 and 8, 8 86.03% of development was observed at a concentration of 100 mg L-1, this result is similar to that obtained by Yang et al. (2017) who worked with Cr solutions at concentrations of 50-300 mg L-1 and observed that mycelium grew well up to 150 mg L-1, but from 200 mg L-1 had partial inhibition and a total of 300 mg L-1.
The same behavior was recorded by da Rocha Ferreira et al. (2019) who measured the radial increase in the mycelium of P. ostreatus in the range of (10-150 mg L-1) of Cr (VI) during 10 days of incubation, without inhibition of growth, implying that high concentrations of Cr metal solutions inhibit the growth of the fungus by possible damage to the cell membrane, induction of lipid peroxidation, formation of reactive oxygen species, as well as damage to DNA and protein structures(Huang et al., 2009: Sazanova et al., 2015; Eliescu et al., 2020).
Figure 2c shows the radial growth of mycelium P. ostreatus in mm per day at different concentrations of Cd solutions, inhibition occurs from the concentration of 60 mg L-1, but growth is observed until day 8 at a concentration of 100 mg L-1 reaching the highest growth and no posterior mycelial advancement was recorded.
This behavior coincides with that reported by Yang et al. (2017) who worked with Cd concentrations of 10-50 mg L-1 and reported inhibition of micellar growth in P. ostreatus from the concentration of 40 mg L-1, in parallel, Miaomiao et al. (2018) discovered two strains of P. ostreatus JINONG21 and SUYIN6 Cd tolerant, observing an inhibition of growth from day 7 of inoculation to 40 mg L-1 of concentration, in this case it can be considered as in previous metals that the behavior and effect of Cd on the growth and viability of mycelium of P. ostreatus, vary according to concentrations and type of element according to Yang et al. (2017); Baldrian (2003).
Description of the effect of metal concentration on the morphology of mycelial structures by scanning electron microscopy (SEM).
Figure 3 shows the internal structural microscopic morphology of mycelium of P. ostreatus before and after making contact with the different experimental metal concentrations of Pb, Cr, and Cd, the structures were analyzed by scanning electron microscopy and X-ray spectrometry.

Figure 3 Microscopic structures of the mycelium of P. ostreatus from the control(a) and mycelium with metal solutions of lead (b), chromium(c) and cadmium (d) respectively.
This figure shows the damage to the mycelial structure due to the action of experimental metals indicating these images that the metal in addition to being adsorbed on the surface of the mycelium, managed to enter the inside of the cells interfering with their metabolism causing alterations in the cell structure and cell morphology.
Figure 3a shows the reproduction structures of the mycelium of P. ostreatus, which are presented developed in the metal-free solid medium (control) and elongated hyphae are seen, hyalines, hydrated, cylindrical, septate, and spherical structures or turgid blast conidia as indicated by Bowman et al. (2006)).
Figure 3b shows changes in mycelium morphology due to the effect of Pb metal solution, also deformed, dehydrated, flattened and broken hypfas are observed, no reproductive structures are observed, this indicates that the metal in addition to being adsorbed on the surface of the mycelial biomass enters the inside of the cells interfering with metabolism causing alterations in the cell structure and cell morphology.
Figure 3c shows the mycelium that was in contact with a metallic solution of Cr, the structures are observed without turrgence, with hyphal rupture and absence of blastoconidia, this is indicative of the interaction of Cr with functional groups OH-, -NH, belonging to carbohydrates and cell wall proteins, but structural cellular damage may be related to the interaction of chromium with groups -CH3 and =CH2 of fatty acids associated with constituent phospholipids of the cell membrane, this implies the interaction of metal with cellular metabolism and its bioaccumulation inside the mycelium as mentioned by Corona et al. (2010); Cervantes et al. (2006).
Figure 3d shows the mycelium developed in solid medium with Cd, which shows greater dehydration and deformation of the hyphae, major rupture and minimal presence of conidial structures. Sections b, c, and d of Figure 3 also an increase in the size or thickening of damaged structures is observed, this may be due to the high tolerance of mycelium to certain metals, therefore, the activation of defense mechanisms such as wall thickening, the melanin synthesis and the production of organic acids (Arango et al., 2009) are initiated, since according to Banerjee and Nayak (2007) and Javaid et al. (2011) these white rot fungi and in particular P. ostreatus have a cell wall composed of β 1-3 and β 1-6 glucans of carboxylic groups, amino, thiol, phosphate and hydroxide in the cell wall that help in the biosorption of metal ions and offer a primary barrier against metal.
Figure 4 shows the analysis of X-ray energy dispersion spectrometry (EDX) to mycelium P. ostreatus, where the Pb achieved greater retention inside the mycelium. Figure 4a presents the EDX analysis to the witness sample of the mycelium of P. ostreatus, in Figure 4b shows the presence of Pb in the mycelium, this confirms that there was bioaccumulation inside the cells and the cell wall as confirmed Morales-Fonseca et al. (2010) and Figures 4c and 4d show the presence of Cr and Cd respectively in the X-ray energy microanalysis test of P. ostreatus mycelium samples.
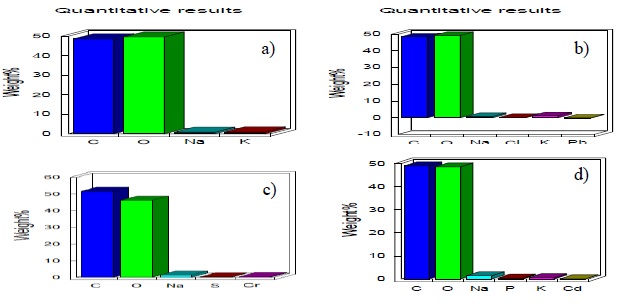
Figure 4 X-ray energy microanalysis of P. ostreatus mycelium samples, control samples (a) where the quantitative content of mycelium is observed presence of Pb (b) in fungal tissue Cr (c) y Cd (d).
In Figure 5, the alterations caused by lead and cadmium in the mycelial biomass of other species of fungi are presented.

Figure 5 Scanning electron microscopy in Phanerochaete chysosporium, a) mycelial biomass mycelium without contact with metals; b) effect of Pb solution at 300 mg L-1; c) effect of Cadmium solution; and d) P. chysosporium mycelium in contact with Pb and Cd. (Morales et al., 2008).
Comparing the images in Figures 3 and 5 it can be observed that alterations to the cell morphology of mycelium are different this is surely related to the metal concentrations and contact times that in this case were 72 h according to Morales et al. (2008).
Conclusions
According to the results analysis it is concluded that P. ostreatus has a great capacity to tolerate and eliminate experimental metals (Pb, Cr and Cd). In relation to the effects caused by each metal on the structure of the mycelium of P.ostreatus it can be concluded that by electron microscopy analysis Pb and Cr caused structural damage to the mycelium cells.
In general, the fungus had an outstanding biosorption capacity of Pb and Cr. Therefore, the use of mycelium of strain of P. ostreatus can be proposed to generate a specific bioremediation model for wastewater contaminated with Pb. Considering economic aspects, it is necessary to produce low-cost, effective and recyclable adsorbents for widespread use.
The biosorption potential of different species should be evaluated comparatively. Looking at the results obtained by the strain of P. ostreatus, it can be considered that the species has the potential to be used as a biosorbent of heavy metals without losing sight that the degree of tolerance is different for each species and for different heavy metals.
Acknowledgments
To the National Council for Science and Technology (CONACYT) for the scholarship it provided for the study in the Environmental Sciences postgraduate course of BUAP (ICUAP), to the University Center for Linking and Transferring Technology VIEP-BUAP (CUVYT).
REFERENCES
Abbas, S. H.; Ismail, I. M.; Mostafa, T. M. and Sulaymon, A. H. 2014. Biosorption of heavy metals a review. J. Chemical Sci. Technol. 3(4):74-102. [ Links ]
Anand, P.; Isar, J.; Saran, S. and Saxena, R. K. 2006. Bioaccumulation of copper by Trichoderma viride. Bio. Technol. 97(8):1018-1025. [ Links ]
APHA. 2018. Standard methods for examination of water and wastewater. American Public Health Association (APHA), (American Water Works Association (WWA), Water Pollution Control Federation (WPCF), Washington, DC. EUA. 1 100 p. [ Links ]
Arango, R. A.; Lebow, P. K. and Green, F. 2009. Correlation between oxalic acid production and tolerance of Tyromyces palustris strain TYP-6137 to N’, N-naphthaloylhydroxamine. International Biodeterioration & Biodegradation. 63(1):46-51. [ Links ]
Awual, M. R.; Ismael, M.; Yaita, T.; El-Safty, S. A.; Shiwaku, H.; Okamoto, Y. and Suzuki, S. 2013. Trace copper (II) ions detection and removal from water using novel ligand modified composite adsorbent. Chem. Eng. J. 222:67-76. https://doi.org/10.1016/ j.cej. 2013.02.042. [ Links ]
Bai, S. R. and Abraham, T. E. 2005. Continuous adsorption and recovery of Cr (VI) in different types of reactors. Biotechnology Progress. 21(6):1692-1699. [ Links ]
Banerjee, A. and Nayak, D. 2007. Biosorption of no-carrier-added radionuclides by calcium alginate beads using ‘tracer packet’technique. Bio. Technol. 98(14):2771-2774. [ Links ]
Beyer, D. 2005. Spent mushroom substrate (SMS) research in the US. American Medical Group Association Journal, Summer Issue. 31-32 pp. [ Links ]
Bou, L. P.; Bernal, I. S.; Duarte, C. L.; Sardiñas, A. M.; Arias, M. E. C. and Valdés, M. E. C. 2018. Biosorción microbiana de metales pesados: características del proceso/Biosorption of heavy metals: characteristics of the process. Rev. Cubana de Cienc. Biol. 6(1):1-13. [ Links ]
Bowman, S. M. and Free, S. J. 2006. La estructura y síntesis de la pared celular fúngica. Bioensayos. 28(8):799-808. [ Links ]
Cervantes, C.; Espino-Saldaña, A. E.; Acevedo-Aguilar, F.; León-Rodríguez, I. L.; Rivera-Cano, M. E.; Avila-Rodríguez, M. and Moreno-Sánchez, R. 2006. Interacciones microbianas con metales pesados. Rev. Latinoam. Microbiol. 48(2):203-210. [ Links ]
Chalmers, W. 1993. Specialty mushrooms: mushroom tissue culture. Mushroom World. (September) 14-18 pp. [ Links ]
Chang, S. T. and Hayes, W. A. 2013. The biology and cultivation of edible mushrooms. Academic press. Inc., New York & London. (Ed.). 819 p. [ Links ]
CONAGUA. 2018. Comisión Nacional del Agua. B Atlas del agua en México. Secretaría de Medio Ambiente y Recursos Naturales. www.conagua.gob.mx. [ Links ]
Da-Rocha, F. G. L.; Vendruscolo, F. and Antoniosi-Filho, N. R. 2019. Biosorption of hexavalent chromium by Pleurotus ostreatus. Heliyon. 5(3):01450. [ Links ]
Deng, L.; Zhang, Y.; Qin, J.; Wang, X. and Zhu, X. 2009. Biosorption of Cr (VI) from aqueous solutions by nonliving green algae Cladophora albida. Minerals Engineering. 22(4):372-377. [ Links ]
Deng, S. and Ting, Y. P. 2005. Characterization of PEI-modified biomass and biosorption of Cu (II), Pb (II) and Ni (II). Water Res. 39(10):2167-2177. [ Links ]
Díaz, R.; Téllez-Téllez, M.; Sánchez, C.; Bibbins-Martínez, M. D.; Díaz-Godínez, G. and Soriano-Santos, J. 2013. Influence of initial pH of the growing medium on the activity, production and genes expression profiles of laccase of Pleurotus ostreatus in submerged fermentation. Electronic J. Biotechnol. 16(4):6-6. [ Links ]
Eliescu, A.; Georgescu, A. A.; Nicolescu, C. M.; Bumbac, M.; Cioateră, N.; Mureșeanu, M. and Buruleanu, L. C. 2020. Biosorption of Pb (II) from aqueous solution using Mushroom (Pleurotus ostreatus) biomass and spent mushroom substrate. Analytical Letters. 1-28 pp. [ Links ]
EPA. 2002. Methods for chemical analysis of water and wastes. U.S. Environmental Protection Agency, Washington, D.C., EPA/600/4-79/020 (NTIS PB84128677). [ Links ]
Flegg, P. B.; Spencer, D. M. and Wood, D. A. 1985. Biology and technology of the cultivated mushroom. Wiley. [ Links ]
Fleites, L. and Calderón, A. 2015. Adsorption of Cu and Cd by the mycelial biomass of three strains of Pleurotus white rot fungus. Rev. Latinoam. Amb. Cienc. 6(14):20-34 [ Links ]
Fox, J. 2005. Getting started with the R commander: a basic-statistics graphical user interface to R. J Stat Softw. 14(9):1-42. [ Links ]
Frutos, I.; García-Delgado, C.; Gárate, A and Eymar, E. 2016. Biosorción de metales pesados por carbono orgánico de sustratos de hongos usados y sus materias primas. Int. J. Environ. Sci. Technol. 13(11):2713-2720. Doi: 10.1007/s13762-016-1100-6. [ Links ]
Huang, H.; Cheng, G.; Chen, L.; Zhu, X. and Xu, H. 2009. Lead (II) removal from aqueous solution by spent Agaricus bisporus: determination of optimum process condition using Taguchi method. Water Air Soil Pollution. 203(1-4):53-63. [ Links ]
Ismadji, S.; Soetaredjo, F. E. and Ayucitra, A. 2015. Clay materials for environmental remediation 25:1-124. Berlin: Springer. [ Links ]
Javaid, A.; Bajwa, R.; Shafique, U. and Anwar, J. 2011. Removal of heavy metals by adsorption on Pleurotus ostreatus. Bio. Bioen. 35(5):1675-1682. [ Links ]
Kulshreshtha, S.; Mathur, N. and Bhatnagar, P. 2014. Mushroom as a product and their role in mycoremediation. AMB Express. 4(1):29-36. [ Links ]
Kumar-Ramasamy, R.; Congeevaram, S. and Thamaraiselvi, K. 2011. Evaluation of isolated fungal strain from e-waste recycling facility for effective sorption of toxic heavy metal Pb (II) ions and fungal protein molecular characterization a mycoremediation approach. Asian J. Exp. Biol. Sci. 2:342-347. [ Links ]
Lozano, V.; Yañez, M. J. y Morales, A. 2014. Principios y práctica de la microscopía electrónica. México: Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). [ Links ]
Miaomiao, C. H. E. N.; Zheng, X; Liang, C. H. E. N. and Xiaofang, L. I. 2018. Cadmium-resistant oyster mushrooms from North China for mycoremediation. Pedosphere. 28(6):848-855. [ Links ]
Morales-Fonseca, D. M. and Ruiz-Tovar, K. J. 2008. Determinación de la capacidad de remoción de cadmio, plomo y níquel por hongos de la podredumbre blanca inmovilizados. [ Links ]
Morales-Fonseca, D.; Ruiz-Tovar, K.; Martínez-Salgado, M. M.; Soto-Guzmán, A. B.; Falcony-Guajardo, C.; Vázquez, R. R. and Pedroza-Rodríguez, A. M. 2010. Desarrollo de un bioadsorbente laminar con Phanerochaete chrysosporium hipertolerante al cadmio, al níquel y al plomo para el tratamiento de aguas. Rev. Iberoam. Micol. 27(3):111-118. [ Links ]
Mosa, K. A.; Saadoun, I.; Kumar, K.; Helmy, M. and Dhankher, O. P. 2016. Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Frontiers Plant Sci. 7:303-311. [ Links ]
Mumpuni, A.; Ekowati, N.; Purnomowati, P. and Purwati, E. S. 2017. Growth and protein content establishment of pleurotus ostreatus on liquid and solid medium. Biosaintifika. J. Biology & Biology Education. 9(3):572.578. [ Links ]
Nasseri, S.; Mazaheri, A. M.; Noori, S. M.; Rostami, K. H.; Shariat, M. and Nadafi, K. 2002. Chromium removal from tanning effluent using biomass of Aspergillus oryzae. Pakistan J. Biol. Sci. 5(10):1056-1059. [ Links ]
Noble, R. 2005. Spent mushroom substrate-an alternative use. AMGA J. Summer. 33-35 pp. [ Links ]
Ogbo, E. M. and Okhuoya, J. A. 2011. Bio-absorption of some heavy metals by Pleurotus tuberregium Fr. Singer (an edible mushroom) from crude oil polluted soils amended with fertilizers and cellulosic wastes. Inter. J. Soil Sci. 6(1):34-40. [ Links ]
Pawar, R. R.; Bajaj, H. C. and Lee, S. M. 2016. Activated bentonite as a low-cost adsorbent for the removal of Cu (II) and Pb (II) from aqueous solutions: Batch and column studies. J. Ind. Eng. Chem. 34:213-223. [ Links ]
PPrakash, V. 2017. Mycoremediation of environmental pollutants. Int. J. Chem. Tech. Res. 10(3):149-155. [ Links ]
Prasenjit, B. y Sumathi, S. 2005. Captación de cromo por Aspergillus foetidus. Revista de Ciclos de Materiales y Gestión de Residuos. 7(2):88-92. [ Links ]
Qazilbash, A. A. 2004. Isolation and characterization of heavy metal tolerant biota from industrially polluted soils and their role in bioremediation. Doctoral dissertation, Quaid-i-Azam University Islamabad, Pakistan. 210-256 pp. [ Links ]
Ramos, D. C. 2018. Adsorción de cadmio, cobre y plomo en bentonita, caolín y zeolita naturales y modificadas: una revisión de los parámetros de operación, isotermas y cinética. Ingeniería. 23(3):252-273. [ Links ]
Sánchez, A. 2017. Saneamiento descentralizado y reutilización sustentable de las aguas residuales municipales en México. Sociedad y Ambiente. 14:119-143. [ Links ]
Sazanova, K.; Osmolovskaya, N.; Schiparev, S.; Yakkonen, K.; Kuchaeva, L. and Vlasov, D. 2015. Organic acids induce tolerance to zinc-and copper-exposed fungi under various growth conditions. Current Microbiol. 70(4):520-527. [ Links ]
SEMARNAT. 1997. NOM-003-SEMARNAT-1997. [ Links ]
Singh, J.; Kant, K.; Sharma, H. B. and Rana, K. S. 2008. Bioaccumulation of cadmium in tissues of Cirrihna mrigala and Catla catla. Asian J. Exp. Sci. 22:411-414. [ Links ]
Smith, D. and Onions, A. H. 1994. The preservation and maintenance of living fungi (Ed). 2. CAB international. [ Links ]
Stamets, P. and Chilton, J. S. 1983. The mushroom cultivator. First Washington. [ Links ]
Tejada-Tovar, C.; Villabona-Ortiz, Á. and Garcés-Jaraba, L. 2015. Adsorción de metales pesados en aguas residuales usando materiales de origen biológico. TecnoLógicas. 18(34):109-123. [ Links ]
Wang, Y.; Yi, B.; Sun, X.; Yu, L.; Wu, L.; Liu, W. and Li, X. 2019. Removal and tolerance mechanism of Pb by a filamentous fungus: a case study. Chemosphere. 225:200-208. [ Links ]
Yang, S.; Sun, X.; Shen, Y.; Chang, C.; Guo, E.; La, G. and Li, X. 2017. Tolerance and removal mechanisms of heavy metals by fungus Pleurotus ostreatus Haas. Water, Air & Soil Pollution. 228(4):130-137. [ Links ]
Yazdani, M.; Yap, C. K.; Abdullah, F. and Tan, S. G. 2010. An in vitro study on the adsorption, absorption and uptake capacity of Zn by the bioremediator Trichoderma atroviride. Environ. Asia. 3(1):53-59. [ Links ]
Received: January 01, 2021; Accepted: March 01, 2021











 texto en
texto en 


