Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.11 n.spe24 Texcoco Apr./May. 2020 Epub May 07, 2021
https://doi.org/10.29312/remexca.v0i24.2364
Articles
Cocksfood forage yield inoculated with PGPB bacteria
1Instituto Politécnico Nacional-Centro de Investigación en Biotecnología Aplicada. Ex Hacienda de San Juan Molino, Carretera Tecuexcomac-Tepetitla km 1.5, Tlaxcala. CP. 90700. (mirobatlx@hotmail.com; jonathan-castro@live.com.mx).
2Instituto de Investigación de Zonas Desérticas-Universidad Autónoma de San Luís Potosí. Altair 200, Colonia del Llano, San Luis Potosí. CP. 78377. (gisela.aguilar@uaslp.mx).
3Instituto Tecnológico del Valle de Oaxaca-División de Estudios de Posgrado. Ex Hacienda de Nazareno, Santa Cruz Xoxocotlán, Oaxaca, México. CP. 71230. (yuriva1968@gmail.com).
4División de Ciencias Biológicas y de la Salud-Universidad Autónoma Metropolitana-Unidad Xochimilco. Calzada del Hueso 1100, Colonia Villa Quietud, Delegación Coyoacán, Ciudad de México, México. CP. 04960. (asolis@correo.xoc.uam.mx).
The PGPB bacteria have beneficial effects on crop yields. The aim of the present study was to determine the effect of five plant growth promoting bacteria on yield, plant height, SPAD units and content of protein in cocksfood defoliated every five weeks in spring and summer, under greenhouse conditions. A completely random design was used, with factorial arrangement 5x2x2, being the experimental unit a pot with ten stems, with four repetitions. The bacteria evaluated were: Ewingella americana (digestate), Ewingella americana (Soil), Pseudomonas clororaphis, Bacillus toyonensis and Microbacterium oxidans; compared with each other, and with the positive (triple 17) and negative (soil without fertilization) controls. The highest values of dry matter yield in spring were recorded by E. americana (3.5 g DM pot), while in summer it was B. Toyonensis. The height values did not register differences (p> 0.05) in both epochs, the SPAD units only in the summer and E. americana registered the lowest values (1.8). The protein content evidenced that the controls were inferior to all the treatments that were inoculated. The evaluated bacteria recorded effects on all evaluated variables and were superior to inorganic fertilization and unfertilized soil.
Keywords: Bacillus toyonensis; Ewingella americana; Microbacterium oxidans and Pseudomonas clororaphis
Las bacterias PGPB tienen efectos benéficos en el rendimiento de los cultivos. El objetivo del presente estudio fue determinar el efecto de cinco bacterias promotoras del crecimiento vegetal sobre el rendimiento, altura de planta, unidades SPAD y contenido de proteína de pasto ovillo defoliado cada cinco semanas en primavera y verano, bajo condiciones de invernadero. Se utilizó un diseño completamente al azar, con arreglo factorial 5 x 2 x 2, siendo la unidad experimental una maceta con diez tallos, con cuatro repeticiones. Las bacterias evaluadas fueron: Ewingella americana (digestato), Ewingella americana (suelo), Pseudomonas clororaphis, Bacillus toyonensis y Microbacterium oxidans, comparados entre sí y con los controles positivo (triple 17) y negativo (suelo sin fertilización). Los valores altos de MS en primavera lo registraron E. americana (3.5 g MS maceta-1), mientras que en verano fue B. Toyonensis. Los valores de altura no registraron diferencias (p> 0.05) en ambas épocas, las unidades SPAD solo en el verano y E. americana registró los menores valores (1.8). El contenido de proteína evidenció que los testigos fueron inferiores a todos los tratamientos que fueron inoculados. Las bacterias estudiadas registraron efectos en todas las variables evaluadas y fueron superiores a la fertilización inorgánica y al suelo sin fertilizar.
Palabras clave: Bacillus toyonensis; Ewingella americana; Microbacterium oxidans y Pseudomonas clororaphis
Introduction
The rhizospheric microorganisms colonize the rhizosphere and improve the development of plants, promoting an efficient use of minerals, through a wide variety of mechanisms such as mineralization of organic matter, biological control against soil pathogens, biological fixation of N, P, K and Zn, among other micronutrients and synthesize siderophores, which encourages the development of roots since, in this component of the plant.
Complex interactions occur between photosynthetic root exudates and other physiological processes of the plant, soil, and microbiome, which is inhabited by a wide range of microorganisms including fungi, bacteria, actinomycetes, algae, and nematodes, where dominance is determined by the product of the root exudates (carbohydrates, amino acids, fatty acids, nucleotides, organic acids, phenolics, growth regulators, putrescence, sterols, sugars and vitamins, among other compounds), which affects the population and functional dynamics of soil microorganisms, which differs from the rhizospheric zone to the rest of the population present in the soil or substrate (De-Bashan et al., 2007; Rashid et al., 2012; Esquivel-Cote et al., 2013; Hungria et al., 2016; Mora et al., 2017; Singh et al., 2017; Posada et al., 2018; Ramakrishna et al., 2019).
Cocksfood (Dactylis glomerata L.) is a species cultivated and exploited for its high nutritional value, rapid regrowth, DM yield, stem production and for being a species associated with legumes that allow more efficient use of radiation and soil resource (Castro et al., 2012; Castro et al., 2013; Villareal et al., 2014; Flores-Santiago et al., 2018).
The information published on the use of biofertilizers or plant growth promoting bacteria (PGPB) in this species is null; however, these have been reported in other pastures of the genus Penissetum (Criollo et al., 2012), Brachiaria (Lopes et al., 2018), Zea (Tejada et al., 2016), Sorgum and Tritricum (Naiman et al. , 2009; Rangel et al., 2014). Reporting differences (p< 0.05) due to the inoculation or effect of the bacteria compared to the control, which generally refer to the unfertilized soil.
Montalvo-Aguilar et al. (2018), evaluated the effect of fertilization with digestate at different concentrations and reported that the increase in yield was gradual (p< 0.01) in relation to the concentration, which also presents an interaction with the frequency of pasture harvest. In this experiment, the effect of digestate (sterile and non-sterile) as a means of inoculating bacteria was evaluated; as well as the effect of bacteria in sterile and non-sterile soil, that is why the objective was to evaluate the effect of bacteria that promote plant growth on the yield of cocksfood in greenhouse conditions.
Materials and methods
The experiment was carried out in a tunnel-type plastic greenhouse with side windows of the Center for Research in Applied Biotechnology of the National Polytechnic Institute (IPN), located in Tepetitla de Lardizabal, Tlaxcala (19° 16’ 50.3” north latitude, 98° 21’ 58.1” west longitude, 2 221 masl). The outdoor temperature is shown in Figure 1.
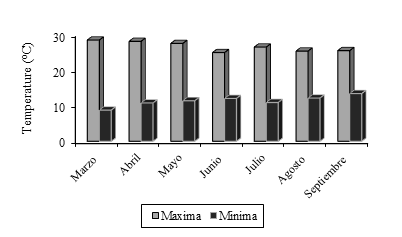
Figure 1 Maximum average temperature and minimum average environmental temperature in Tepetitla de Lardizabal, Tlaxcala. https://www.accuweather.com/es/mx/tepetitla/240244/ weather-forecast/240244.
Cocksfood seeds (Dactylis glomerata L.) were donated by the forage laboratory of the Postgraduate in Livestock of the College of Postgraduates. The digestate was obtained from the Experimental Farm of the Department of Zootechnics of the Autonomous University Chapingo (UACH). The compost made from garden waste was donated by the Zacatenco composting unit of the National Polytechnic Institute. The soil used as substrate was obtained from the experimental plot of the CIBA IPN Unit Tlaxcala, which was identified as Fluvisol with a sandy texture.
Sowing of cocksfood was performed by placing 15 seeds in the substrate containing 1.5 kg of soil in plastic pots (experimental unit). Once the seedlings emerged, a manual thinning was performed to leave only 10 stems per pot and an establishment period of 45 days was left after sowing, later a uniform cut was made at 5 cm in height, to reduce the effect of covariate and plant growth-promoting bacteria (PGPB) were inoculated directly into the rhizosphere of the grass by means of plastic syringes.
Selection and inoculation of PGPB batteries
Serial dilutions were made of the soil, compost and digestate samples. 1 mL was grown in Petri dishes at three dilutions 10-2, 10-4 and 10-6, with an incubation period of 24 h at 30 °C. Pure cultures were obtained to describe the particular characteristics and with the selected morphotypes they were inoculated into the selective and specific media: Paenibacillus, Variovorax, Lysobacter, Azospirillum, Streptomyces, Streptomyces, Pikovskaya, Ashby, NFIP and NBRIP (Bashan and Holguin, 1997; Noumavo et al., 2013; Beghalem et al., 2017).
Bacteria that grew in these media were re-inoculated into Pikovskaya, Ashby, NFb and NBRIP specific media to evaluate their potential as potassium solubilizers, nitrogen fixers and phosphorus solubilizers, respectively. The strains selected for this experiment were identified as Ewingella americana, Pseudomonas clororaphis, Bacillus toyonensis and Microbacterium oxidans, which were previously identified by means of 16S rRNA sequencing.
The inoculation of the bacteria was carried out directly in the soil, in the rhizosphere of the tillers at the beginning of the season, at a concentration of 1 x 108 CFU mL-1 and the inoculation medium was the liquid digestate. A completely czar design with a 5 x 2 x 2 factorial arrangement was used, where the factors were: bacteria (five strains of bacterial), inoculation medium (sterile and non-sterile digestate) and substrate (sterile soil and non-sterile soil). In addition to this, experimental units with uninoculated soil (negative control) and experimental units with chemical fertilization (triple 17) were established as positive control. Protein values were plotted in SigmaPlot V.10.0 and statistical analyzes were performed using the GLM procedure in SAS® Statistical Software Version 9.0 for Windows®. The treatment means were compared using Tukey at a significance level of 5%. The media and substrates were sterilized in a Prado brand autoclave, model AH-80170.
Variables evaluated
The DM yield was determined every five weeks (Velasco et al., 2001) at a harvest height of five centimeters, placing all the cut forage in previously identified paper bags. The harvested plant material was washed and weighed fresh, and then dried in a forced air stove at 70 °C, for 48 h to a constant weight, and the dry matter content was determined.
Before each defoliation, the height of the forage was recorded with a fifty centimeter ruler and an accuracy of 0.1 cm, in randomly chosen plants, with the ruler placed completely vertical from the base of the plant to the youngest top leaf (Castillo et al., 2009; Castro et al., 2011). Likewise, the chlorophyll content (SPAD units) was measured before each cut, taking 3 samples per bunch, placing the sensor of the Apogee instruments MC-100 instrument on the exposed upper leaves with the ligule well differentiated.
The protein content in leaves was obtained from a sample of each treatment in the middle of each evaluated period, which was ground in a porcelain mortar and sieves with 0.5 and 0.17 mm opening were used, the material obtained was introduced into 1.5 ml Appendort tubes, which were analyzed by the Thermo Scientific team (Flash 2000 Series, Organic Elemental Analyzer).
Results and discussion
Cocksfood forage yield recorded that both in spring and summer there were differences (p< 0.05) due to the effects of different bacteria, in spring P. clororaphis was the one with the lowest record (2.74 g DM pot-1) and it was not different (p< 0.05) from the rest of the bacteria except for E. mericana (soil), which was the one that registered (p< 0.05) the highest value (3.5 g DM pot), surpassing P. chlororaphis 27% (lowest record), in 20% to M. oxidans and B. toyonensis, respectively and in 5% to the same species, which was obtained from the digestate. While in the summer the behavior of the yield changed and the bacteria (E. mericana (soil)) that obtained the highest yield in spring, now registered (p< 0.05) the lowest yield (2.98 g DM pot-1), but it was not different (p> 0.05) to its same species that was obtained from the digestate, nor to P. clororaphis and M. oxidans, where the latter were similar (p> 0.05) to B. toyonensis, which was the one that registered (p< 0.05) the highest value (3.74 g DM pot-1), exceeding M. oxidans and P. clororaphis by 14 and 18%, respectively, and E. mericana of digestate and compost, respectively, and 21 and 25%.
The inoculation medium (digestate) did not register differences (p> 0.05) between being sterilized and non-sterilized, which infers that the result in performance is due to bacteria and not to the medium, while the same result was obtained with the ground; that is, the effect is the same whether the soil is sterile or not, at the time of inoculation. Regarding the effect of the main factor, it was only registered that the strain (p> 0.05) had an effect on the yield both in spring and summer, while the soil and digestate did not (p> 0.05) and in the interactions only in the summer it was recorded that the strain*soil was the one that had an effect on yield (Table 1).
Table 1 Yield of dry matter (g DM pot), SPAD units and height of (cm) of cocksfood, inoculated with PGPB bacteria in sterilized and non-sterilized media and substrates.
| Factor | Yield | SPAD | Height | ||||||
| Spring | Summer | Spring | Summer | Spring | Summer | ||||
| Bacteria | Microbacterium oxidans | 2.92 AB | 3.26 AB | 2.07 | 2.3 AB | 22.3 | 19.7 | ||
| Bacillus toyonensis | 2.92 AB | 3.74 A | 2.31 | 3.1 A | 23.2 | 22.9 | |||
| Pseudomonas clororaphis | 2.74 B | 3.16 AB | 2.27 | 2.9 A | 22.7 | 20.9 | |||
| Ewingella americana Soil | 3.5 A | 2.98 B | 2.07 | 1.8 B | 22.2 | 19.7 | |||
| Ewingella americana Digestate | 3.31 AB | 3.09 B | 2.71 | 3.0 A | 24.5 | 18.4 | |||
| Digestate | Sterile | 2.91 | 3.29 | 2.34 | 2.49 | 23.5 | 20.1 | ||
| Not sterile | 3.25 | 3.24 | 2.23 | 2.81 | 23.7 | 20.4 | |||
| Soil | Sterile | 3.18 | 3.28 | 2.05 | 2.59 | 23.5 | 19.5 | ||
| Not sterile | 2.98 | 3.24 | 2.52 | 2.71 | 23.7 | 21 | |||
| Sign. | Strain | * | * | ns | * | ns | ns | ||
| Soil | ns | ns | ns | ns | ns | ns | |||
| Digestate | ns | ns | ns | ns | ns | ns | |||
| Strain*Soil | ns | * | ns | ns | ns | * | |||
| Strain*Digestate | ns | ns | ns | ns | ns | ns | |||
| Digestate*Soil | ns | ns | ns | ns | ns | ns | |||
Different capital letters in columns are statistically different Tukey (p< 0.05); Sing.= significance; *= 0.05; ns= not significant.
The SPAD units that are closely related to the chlorophyll content and as a consequence of the nitrogen in the plant, did not register differences in spring (p> 0.05) between bacteria; however, in the summer, with the exception of E. americana (soil), which registered the lowest value (1.8) (p< 0.05), in the rest of the bacteria there were no differences (p< 0.05), registering that, B. toyonensis , E. americana (digestate) and P. chlororaphis were the best (p< 0.05) despite the fact that there was no difference between them and exceeding the one with the lowest value by an average of 70%. In the factorial analysis it was recorded that neither the medium nor the substrate had an effect (p> 0.05). However, there was only an effect of the strain in the summer season, while the rest of the factors and the interactions were not significant (Table 1).
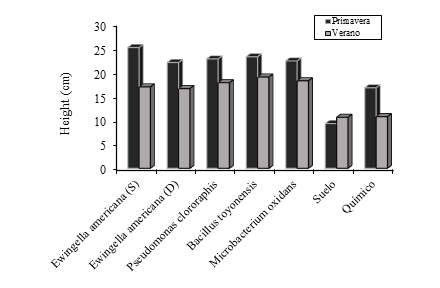
Regarding the height of the forage, both in spring and summer, there were no differences (p> 0.05). However, in the summer time in the factor analysis the interaction strain*soil, recorded an effect on height. However, the mean test did not show differences, despite the fact that the difference in height between the highest value (B. toyonensis) and the lowest (M. oxidans and E. americana) was 16% (Table 1).
Protein content recorded that with the exception of P. clororaphis in the rest of the treatments, higher protein content was recorded in the spring than in the summer (Figure 2). Likewise, in the spring it was evident that with the exception of the negative control (soil only) and P. chlororaphis, in the rest of the treatments a higher nitrogen content was found in the leaves, with E. americana (digestate) having the highest value (23%), surpassing 90% to the soil without fertilizer and 20% to chemical fertilizer. In the summer, the behavior of the protein in leaves registered that P. chlororaphis was the highest (15%), exceeding the control treatments on average by 61% (Figure 2).
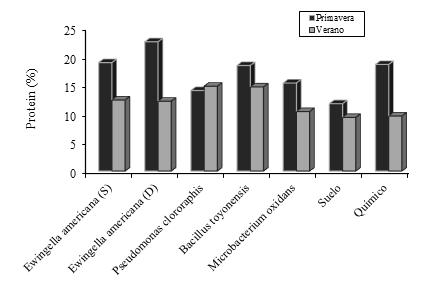
Figure 2 Protein content in cocksfood leaf, fertilized with PGPB bacteria, negative control (soil) and chemical fertilizer in the spring and summer of 2018.
Different authors, such as Hernández-Garay et al. (1997); Moliterno (2002), Ganderats et al. (2003), Mendoza-Pedroza et al. (2018), Gaytan et al. (2019), mention that forage yield is determined by the interaction of different environmental factors in a given climate or place, but the environmental variable that most influences the performance of a forage species is the environmental temperature (McKenzie et al., 1999).
However, in this experiment it is shown that this variable affects not only the plant but also the soil and, consequently, the microbiota of the rhizosphere, the difference between the spring and summer season was 1.7 °C in the maximum temperature recorded (Figure 1) and this difference was sufficient to show that in spring Ewingella americana registered the greatest effect on yield, while in summer it recorded Bacillus toyonensis (Figure 3).
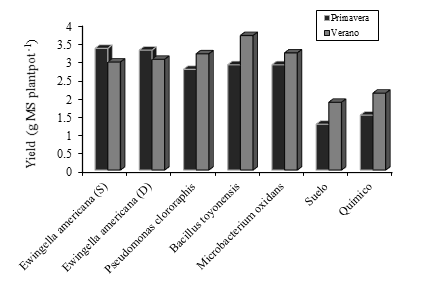
Figure 3 Cocksfood dry matter yield, fertilized with PGPB bacteria, negative control (soil) and chemical fertilizer in the spring and summer of 2018.
Meanwhile Psedumona. clororaphis, Bacillus toyonensis and the controls had more effect on the yield than in spring, which allows us to infer that, at different times of the year, the populations of rhizosphere bacteria that have symbiosis with grasses differ depending on the ambient temperature. . However, other publications have only evidenced and suggested that inoculating PGPB bacteria has effects on yield in grasses (Lopes et al., 2016), for grain production (Rangel et al., 2014), in forages of tropical climate (Humgria et al., 2016), in legumes (Pérez-Montaño et al., 2014) and in temperate pastures (Criollo et al., 2012), but in no article did they mention the effect of temperature or a conclusion regarding this variable.
SPAD units is a colorimetric of the leaves, which are correlated with the nitrogen content in the plant, and this element is at the same time the protein content. In this experiment it was recorded that in spring E. americana (soil), was the one that showed the highest value of SPAD units and at the same time of protein, while, in the summer, the lowest value of protein was obtained by M. oxidants (Figure 2) that also recorded the lowest values of SPAD units (Table 1). Rodríguez et al. (1998) and Gonzáles-Torres et al. (2009), mention that the determination coefficients between SPAD units and the chlorophyll content in the plant is 0.79 on average. However, these authors made the nitrogen determination in the entire plant and correlated it with the values obtained in the chlorophyll meter. Whereas, in this experiment, only the record of the portable meter was taken in the leaves, which are the ones that contain the highest protein content in a grass.
Regarding the digestate and the soil, the results obtained show that the condition of the application (sterile or not) does not influence the dry matter yield of cocksfood, despite the fact that the non-sterile medium was 12% higher than the sterilized one (digestate) and in the soil only a difference of 3% was registered. In the case of digestate, the difference in the percentage could be due to the microbial load that has beneficial effects on the soil, while the nutrient supply remains the same.
This statement coincides with what Tilvikiene et al. (2018), who reported the benefits of microorganisms and the contribution of organic matter when fertilizing cocksfood with digestate for five consecutive years. Montalvo-Aguilar et al. (2018), reported that the higher the concentration of the digestate, the forage yield has an upward trend in the yield of Lolium perenne; likewise, Walsh et al. (2012) reported that fertilization with digestate in Ballico perennial, exceeds (p< 0.05) in the yield to grasslands associated with white clover (Trifolium repens). Tempere and Viiralt (2014) reported that in temperate pastures fertilizing with digestate increases the yield by an average of 2.4 t DM ha-1, compared to unfertilized meadows and is 85% higher than chemical fertilization.
Regarding forage height, it was recorded that, with the exception of the negative control (p< 0.05), in the rest of the treatments in spring, higher records were obtained (Figure 4), and the treatments with bacteria were higher (p< 0.05) to the control in this variable. Some authors have shown that the forages with the highest heights are not necessarily the most productive, since as the height of the forage species increases and yield increases, in the lower strata, the dead or senescent material also accumulates, suggesting that as plant height increases, forage yield is a result of green or live biomass and decomposing tissue (Hernández-Garay et al., 1997; Velasco et al., 2003; Montes et al., 2016; Mendoza-Pedroza et al., 2018; Gaytan et al., 2019).
Conclusions
Plant growth promoting bacteria have a direct effect on the forage yield of cocksfood, and the level of influence is determined by the environmental temperature, so more should be evaluated in this regard, and thus a management of the inoculation with PGPB bacteria at different times of the year. In this experiment, all bacteria treatments were superior to controls, regardless of whether the media (digestate) and substrates (soil) were sterile or not.
Acknowledgments
To the MC Stefani Aletse Meza Zamora for the data collection and to the MC Laura Jeannette Garcia Barrera, for all the support in the isolation and identification of the bacteria evaluated in this work. To the National Polytechnic Institute, Research and Postgraduate Secretariat for financing the multidisciplinary project SIP 20180320.
REFERENCES
Bashan, Y., and Holguin, G. 1997. Azospirillum-plant relationships: environmental and physiological advances (1990 1996). Canada. Canadian J. Microbiol. 43(2):103-121. [ Links ]
Beghalem, H.; Aliliche, K.; Chriki, A. and Landoulsi, A. 2017. Molecular and phenotypic characterization of endophytic bacteria isolated from sulla nodules. Netherlands. Microbial Pathogenesis. 111:225-231. https://doi.org/10.1016/j.micpath.2017.08.049. [ Links ]
Castillo, E. G; Valles M. B. y Jarillo, R. J. 2009. Relación entre materia seca presente y altura en gramas nativas del trópico mexicano. México. Téc. Pec. Méx. 47(1):79-92. [ Links ]
Castro, R. R.; Hernández, G. A.; Aguilar, B. G. y Ramírez, R. O. 2011. Comparación de métodos para estimar rendimiento de forraje en praderas asociadas. México. Naturaleza y Desarrollo. 9(1):38-46. [ Links ]
Castro, R. R.; Hernández, G. A.; Ramírez, R. O.; Aguilar, B. G., Enríquez, Q. J. F y Mendoza, P. S. I. 2013. Crecimiento en longitud foliar y dinámica de población de tallos de cinco asociaciones de gramíneas y leguminosa bajo pastoreo. México. Rev. Mex. Cienc. Pec. 4(2):201-215. [ Links ]
Castro, R. R.; Hernández, G. A.; Vaquera, H. H.; Hernández G. J. P.; Quero, C. A. R; Enríquez, Q. J. E. y Martínez H. P. A. 2012. Comportamiento productivo de asociaciones de gramíneas con leguminosas en pastoreo. México. Rev. Fitotec. Mex. 35(1):87-95. [ Links ]
Criollo, P. J.; Obando, M.; Sánchez, M. L. and Bonilla R. 2012. Efecto de las bacterias promotoras de crecimiento vegetal (PGPR) asociadas a Penissetum clandestinum en el altiplano cundiboyacense. Colombia. Revista CORPOICA-Ciencia y Tecnología Agropecuaria. 13(2):189-195. [ Links ]
De-Bashan, L. E.; Holguin, G.; Glick, B. R. and Bashan, Y. 2007. Bacterias promotoras de crecimiento en plantas para propósitos agrícolas y ambientales. In: Microbiología Agrícola: hongos, bacterias y microfauna, control biologico, planta-microorganismo. (Eds.). Ferrara-Cerrato, R. and Alarcon, A. Chapter 8. Published by Ed. Trillas, México city. 170-224 pp. [ Links ]
Esquivel-Cote, R.; Gavilanes-Ruiz, M.; Cruz-Ortega, R. y Huante P. 2013. Importancia agrobiotecnológica de la enzima ACC desaminasa en rizobacterias, una revisión. Rev. Fitotec. Mex. 36(3):251-258. [ Links ]
Flore-Santiago, E. J.; Guerrero-Rodríguez, J. D.; Cadena-Villegas, S.; Alejos-de la Fuente, J. I.; Mendoza-Pedroza, S. I.; Luna-Guerrero, M. J.; Peña-Aguilar, M. A. y Hernández-Garay, A. 2018. Dinámica de tallos de pasto Ovillo (Dactilis glomerata L.) solo y asociado con Ryegrass perenne (Lolium perenne L.) y Trébol blanco (Trifolium repens L.). Agroproductividad. 11(5):10-17. [ Links ]
Ganderats, F. S. y Hepp, K. C. 2003. Mecanismos de crecimiento de Lolium perenne, Festuca arundinacea y Dactylis glomerata en la zona intermedia de Aysén. Chile. Agric. Téc. 63(3):259-265. [ Links ]
Gaytan, V. J. A.; Castro, R. R.; Villegas, A. Y.; Aguilar, B. G.; Solís, O. M. M.; Carrillo, R. J. C. y Negrete, S. L. O. 2019. Rendimiento de alfalfa (Medicago sativa L.) a diferentes edades de la pradera y frecuencias de defoliación. México. Rev. Mex. Cienc. Pec. 10(2):353-366. [ Links ]
González-Torres, A.; Figueroa-Viramontes, U.; Delgado, J. A.; Nuñez-Hernández, G.; Cueto-Wong, J. A.; Preciado-Rangel, P. y Palomo-Gil A. 2009. Calibración del SPAD-502 para evaluar requerimientos de nitrógeno en maíz forrajero. México. Terra Latinoam. 27(4):303-309. [ Links ]
Hernández-Garay, A.; Matthew, C. and Hodgson, J. 1997. Effect of spring grazing management on perennial ryegrass and ryegrass-white clover pastures. 1. Tissue turnover and herbage accumulation. New Zealand. New Zealand J. Agric. Res. 40:25-35. [ Links ]
Hungria, M.; Nogueira, M. A. and Silva, A. R. 2016. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: an environment-friendly component in the reclamation of degraded pastures in the tropics. Netherlands. Agric. Ecosys. Environment. 221:125-131. [ Links ]
Lopes, M. J. S.; Dias-Filho, M. B.; Castro, T. H. R. and Silva, G. B. 2016. Light and plant growth-promoting rhizobacteria effects on Brachiaria brizanta growth and phenotypic plasticity to shade. United Kingdom Grass and Forage Science. 73(2):493-499. [ Links ]
McKenzie, B. A.; Kemp, P. D.; Moot, D. J.; Matthew, C. and Lucas, R. J. 1999. Environmental effects on plant growth and development. In: White, J. and Hodgson, J. (Ed.). New Zealand Pasture and Crop Science. Auckland, New Zealand. Oxford University Press. 29-44 pp. [ Links ]
Mendoza-Pedroza, S. I.; Cadena-Villegas, S.; Hernández-Garay, A.; Vaquera-Huerta, H.; Villareal-González, J. A. y Flores-Santiago, E. J. 2018. Cambios en la frecuencia de defoliación para recuperar la densidad de plantas en una pradera de alfalfa (Medicago sativa L.). Agroproductividad. 11(5):51-55. [ Links ]
Moliterno, E. A. 2002. Variables básicas que definen el comportamiento productivo de mezclas forrajeras en su primer año. Agrociencia. 6(1):40-52. [ Links ]
Montalvo-Aguilar, K. J.; Castro-Rivera, R.; Solís-Oba, M. M.; Aguilar-Benitez, G.; GarcÍa-Barrera L. J. y Hernández-Garay, A. 2018. Efecto de la frecuencia de defoliación y la fertilización con digestato en los componentes del rendimiento de Ballico perenne (Lolium perenne L.). Agroproductividad. 11(5):3-9. [ Links ]
Montes, C. F. J.; Castro, R. R.; Aguilar, B. G.; Sandoval, T. S.; Solís, O. M. M. 2016. Acumulación estacional de biomasa aérea de alfalfa var. Oaxaca criolla (Medicago sativa L.) Rev. Mex. Cienc. Pec. 7(4):539-552. [ Links ]
Mora, M.; Demanet, R.; Acuña, J. J.; Viscardi, S.; Jorquera, M.; Rengel, Z. and Durán, P. 2017. Aluminum-tolerant bacteria improve the plant growth and phosphorus content in ryegrass grown in a volcanic soil amended with cattle dung manure. Netherlands. Appl. Soil Ecol. 115:19-26. [ Links ]
Naiman, A. D.; Latrónico, A. and García de Salomone, I. E. 2009. Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: Impact on the production and culturable rhizosphere microflora. France. Eur. J. Soil Biol. 45:44-51. [ Links ]
Noumavo, P. A.; Kochoni, E.; Didagbé, Y. O.; Adjanohoun, A.; Allagbé, M.; Sikirou, R. and Baba-Moussa, L. 2013. Effect of different plant growth promoting Rhizobacteria on maize seed germination and seedling development. USA. Am. J. Plant Sci. 04(05):1013-1021. [ Links ]
Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R. A.; Del Cerro, P.; Espuny, M. R.; Jiménez-Guerrero, I. and Cubo, T. 2014. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Germany. Microbiol. Res. 169(5-6):325-336. [ Links ]
Posada, L. F.; Álvarez, J. C.; Romero-Tabarez, M.; de-Bashan, L. and Villegas-Escobar V. 2018. Enhanced molecular visualization of root colonization and growth promotion by Bacillus subtilis EA-CB0575 in different growth system. Germany. Microbiol. Res. 217:69-80. [ Links ]
Ramakrishna, W.; Yadav, R. and Li, K. 2019. Plant growth promoting bacteria in agriculture: Two sides of a coin. Netherlands. Appl. Soil Ecol. 138:10-18. [ Links ]
Rangel, L. J. A.; Ramírez, G. R. M.; Cervantes, O. F.; Mendoza, E. M.; García, M. E. y Rivera, R. J. G. 2014. Biofertilización de Azospirillum spp. y rendimiento de grano de maíz, sorgo y trigo. Argentina. Revista de la Facultad de Ciencias UNCUYO. 46(2):231-238. [ Links ]
Rashid, S.; Charles, T. C. and Glick, B. R. 2012. Isolation and characterization of new plant growth-promoting bacterial endophytes. Netherlands. Appl. Soil Ecol. 61:217-224. [ Links ]
Rodríguez, M. M. N.; Alcántar, G. G.; Aguilar, S. A.; Etchevers, B. J. and Santizó, R. A. 1998. Estimación de la concentración de nitrógeno y clorofila en tomate mediante un medidor portatil de clorofila. México. Terra Latinoam. 16(2):135-141. [ Links ]
Singh, M. V.; Kumari, M. S.; Prakash, V. J.; Kumar, A.; Aeron, A.; Kumar, M P.; Kumar, B. J.; Pattanayak, A.; Neveed, M. and Dotoniya, M. L. 2017. Plan beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Netherlands. Ecol. Eng. J. Ecotechnol. 107:8-12. [ Links ]
Tejada, M.; Rodríguez-Morgado, B.; Gómez, I.; Franco-Andreu, L.; Benítez, C. and Parrado, J. 2016. Use of biofertilizers obtained from sewage sludges on maize yield. Netherlands. Eur. J. Agron. 78:13-19. [ Links ]
Tempere, M. and Viiralt, R. 2014. The efficiency of biogas digestate on grassland compared mineral fertilizer and cattle slurry. Latvia. Research for Rural Development. 1:89-94. [ Links ]
Tilvikiené, V.; Slepetiené. A. and Kadziuliené. 2018. Effects of 5 years of digestate application on biomass production and quality of cocksfoot (Dactylis glomerata L.). United Kingdom. Grass and Forage Science. 73(1):206-2017. [ Links ]
Velasco, Z. M. E.; Hernández, G. A.; González, H. V. A.; Pérez, P. J.; Vaquera, H. H. y Galvis, S. A. 2003. Curva de crecimiento y acumulación estacional del pasto ovillo (Dactylis glomerata L.). Téc. Pec. Méx. 39(1):1-14. [ Links ]
Villarreal, G. J. A.; Hernández, G. A.; Martínez, H. P. A.; Guerrero, R. J. D. y Velasco, Z. M. E. 2014, Rendimiento y calidad de forraje del pasto ovillo (Dactylis glomerata L.) al variar la frecuencia e intensidad del pastoreo. México. Rev. Mex. Cienc. Pec. 5(2):231-245. [ Links ]
Walsh, J. J.; Jones, D. L.; Edwards-Jones, G. and Williams, A. P. 2012. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. United Kingdom. J. Plant Nutr. Soil Sci. 175(6):840-845. [ Links ]
Received: January 01, 2020; Accepted: March 01, 2020











 text in
text in