Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.11 no.spe24 Texcoco abr./may. 2020 Epub 07-Mayo-2021
https://doi.org/10.29312/remexca.v0i24.2363
Articles
Effect of PGPB bacteria, compost and digestate on the dry matter yield of cocksfood
1Instituto de Investigación de Zonas Desérticas-Universidad Autónoma de San Luís Potosí. Altair 200, Colonia del Llano, San Luis Potosí. CP. 78377. (gisela.aguilar@uaslp.mx).
2Instituto Politécnico Nacional-Centro de Investigación en Biotecnología Aplicada. Ex Hacienda de San Juan Molino, Carretera Tecuexcomac-Tepetitla km 1.5, Tlaxcala. CP. 90700 (mirobatlx@hotmail.com; valgayou@gmail.com).
3Facultad de Agronomía y Veterinaria-Universidad Autónoma de San Luis Potosí, carretera San Luis Potosí-Matehuala km 14.5, Ejido Palma de la Cruz, Soledad de Graciano Sánchez, SLP. CP. 78439. (pablo.lara@uaslp.mx).
4Instituto Tecnológico del Altiplano de Tlaxcala. Carretera federal San Martín Texmelucan-Tlaxcala km 7.5, San Diego Xocoyucan, Tlaxcala. CP. 90122. (mael071260@hotmail.com ).
The aim of the present study was to determine the effect of compost, digestate and PGPB (plant growth-promoting bacteria) on yield forage accumulation curve, growth rate, forage height and SPAD (soil plant analysis development) units in newly established of cocksfood under greenhouse conditions. The treatments were digestate (60%); compost (10% on dry soil based); bacteria: Brevibacterium frigoritolerans, Bacillus simplex and Pseudomonas putida; positive control (triple 17 fertilization) and negative control (soil without fertilization). A completely random experimental design was used, the experimental unit was a pot with ten tillers, with four replicates by treatment. The highest values (p< 0.05) of dry matter yield (6.4 g DM pot), growth rate (0.15 g DM pot d-1) and forage height (18.3 cm) were recorded in treatment with compost, with which the final yield was 200% higher than the negative control. The treatment with digestate showed lower values than those obtained with compost but surpassed the rest of the treatments. The best PGPB were Pseudomonas putida and Bacillus simplex, which outperformed Brevibacterium frigoritolerans and negative control by 25 and 37%, respectively. The PGPB bacteria can be a fertilization alternative since the yield was higher than with the negative control and was equal to the yield with chemical fertilization; however, the two organic fertilizers (compost and digestate) favored the higher yield.
Keywords: Bacillus simplex; Brevibacterium frigoritolerans; Dactylis glomerata; Pseudomonas putida; forage yield and
El objetivo fue determinar el efecto de composta, digestato y bacterias plant growth-promoting bacteria (PGPB) en la curva de crecimiento, acumulación de biomasa, tasa de crecimiento, altura de planta y unidades soil plant analysis development (SPAD) en pasto ovillo recién establecido, bajo condiciones de invernadero. Los tratamientos fueron: digestato (60%), composta (10% en base seca del suelo), bacterias: Brevibacterium frigoritolerans, Bacillus simplex, Pseudomonas putida, control positivo (fertilización con triple 17) y el control negativo (suelo sin fertilización). Se utilizó un diseño experimental completamente al azar, la unidad experimental fue una maceta con diez tallos de pasto ovillo, con cuatro repeticiones por tratamiento. Los valores más altos (p< 0.05) de materia seca (6.4 g MS maceta), tasa de crecimiento (0.15 g MS maceta d-1) y altura de forraje (18.3 cm) se registraron en el tratamiento con composta; donde el rendimiento final de materia seca superó 200% al testigo negativo. El tratamiento con digestato evidenció valores inferiores a los obtenidos con composta, pero superó al resto de los tratamientos. Las mejores bacterias PGPB fueron Pseudomonas putida y Bacillus simplex que superaron el rendimiento de materia seca 25 y 37% con respecto a Brevibacterium frigoritolerans y al control negativo, respectivamente. Las bacterias PGPB pueden ser una alternativa de fertilización ya que el rendimiento de materia seca fue mayor que con el control negativo y se igualó al rendimiento obtenido con fertilización química; sin embargo, los dos fertilizantes orgánicos (composta y digestato) favorecieron el mayor rendimiento de materia seca.
Palabras clave: Bacillus simplex; Brevibacterium frigoritolerans; Dactylis glomerata; Pseudomonas putida; producción de forraje
Introduction
Forage yield is determined by various factors such as temperature, humidity and fertilization (Ahmad et al., 2016). Mineral fertilization of forage grasses is a necessary activity to increase yields and to cope with the low natural or induced fertility of the soils, this practice is expensive and has adverse environmental implications if done improperly. An alternative to the application of mineral fertilizers in forage crops is organic fertilizers, which provide N in organic forms such as proteins and amino acids, more or less stable, which gradually become mineralized in forms assimilable by plants (Ramos and Terry, 2014).
The use of plant growth promoting bacteria (PGPB) promotes better absorption of nutrients in the soil due, among others, to the ability of bacteria to solubilize phosphorous, fix nitrogen and synthesize siderophores, also produce phytostimulant substances (auxins, gibberellins, cytokinins) or can act as stress controllers (biological control through antagonistic activity against phytopathogenic microorganisms) in the plant (De-Bashan et al., 2007; Pérez-Montaño et al., 2014; Menna et al., 2017; Singh et al., 2017).
Lopes et al. (2018) evaluated the effect of inoculation of PGPB bacteria on forage yield in Brachiaria brizanta, subjected to different radiation intensities, the results were higher yield, chlorophyll content, leaf area index and root weight (p< 0.05) with pure and associated bacteria treatments compared to controls.
Digestates are the liquid by-product of anaerobic digestion for the production of biogas, from organic solid waste. This by-product is used as a fertilizer since they contain phytohormones (gibberellins and indolacetic acid) dissolved in organic matter, a considerable amount of microorganisms, as well as other bioactive compounds that have the potential to promote plant growth and increase stress tolerance biotic and abiotic (Yu et al., 2010).
The benefits of compost in crops have been reported in different works. Beltrán et al. (2017) estimated the forage yield of Triticale fertilized with compost obtained from feces of dairy cattle and observed that the combination of this fertilizer with inorganic fertilizer promotes a higher dry matter yield, forage height and higher stem density, compared to unfertilized plants and fertilized only with inorganic fertilizer. For the foregoing, the aim of the present work was to evaluate the effect of the different types of organic fertilization on the regrowth pattern of cocksfood, under greenhouse conditions.
Materials and methods
Description of the experimental area
The experiment was carried out in a tunnel-type plastic greenhouse with side windows of the Center for Research in Applied Biotechnology of the National Polytechnic Institute, located in Tepetitla de Lardizabal, Tlaxcala (19° 16’ 50.3” north latitude, 98° 21’ 58.1” west longitude, 2 221 masl). The average outdoor temperature is shown in Figure 1.
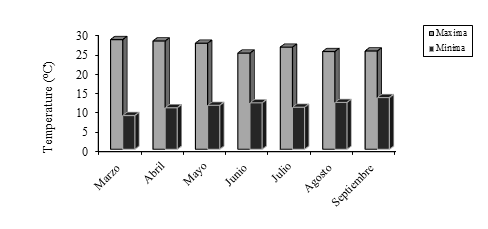
Figure 1 Maximum average temperature and minimum average environmental temperature, in Tepetitla de Lardizabal, Tlaxcala. https://www.accuweather.com/es/mx/tepetitla/240244/ weather-forecast/ 240244.
The cocksfood seeds (Dactylis glomerata L.) were donated by the Forage Laboratory of the Livestock Program of the Postgraduate College. The digestate was obtained from the Experimental Farm of the Department of Zootechnics of the Autonomous University Chapingo (UACH). The compost was donated by the Zacatenco composting unit of the National Polytechnic Institute. The soil used as a substrate was obtained from the experimental plot of the CIBA IPN Unidad Tlaxcala, which was identified as fluvisol with a sandy texture.
Experiment development
Cocksfood was sown by placing 15 seeds in plastic pots containing 1.5 kg of soil (experimental unit). Once the seedlings emerged, manual thinning was performed to leave only 10 stems per pot and an establishment period of 45 days was left after planting. Subsequently, a uniform cut was made at a height of five cm, to reduce the effect of the covariate, and the PGPB bacteria were inoculated and organic fertilizers were applied in the early spring of 2018.
Selection and inoculation of PGPB batteries
Serial dilutions were made of the soil, compost and digestate samples. 1 ml was grown in Petri dishes at three dilutions 10-2, 10-4 and 10-6, with an incubation period of 24 h at 30 °C. Pure cultures were obtained to describe the particular characteristics and with the selected morphotypes, Paenibacillus, Variovorax, Lysobacter, Azospirillum, Streptomyces, Streptomyces, Pikovskaya, Ashby, NFb and NBRIP were inoculated into the selective media (Bashan and Holguin, 1997; Noumavo et al., 2013; Beghalem et al., 2017).
Bacteria that grew in these media were re-inoculated into Pikovskaya, Ashby, NFb and NBRIP specific media to evaluate their potential as potassium solubilizers, nitrogen fixers and phosphorus solubilizers, respectively. The strains selected for this experiment were identified as Pseudomonas putida, Bacillus simplex and Brevibacterium frigoritolerans, which were previously identified by means of 16S rRNA sequencing.
The inoculation of the bacteria was carried out directly in the soil, in the rhizosphere of the tillers, at the beginning of the season, with a sterile 1 mL syringe of nutrient broth at a concentration of 1 x 108 CFU mL-1 per experimental unit.
Treatment and experimental design
A completely randomized design and four repetitions per treatment were used, which were: concentration of digestate (60%), compost (10% in dry soil), bacteria: Brevibacterium frigoritolerans, Bacillus simplex, Pseudomonas putida, positive control (fertilization with triple 17) and the negative control (soil without fertilization).
Variables evaluated
The dry matter yield was obtained from cuts at weeks one, two, three, four, five, six and seven. The harvest height was five centimeters, placing all the cut forage in previously labeled paper bags. The harvested plant material was washed and weighed fresh, and then dried in a forced air stove at 70 °C, for 48 h to a constant weight, and the proportion of dry matter was determined.
Before each cut, the height of the forage to the recently exposed top leaf was recorded in randomly chosen plants, with a graduated ruler of 30 cm and an accuracy of 0.1 cm (Castillo et al., 2009).
The growth rate (TC) was calculated with the dry matter yield data per cut using the following formula.
Where: FC= forage harvested (g MV pot) and t= days elapsed between one cut and the next. The chlorophyll content (SPAD units) were recorded before each cut, taking 3 samples per experimental unit, placing the Apogee instruments MC-100 instrument sensor on the exposed upper leaf with the ligule well differentiated.
Statistical analysis
The values grouped by week of growth were plotted using the SigmaPlot V.10 statistical software and analyzed using the GLM and PROC MIXED procedures of the Statistical Software SAS® version 9.0 for Windows®. The treatment means comparison was made using Tukey at a significance level of 5%.
Results and discussion
The highest average height of the cocksfood was obtained with the compost treatment (10.5 cm); however, statistically there was no difference (p> 0.05) with the treatment with digestate and Pseudomonas putida, in addition these last two treatments did not present differences (p> 0.05) with the rest of the treatments. The average height was higher (p> 0.05) in the plants harvested at seven weeks and this variable showed no difference (p> 0.05) in the cuts at five and six weeks, despite the fact that at six weeks the higher forage yield (Table 1).
Table 1 Height of cocksfood plant (Dactylis glomerata L.) at different weeks of growth after a homogenization cut.
| Treatments | Growth weeks | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Pom. | SEM | Sig. | |
| Compost | 6.13Ad | 6.9Ad | 8.6Ac | 9.6Ac | 11.3Ab | 12.6Ab | 18.3Aa | 10.5A | 0.42 | ** |
| Digestate | 5.1Bd | 5.6Bd | 6.9BCd | 9.1Ac | 9.6Bbc | 11.4Ab | 16.2Aa | 9.13AB | 0.55 | ** |
| P. putida | 5.2Be | 5.3Be | 7.7ABd | 8.7Acd | 10.4ABcb | 12.4Ab | 15ABa | 9.25AB | 0.59 | ** |
| B. simplex | 5.2Bc | 5.6Bc | 7.1BCbc | 7.1Bbc | 7.8Cb | 8.3Bb | 10.3Ca | 7.36B | 0.54 | ** |
| B. frigoritolerans | 5.3Bc | 5.4Bc | 5.3Dc | 5.9Bbc | 6.3Cbc | 7.7Bb | 13.8ABa | 7.12B | 0.56 | ** |
| Chemical | 5.1Bd | 5.2Bd | 5.5Dd | 6.3Bcd | 7.6Cbc | 9.3Bb | 12.2Ca | 7.31B | 0.56 | ** |
| Soil | 5.3Bd | 5.9ABcd | 6.3CDcd | 6.4Bcd | 7.3Cbc | 8.6Bab | 9.5Da | 7.06B | 0.42 | ** |
| Average | 5.35e | 5.73 e | 6.76ed | 7.58cd | 8.64 bc | 10.03b | 13.63a | ** | ||
| SEM | 0.15 | 0.32 | 0.35 | 0.39 | 0.43 | 0.61 | 0.86 | |||
| Sig. | ** | ** | ** | ** | ** | ** | ** | ** | ||
Different lowercase literals in a row are statistically different Tukey (p< 0.05). Different capital letters in columns are statistically different Tukey (p< 0.05). Average (Prom.); significance (Sig.); *= 0.05; **= 0.01.
Likewise, the compost treatment promoted the highest heights (p< 0.05) in all the cutting weeks, while P. putida and the digestate showed the highest heights from week four, but their values were lower than those obtained with compost, although superior to the rest of the treatments.
The SPAD units in the compost and digestate treatments were higher (p< 0.05), during all the weeks of growth evaluated, whereas with B. simplex, B. frigoritolerans and in the positive and negative controls the values were not different (p< 0.05). With the exception of the treatments with inorganic fertilizer and B. frigoritolerans, in the rest of the treatment’s differences were observed (p< 0.05) in the SPAD values registered in the different weeks of growth (Table 2). With S. putida in week one and two there were no differences with respect to the rest of the treatments, but from the third week the SPAD units increased, equaling the values obtained with compost and digestate.
Table 2 Chlorophyll content (SPAD units cm2) of cocksfood (Dactylis glomerata L.) in the different weeks of growth after a uniform cut.
| Treatments | Growth weeks | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Prom. | SEM | Sig. | |
| Compost | 3.2 A | 3.1 | 3.2A | 3.2A | 3.3A | 3.5A | 3.4A | 3.28A | 0.15 | ** |
| Digestate | 3.1A | 2.9 | 3.2A | 3AB | 3.1AB | 3.5A | 3.2A | 3.16A | 0.32 | ** |
| P. putida | 3.3A | 3 | 2.1B | 2.9AB | 2.2C | 2.6B | 3.1A | 2.75B | 0.42 | ** |
| B. simplex | 2.1Bb | 2.1b | 2.2b | 2.2 ABb | 2.4BCab | 2.3Bb | 2.9ABa | 2.3C | 0.17 | ** |
| B. frigoritolerans | 2.2B | 2.3 | 2.1B | 2.3AB | 2.1C | 2.4B | 2.1C | 2.24C | 0.15 | ** |
| Chemical | 2.1B | 2.4 | 2.2B | 2.2AB | 2.2C | 2.2B | 2.3BC | 2.25C | 0.19 | ** |
| Soil | 1.2Cc | 2.1b | 1.9Bb | 2.1Bb | 2.2Cb | 2.3Bb | 2.9ABa | 2.08C | 0.14 | ** |
| Prom. | 2.46 | 2.57 | 2.42 | 2.56 | 2.49 | 2.69 | 2.87 | ns | ||
| SEM | 0.11 | 0.37 | 0.14 | 0.28 | 0.24 | 0.23 | 0.17 | |||
| Sig. | ** | ** | ** | ** | ** | ** | ** | ** | ||
Different lowercase literals in a row are statistically different Tukey (p< 0.05). Different capital letters in columns are statistically different Tukey (p< 0.05). Average (Prom.); Significance (Sig.); *= 0.05; **= 0.01.
Regarding the growth rate, the highest values were obtained in the compost and digestate treatments. For this variable, P. putida outperformed (p< 0.05) the other PGPB bacteria and both controls, and it is observed that the treatment with compost, during the entire evaluation period, was the one with the highest growth rate, otherwise, with digestate this variable only increased in week six (Table 3).
Table 3 Growth rate (g DM pot d-1) of cocksfood (Dactylis glomerata L.) in the different weeks of growth after a uniform cut.
| Treatments | Growth weeks | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Prom. | SEM | Sig. | |
| Compost | 0.13Ab | 0.1Ac | 0.11Ac | 0.11Ac | 0.11Ac | 0.15Aa | 0.11Ab | 0.11A | 0.003 | ** |
| Digestate | 0.09Ba | 0.06BCb | 0.058Bb | 0.049BCb | 0.055BCb | 0.099Ba | 0.06BCb | 0.06C | 0.006 | ** |
| P. putida | 0.08BCa | 0.07Bab | 0.059Bbc | 0.051Bcd | 0.059Bc | 0.073Ca | 0.05BCd | 0.07B | 0.003 | ** |
| B. simplex | 0.08Ca | 0.06BCb | 0.053BCbc | 0.048CDc | 0.056BCDbc | 0.085BCa | 0.057Bbc | 0.05D | 0.004 | ** |
| B. frigoritolerans | 0.08BCa | 0.064BCab | 0.047CDbc | 0.041Ec | 0.04Ec | 0.066CDab | 0.031Cc | 0.054DE | 0.005 | ** |
| Chemical | 0.077BCa | 0.051CDb | 0.046CDb | 0.043EDb | 0.043EDb | 0.075Ca | 0.047BCb | 0.054DE | 0.005 | ** |
| Soil | 0.064Ca | 0.046Dab | 0.037Db | 0.036Fb | 0.046CDEb | 0.05Dab | 0.042BCb | 0.051E | 0.005 | ** |
| Prom. | 0.085a | 0.065b | 0.058c | 0.054c | 0.058 c | 0.083a | 0.064b | 0.0011 | ** | |
| SEM | 0.006 | 0.004 | 0.003 | 0.001 | 0.003 | 0.006 | 0.006 | |||
| Sig. | ** | ** | ** | ** | ** | ** | ** | ** | ||
Different lowercase literals in a row are statistically different Tukey (p< 0.05). Different capital letters in columns are statistically different Tukey (p< 0.05). Average (Prom.); Significance (Sig.); *= 0.05; **= 0.01.
The highest dry matter yield and the highest forage accumulation (p< 0.05) were achieved in week six, regardless of the treatment evaluated. However, the highest average value (p< 0.05) per treatment was obtained with compost (3.4 g DM pot), followed by digestate (1.9 g DM pot). The lowest values (p< 0.05) of dry matter were obtained with the negative control and Brevibacterium frigoritolerans and the treatments of Pseudomonas putida, Bacillus simplex and the chemical fertilizer showed no differences (Table 4). The highest yield from week one to seven was achieved with the compost, with the digestate no differences were distinguished from week one to five and it was until week six that it was different (p< 0.05) from the rest of the treatments (Figure 2).
Table 4 Forage yield (g DM pot), of cocksfood (Dactylis glomerata L.) in the different weeks of growth after a uniform cut.
| Treatments | Growth weeks | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Prom. | SEM | Sig. | |
| Compost | 0.92Ag | 1.46Af | 2.3Ae | 3.14Ad | 4.03Ac | 6.4Aa | 5.37Ab | 3.4A | 0.13 | ** |
| Digestate | 0.67Bd | 0.86BCDd | 1.23BCcd | 1.39BCcd | 1.95BCc | 4.18Ba | 2.96BCb | 1.9B | 0.23 | ** |
| P. putida | 0.53BCe | 0.99Bd | 1.25Bcd | 1.45Bc | 2.1Bb | 3.1Ca | 2.3BCb | 1.7C | 0.091 | ** |
| B. simplex | 0.55BCg | 0.87BCde | 1.3BCde | 1.35BCcd | 1.99Bc | 3.6BCa | 2.8Bb | 1.6C | 0.22 | ** |
| B. frigoritolerans | 0.54BCc | 0.9BCbc | 0.98CDbc | 1.15CDbc | 1.45Cb | 2.8CDa | 1.5Cb | 1.3D | 0.21 | |
| Chemical | 0.54BCd | 0.71CDd | 0.96BCDcd | 1.2BCDcd | 1.5BCc | 3.2Ca | 2.3BCb | 1.6C | 0.2 | ** |
| Soil | 0.44Ce | 0.65Dde | 0.8Dcd | 1Dc | 1.6BCb | 2.1Da | 2.06BCa | 1.2D | 0.09 | ** |
| Average | 0.59g | 0.91f | 1.22e | 1.52d | 2.05c | 3.68a | 2.69 b | 0.044 | ** | |
| SEM | 0.053 | 0.067 | 0.088 | 0.064 | 0.16 | 0.26 | 0.3 | 0.06 | ||
| Sig. | ** | ** | ** | ** | ** | ** | ** | ** | ||
Different lowercase literals in a row are statistically different Tukey (p< 0.05). Different capital letters in columns are statistically different Tukey (p< 0.05). Average (Prom.); Significance (Sig.); *= 0.05; **= 0.01.
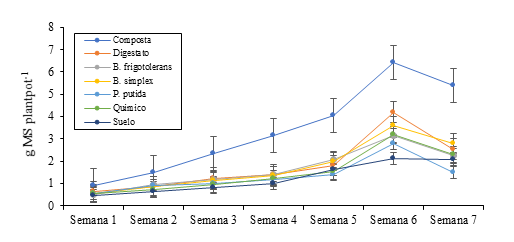
Figure 2 Cocksfood dry matter yield under different fertilization schemes, during the spring time of 2018.
In the literature it has been reported that the factors that determine the speed and magnitude of growth of a forage plant are: climate (radiation, photoperiod, temperature, humidity and precipitation) (McKenzie et al., 1999), soil (physical, chemical characteristics, topographic and fertilization); species (photosynthetic route, genetic potential of different species and varieties); and grassland management (intensity and frequency of defoliation) (Mendoza-Pedroza et al., 2018). All the factors do not act separately and the growth of the plant responds to an interaction between them (Jiménez and Martínez, 1984; Hernández-Garay et al., 1997; Moliterno, 2002; Ganderats et al., 2003). However, in this experiment the forage species evaluated was subjected to the same conditions of temperature, humidity and radiation, modifying the source of fertilization; therefore, it is evident that the fertilization source affects the yield of the species.
It has been reported that the addition of compost modifies the physical, chemical and microbiological properties of the soil, generating more favorable conditions for the plant, which is reflected in a higher yield (Ramos and Terry 2014; Liu et al., 2016). Furthermore, the benefits of compost have been evidenced in various studies with vegetables and there are few studies published in temperate pastures, so the results presented in this document provide a first of study in forage grasses with temperate climates.
Regarding the results obtained with the digestate, these coincide with what was stated by Rancane et al. (2015); Walsh et al. (2012), who observed an increase in forage yield with the addition of digestate. For their part, Tilvikiene et al. (2018) evaluated the biomass yield in cocksfood fertilized with different concentrations of digestates for five consecutive years and noted that fertilization with this fertilizer causes high concentrations of structural components (cellulose and hemicellulose) in the biomass, which has a greater impact forage yield.
Montalvo-Aguilar et al. (2018), warned that irrigation with digestate (60%) after each cut favors the increase in forage yield of Lolium perenne, obtaining more than 150% of yield compared to the control (soil alone). Likewise, with the highest concentrations of digestate, the highest protein levels were observed (28% at the four-week cutoff frequency), and this variable is correlated with the values obtained in this experiment with the SPAD units (González-Torres et al., 2009).
The results obtained with the different PGPB bacteria coincide with that reported by Lopes et al. (2018), since they showed higher yields compared to the control that was unfertilized and inoculated soil. However, in the results obtained in this work only with Pseudomonas putida, high values were obtained in the evaluated variables. Rangel et al. (2014) report that when evaluating PGPB bacteria in the yield of corn, wheat and sorghum, only Azospirillum brasilense registered beneficial effects, exceeding 55 and 49% in soil alone and inorganic fertilizer, respectively.
Criollo et al. (2012) evaluated the effect of PGPB bacteria associated with Penissetum clandestinum, at different regrowth times (70, 100 and 130 days after sowing), reporting that bacteria of the Pseudomonas genus showed an effect on yield up to 130 days after regrowth , surpassing 150% the root weight with respect to the control. In C4 grasses, it is mentioned that for the Brachiaria genus, inoculating with PGPB bacteria is equivalent to a dose of 40 kg of nitrogen per hectare (Hungria et al., 2016).
The bacteria evaluated in this experiment have been reported as PGPB bacteria, by different authors, and their benefits as phosphate solubilizers, nitrogen fixers, and siderophore synthesizers have been mainly evaluated in culture media and in vitro conditions (Rashid et al. (2012). Other authors have evaluated them in the field such as Terry et al. (2005) who reported the beneficial effects of the genera Pseudomonas and Bacillus on tomato (Lycopersicum esculentum L.). However, the species evaluated in this work have not been evaluated in grasses of zootechnical interest and have not been reported in scientific articles.
Conclusions
PGPR bacteria can be a fertilization alternative since the evaluated variables increased their values with respect to the soil without fertilization and were equal to chemical fertilization; however, the two organic fertilizers (compost) and the digestate were superior to all the treatments evaluated. From an economic point of view, bacteria may be the best fertilization option, since these were only inoculated at the beginning of the experiment, while digestates were applied at each uniform cut and the percentage of compost in the soil is equivalent to one approximate dose of 60 t ha-1.
Acknowledgments
To the MC Laura Jeannette García Barrera, for all the support in the isolation and identification of the bacteria evaluated in this work. To the National Polytechnic Institute, Research and Postgraduate Secretariat for financing the multidisciplinary project SIP 20180320.
REFERENCES
Ahmad, J.; Iqbal, A.; Ayub, M. and Akhtar, J. 2016. Forage yield potential and quality attributes of alfalfa (Medicago sativa L.) under various agro-management techniques. Pakistan. The J. Animal Plant Sci. 26(2):465-474. [ Links ]
Beghalem, H.; Aliliche, K.; Chriki, A. and Landoulsi, A. 2017. Molecular and phenotypic characterization of endophytic bacteria isolated from sulla nodules. Netherlands. Microbial Pathogenesis. 111:225-231. https://doi.org/10.1016/j.micpath.2017.08.049. [ Links ]
Beltrán, S. M.A.; Álvarez, F. G.; Pinos, R. J. M.; García, L. J. C. y Castro, R. R. 2017. Abonos obtenidos del composteado de heces de ganado de leche vs. fertilizante en la producción de triticale (X Tritricum secale Wittmack). Argentina. Rev. Facultad UNCUYO. 49(1):95-104. [ Links ]
Castillo, E. G; Valles M. B. y Jarillo, R. J. 2009. Relación entre materia seca presente y altura en gramas nativas del trópico mexicano. México. Téc. Pec. Méx. 47(1):79-92. [ Links ]
Criollo, P. J.; Obando, M.; Sánchez, M. L. y Bonilla R. 2012. Efecto de las bacterias promotoras de crecimiento vegetal (PGPR) asociadas a Penissetum clandestinum en el altiplano cundiboyacense. Colombia. Rev. CORPOICA-Ciencia y Tecnología Agropecuaria. 13(2):189-195. [ Links ]
De-Bashan, L. E.; Holguin, G.; Glick, B. R. y Bashan, Y. 2007. Bacterias promotoras de crecimiento en plantas para propósitos agrícolas y ambientales. In: microbiología agrícola: hongos, bacterias y microfauna, control biologico, planta-microorganismo. (Eds.). Ferrara-Cerrato, R. and Alarcon, A. Chapter 8. Published by Ed. Trillas, México city. 170. 224 p. [ Links ]
Ganderats, F. S. y Hepp, K. C. 2003. Mecanismos de crecimiento de Lulium perenne, Festuca arundinacea y Dactylis glomerata en la zona intermedia de Aysén. Chile. Agric. Téc. 63(3):259-265. [ Links ]
González-Torres, A.; Figueroa-Viramontes, U.; Delgado, J. A.; Nuñez-Hernández, G.; Cueto-Wong, J. A.; Preciado-Rangel, P. y Palomo-Gil A. 2009. Calibración del SPAD-502 para evaluar requerimientos de nitrógeno en maíz forrajero. México. Terra Latinoam. 27(4):303-309. [ Links ]
Hernández-Garay, A.; Matthew, C. and Hodgson, J. 1997. Effect of spring grazing management on perennial ryegrass and ryegrass-white clover pastures. 1. Tissue turnover and herbage accumulation. New Zealand. New Zealand J. Agric. Res. 40(1):25-35. [ Links ]
Hungria, M.; Nogueira, M. A. and Silva, A. R. 2016. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: an environment-friendly component in the reclamation of degraded pastures in the tropics. Netherlands. Agriculture, Ecosystems and Environment. 221:125-131. [ Links ]
Jiménez, M. A. y Martínez, H. P. A. 1984. Utilización de praderas. Departamento de Zootecnia. Universidad Autónoma Chapingo (UACH). Chapingo, Estado de México, México. 85 p. [ Links ]
Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H. and Whalen, J. K. 2016. Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Netherlands Agric. Ecosyst. Environ 232:199-207. [ Links ]
Lopes, M. J. S.; Dias-Filho, M. B.; Castro, T. H. R. and Silva, G. B. 2018. Light and plant growth-promoting rhizobacteria effects on Brachiaria brizanta growth and phenotypic plasticity to shade. United Kingdom Grass and Forage Science. 73(2):493-499. [ Links ]
McKenzie, B. A.; Kemp, P. D.; Moot, D. J.; Matthew, C. and Lucas, R. J. 1999. Environmental effects on plant growth and development. In: White James and Hodgson John editors. New Zealand Pasture and Crop Science. Auckland, New Zealand: Oxford University Press. 29-44 pp. [ Links ]
Mendoza-Pedroza, S. I.; Cadena-Villegas, S.; Hernández-Garay, A.; Vaquera-Huerta, H.; Villareal-González, J. A. y Flores-Santiago, E. J. 2018. Cambios en la frecuencia de defoliación para recuperar la densidad de plantas en una pradera de alfalfa (Medicago sativa L.). México. Agroproductividad. 11(5):51-55. [ Links ]
Menna, V. S.; Menna, S. K.; Verna, J. P.; Kumar, A.; Aeron, A.; Mishra, P. K.; Bisht, J. K.; Pattanayak, A.; Navved, M. and Dotaniya, M. L. 2017. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use effciency: A review. Netherlands. Ecol. Eng. J. Ecotechnol. 107:8-32. [ Links ]
Moliterno, E. A. 2002. Variables básicas que definen el comportamiento productivo de mezclas forrajeras en su primer año. México. Agrociencia. 6(1):40-52. [ Links ]
Montalvo-Aguilar, K. J.; Castro-Rivera, R.; Solís-Oba, M. M.; Aguilar-Benítez, G.; García-Barrera L. J. y Hernández-Garay, A. 2018. Efecto de la frecuencia de defoliación y la fertilización con digestato en los componentes del rendimiento de Ballico perenne (Lolium perenne L.). México. Agroproductividad.11(5):3-9. [ Links ]
Noumavo, P. A.; Kochoni, E.; Didagbé, Y. O.; Adjanohoun, A.; Allagbé, M.; Sikirou, R. and Baba-Moussa, L. 2013. Effect of different plant growth promoting rhizobacteria on maize seed germination and seedling development. USA. Am. J. Plant Sci. 04(05):1013-1021. [ Links ]
Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R. A.; Del Cerro, P.; Espuny, M. R.; Jiménez-Guerrero, I. and Cubo, T. 2014. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Germany. Microbiol. Res. 169(5-6):325-336. [ Links ]
Ramos, D. and Terry, E. 2014. Generalidades de los abonos orgánicos: importancia del Bocashi como alternativa nutricional para suelos y plantas. Cuba. Cultivos Tropicales. 35(4):52-59. [ Links ]
Rancane, S.; Karklins, A.; Lazdina, D. and Berzins, P. 2015. Biomass yield and chemical composition of perennial grasses for energy production. Latvia. Eng. Rural Development. 20:546-551. [ Links ]
Rangel, L. J. A.; Ramírez, G. R. M.; Cervantes, O. F.; Mendoza, E. M.; García, M. E. y Rivera, R. J. G. 2014. Biofertilización de Azospirillum spp. y rendimiento de grano de maíz, sorgo y trigo. Argentina. Rev. Facultad de Ciencias UNCUYO. 46(2):231-238. [ Links ]
Rashid, S.; Charles, T. C. and Glick, B. R. 2012. Isolation and characterization of new plant growth-promoting bacterial endophytes. Netherlands. Appl. Soil Ecol. 61:217-224. [ Links ]
Singh, M. V.; Kumari, M. S.; Prakash, V. J.; Kumar, A.; Aeron, A.; Kumar, M P.; Kumar, B. J.; Pattanayak, A.; Neveed, M. and Dotoniya, M. L. 2017. Plan beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Netherlands. Ecol. Eng. J. Ecotechnol. 107:8-12. [ Links ]
Terry, A E.; Leyva, Á. y Hernández, A. 2005. Microorganismos benéficos como biofertilizantes eficientes para el cultivo de tomate (Lycopersicum esculentum L.). Colombia. Rev. Colomb. Biotecnol. 7(2):47-54. [ Links ]
Tilvikiené, V.; Slepetiené. A. and Kadziuliené. 2018. Effects of 5 years of digestate application on biomass production and quality of cocksfoot (Dactylis glomerata L.). United Kingdom. Grass Forage Sci. 73(1):206-2017. [ Links ]
Walsh, J. J.; Jones, D. L.; Edwards-Jones, G. and Williams, A. P. 2012. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. United Kingdom. J. Plant Nutr. Soil Sci. 175(6): 840-845. [ Links ]
Yu, F. B.; Luo, X. P.; Song, C. F. and Shan, S. D. 2010. Concentrated biogas slurry enhanced soil fertility and tomato quality. United Kingdom. Acta Agriculturae Scandinavica Section B. Soil Plant Sci. 60(3):262-268. [ Links ]
Received: December 01, 2019; Accepted: March 01, 2020











 texto en
texto en 


