Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.11 n.6 Texcoco Aug./Sep. 2020 Epub Oct 11, 2021
https://doi.org/10.29312/remexca.v11i6.2614
Articles
Effectiveness of biofunguisides for the control of rust in coffee seedlings
1Instituto Tecnológico del Valle de Oaxaca-Tecnológico Nacional de México. Ex-Hacienda de Nazareno s/n. Santa Cruz Xoxocotlán, Oaxaca, México. CP. 71230. Tel. 951 5170788. (roan.rmdz@outlook.com; gissant68@hotmail.com; maria.pl@voaxaca.tecnm.mx; lozanos2004@prodigy.net.mx).
2Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional-Instituto Politécnico Nacional Unidad Oaxaca (CIIDIR-IPN). Calle Hornos 1003, Santa Cruz Xoxocotlán, Oaxaca, México. CP. 71230. (crobles-38@yahoo.it).
Rust (Hemileia vastatrix) is the most destructive and economically important disease in coffee worldwide, especially in Coffea arabica L. It occurs throughout the plant cycle and chemical, biological and cultural methods are used to control it. integrated management and genetic resistance; although some are ineffective, expensive and polluting. The objective was to evaluate the incidence and severity of rust in coffee seedlings var. Geisha under the effect of different biofungicides. The research was carried out at the El Nueve farm, Santa María Huatulco, Oaxaca; during 2018 in nursery. Different treatments were evaluated: control, products based on microorganisms: Baci-Sur subtilis, Bacit-Sur, Michoderma, Blite Free F-07/Guanobras; the minerals: Copper Oxychloride, Bordeaux Broth, Visous Mineral Broth, Sulfocal and a homeopathic (Nat-Rx), plus some combinations, for a total of 24 treatments. The design was completely randomized with 10 repetitions. The variables to assess incidence were total leaves, healthy leaves and rust damaged. Photographs of damaged leaves were used to assess visual severity using the logarithmic-diagrammatic scale. To assess digitized severity using Adobe Photoshop® CC 2017 software, total leaf area, rust-damaged area, and healthy area were measured. They were analyzed by the Kruskal-Wallis test in SAS software. Significant statistical differences were found between the biofungicides at 150 dda on the incidence of rust (IR), without significant differences in severity. The treatments with the lowest IR were the combination of microorganisms Bacit-Sur+Nat-Rx, Baci-Sur subtilis+Blite FreeF-07/Guanobras and Michoderma+Blite Free F-07/Guanobras.
Keywords: Coffea arabica; Hemileia vastatrix; Biofungicides; incidence; seedlings; severity
La roya (Hemileia vastatrix) es la enfermedad más destructiva y mayor importancia económica en el café a nivel mundial en Coffea arabica L. Se presenta en el ciclo de las plantas y para su control se utilizan métodos químicos, biológicos, culturales, manejo integrado y resistencia genética; aunque algunos son inefectivos, caros y contaminantes. El objetivo fue evaluar la incidencia y severidad de la roya en plántulas de café var. Geisha bajo el efecto de diferentes biofungicidas. La investigación se realizó en la finca El Nueve, Santa María Huatulco, Oaxaca; durante 2018 en vivero. Se evaluaron distintos tratamientos: testigo, productos a base de microorganismos: Baci-Sur subtilis, Bacit-Sur, Michoderma, Blite Free F-07/Guanobras; los minerales: Oxicloruro de cobre, Caldo Bordelés, Caldo mineral visosa, Sulfocal y un homeopático (Nat-Rx), más algunas combinaciones, para un total de 24 tratamientos. El diseño fue completamente al azar con 10 repeticiones. Las variables para evaluar incidencia fueron: total de hojas, hojas sanas y dañadas por roya. Se usaron fotografías de hojas dañadas para evaluar severidad visual mediante la escala logarítmica-diagramática. Para evaluar severidad digitalizada con Adobe Photoshop® CC 2017, se midió área foliar total, área dañada por roya y área sana. Se analizaron por la prueba de Kruskal-Wallis en SAS. Se encontraron diferencias estadísticas significativas entre los biofungicidas a los 150 dda sobre la incidencia de roya (IR), sin diferencias significativas en severidad. Los tratamientos con menor IR fueron la combinación de microorganismos Bacit-Sur+Nat-Rx, Baci-Sur subtilis+Blite FreeF-07/Guanobras y Michoderma+Blite Free F-07/Guanobras.
Palabras clave: Coffea arabica; Hemileia vastatrix; Biofungicidas; incidencia; plántulas; severidad
Introduction
Coffee production (Coffea arabica L.) has a high economic, social and environmental value in the countries where it is grown (Flores, 2015). However, the crop is affected by the attack of pests and diseases, aggravating due to climatic conditions (Waller, 1982; SARH, 1993). The affectation goes from the germination of the plant to the productive stage, which generates large losses in production and a decrease in the quality of the grain (CEPAL and CAC/SICA, 2014; Chemura et al., 2017).
Rust (Hemileia vastatrix Berk. & Br.) is the most important coffee disease (Haddad et al., 2014; Oliveira, et al., 2014; Barka et al., 2017), when using this crop as its main host (Brown and Hovmøller 2002). It generally causes the loss of leaves of up to 50% (Avelino et al., 2004; Bonilla, 2018) and 30% of the yield in some varieties of C. arabica L., which means a great economic impact worldwide (Oliveira, et al., 2014; Barka et al., 2017). In the 2013-2014 cycle, production in Central America and Mexico was affected by 3.3 million bags (Flores, 2015), so in 2016 Mexico implemented the Phytosanitary Epidemiological Surveillance Program in the states of Hidalgo, Jalisco, State of Mexico, Nayarit, Querétaro, Chiapas, Veracruz, Puebla, Guerrero, San Luis Potosí and Oaxaca to determine the incidence, severity and implement preventive management actions against rust (SENASICA, 2016).
H. vastatrix is controlled through the use of agrochemicals, fungicides, biofungicides, resistant varieties, biological control, cultural control and integrated management (Obando et al., 2013; Hernandez-Martinez and Velazquez-Premio, 2016). Agrochemicals generate pollution to the environment and increase production costs (Romero, 2010; Gonza et al., 2013), therefore, viable alternatives have been sought that guarantee sustainability in agricultural production (Gonza et al., 2013).
An example of this is the use of cupric fungicides (copper oxychloride and mineral broths) considered the most efficient for the control of rust, since they do not alter the biota of the agroecosystem (Capucho et al., 2013; Melchor et al., 2018), these penetrate the leaf tissue and have curative effects (McCook, 2009) attack the fungus during mycelial growth and pustule formation (Duicela and Ponce, 2015) as well as the use of microorganisms such as fungi and bacteria (Gómez-De La Cruz et al., 2017), with the ability to survive at the expense of the fungus (Boosalis, 1964), affecting the reproductive structures of the pathogen (Barros et al., 1999).
The incidence and severity of rust is obtained through visual evaluations by trained personnel (Zambolim, 2016). However, the use of logarithmic scales with illustrations of leaves in different degrees of damage is common, being a method to quantify visual severity (Nascimento et al., 2005; Hernández and Sandoval, 2015). Digitized severity is obtained with a leaf area integrator (Chavarra et al., 2015) and the use of software such as ImageJ, Image Tool and Adobe Photoshop, allowing image areas to be determined with greater accuracy and precision (Sussel et al., 2009; Rincón et al., 2012).
Based on the above, the objective of the present study was to evaluate the incidence and severity of rust (Hemileia vastatrix Berk. & Br.) in coffee seedlings (Coffea arabica L.) var. Geisha under the effect of different biofungicides.
Materials and methods
The study was carried out from February to August 2018 at the El Nueve farm, Santa María Huatulco, Oaxaca, located at coordinates 15° 55’ 56’’ north latitude and 96° 17’ 08’’ west longitude, with an altitude range of 1 200 to 1 300 m. The type of vegetation is median sub-evergreen forest (INEGI, 2019). To know the fertility level of the substrate in the nursery (mixture of crop residues and forest soil ratio 1:1), a physical-chemical analysis was carried out according to NOM-021-RECNAT-2000 (SEMARNAT, 2002).
Three-month-old var. Geisha coffee seedlings were used, transplanted into 13 x 20 cm polyethylene bags, standardized in terms of height (10 cm) and size. 24 treatments with 10 repetitions were established, each repetition was considered a seedling. The products evaluated were: a single effect control, four based on microorganisms, four based on minerals, one homeopathic and the combinations with the same dose (Bss+Nr, Bs+Nr, M+Nr, Bfg+Nr, Oc+Nr, Cb+Nr, Cmv+Nr, S+Nr, Bss+Bfg, Bs+Bsg, M+Bfg, Bss+Nr+Bfg, Bs+Nr+Bfg and M+Nr+Bfg) (Table 1).
Table 1 Products evaluated for their simple and combined effect on the incidence and severity of H. vastatrix Berk. & Br. in seedlings of C. arabica L. var. Geisha.
| Treatment | Composition | *Dose | |
| T | Control | - | Without application |
| Bss | Baci-Sur subtilis | Bacillus subtilis 5x1020 colonies ml | 0.15 ml + 15 ml water |
| Bs | Bacit-Sur | Bacillus subtilis 5x1020 colonies ml, Trichoderma spp. 1.3x1012 spores ha-1 | 0.15 ml + 15 ml water |
| M | Michoderma | Trichoderma harzianum 1.2x8 g/l/spore dose | 0.01 g + 15 ml water |
| Bfg | Blite Free F-07/Guanobras | Streptomyces spp. 1x10 CFU/ml 60%, glucosamine 1%, OM 3%, ac. humic 0.65%, ac fulvic 0.15% | 0.2 ml + 0.15 ml + 15 ml water |
| Oc | Copper oxychloride | Metallic copper59% | 0.01 g + 4 ml water |
| Cb | Bordeaux soup | Copper sulfate, calcium hydroxide, | 0.04 g CuSO4 + 0.04 g Ca(OH)2 + 4 ml water |
| Cmv | Broth mineral visosa | Copper sulfate, calcium hydroxide, zinc sulfate, magnesium sulfate, ac. boric | 0.02 g CuSO4 + 0.02 g Ca(OH)2 + 0.02 g ZnSO4 + 0.02 g MgSO4 + 0.02 g H3BO3 + 4 ml water |
| S | Sulfocal | Sulfur and quicklime | 0.1 ml + 7.5 ml water |
| Nr | Nat-Rx | Hepar sulphur 15%, Bovista plumbea 5%, omeopathic plant extract 10%, water | 0.7 ml + 30 ml water |
*= dose per seedling per application.
The treatments were applied at two times one month apart (February 15 and March 15). The applications were made in the morning and the dosages were adjusted to 10% of what is applied in the field based on the recommendations issued by the manufacturers and specified on the label of the products.
The incidence of rust (INR) was evaluated using the methodology of Samayoa-Juarez and Sanchez-Garita (2000), adapted to the research work that consisted of counting the total of diseased leaves for each repetition and dividing by the total number of leaves, multiplying the result by 100. The following variables were considered for each repetition: total leaves (HT), healthy leaves (HS) and leaves damaged by rust (HDR). Five evaluations were made every 30 days after applying the biofungicides (dda). At 150 dda, the visual severity (SVR) of the HDR was determined, using the logarithmic-diagrammatic scale proposed by SENASICA (2016) (Figure 1).
At the same time, HDR photographs were taken to determine the digitized severity (SDR), considering the total leaf area (AFT), rust damaged area (ADR) and the healthy area (AS), analyzing the photographs with Adobe Photoshop® software CC 2017. For this, 1 cm of the image was measured by standardizing the measurement scale (370 pixels= 1 cm), for all images. A completely randomized design was used. The assumptions of normality and homogeneity were applied to the data. As the test was not completed, even after the data had been transformed, they were analyzed using nonparametric statistics using the Kruskal-Wallis test using statistical software SAS/STAT 9.3 (SAS Institute Inc., 2011).
Results and discussion
The results of the analysis indicate that the texture of the substrate is loamy sand (Ac), pH of 6.9, electrical conductivity of 0.23 dS m-1 (negligible effects of salinity) organic matter of 5.82% (high levels), phosphorus of 20.86 mg kg-1 (average levels), calcium of 0.0011 cmol kg soil (low), magnesium of 0.0016 cmol kg soil and nitrogen of 0.38%.
The percentage of leaves with rust (PHR) showed significant differences (0.041) between biofungicides up to 150 dda, with a value of p≤ 0.05. However, from 30 to 120 dda there was no response from biofungicides on the incidence of H. vastatrix Berk. & Br. in INR microorganism-based biofungicides were more effective. Combinations stand out Bacit-Sur + Nat-Rx; Baci-Sur subtilis + Blite Free F-07/Guanobras and Michoderma + Blite Free F-07/Guanobras, which generated the greatest control over the INR of H. vastatrix with average ranges of 93.5 (Figure 2).

Figure 2 Biofungicide response to the incidence of H. vastatrix Berk. & Br. in seedlings of C. arabica L. var. Geisha at 150 dda. Low values of medium ranges equal a lower incidence.
According to Cacefo et al. (2016) when applying B. subtilis in the var. Icatu and Mundo Novo obtained a control of 24 and 17%, respectively. Likewise, Dorighello (2013) when using isolates of B. subtilis QST 713 and AP-3 in the control of soybean rust (Phakopsora pachyrhizi), observed a reduction in the germination of uredospores. Li et al. (2013) obtained a significant reduction in the incidence of Puccinia striiformis f. sp. tritici (Pst) (soybean rust) with the application of B. subtilis E1R-j under greenhouse conditions with two different formulations at 24 and 0 h before Pst inoculation.
Leonel and Barros (2013) obtained a lower incidence of H. vastatrix using a homeopathic complex, observing higher vegetative vigor. Likewise, Rissato et al. (2016) obtained the lowest incidence of white bean mold (Sclerotinia sclerotiorum) with homeopathic medicine applications. For their part, El-Sharkawy et al. (2015) used Streptomyces viridosporus in combination with Trichoderma harzianum for the control of greenhouse rust of wheat (Puccinia triticina), with which they obtained a decrease in the number of pustules per unit area (cm2).
For the SVR and SDR there were no significant differences; however, it was numerically observed that the combinations of Bs + Nr; Bss + Bfg and M + Bfg generated greater control over the severity in both variables (Figures 3a and 3b). Cisneros-Rojas et al. (2017) reported greater growth and development of coffee seedlings applying B. subtilis with individual effect and in combination with the Kocuria sp., Cacefo et al. (2016) point out that B. subtilis stimulates the plant to activate its defense mechanisms for greater resistance, mitigating the harmful effects of pathogens. According to Pérez et al. (2015) the biocontroller effect of Trichoderma spp. it has a mode of action that regulates the development of phytopathogenic fungi.
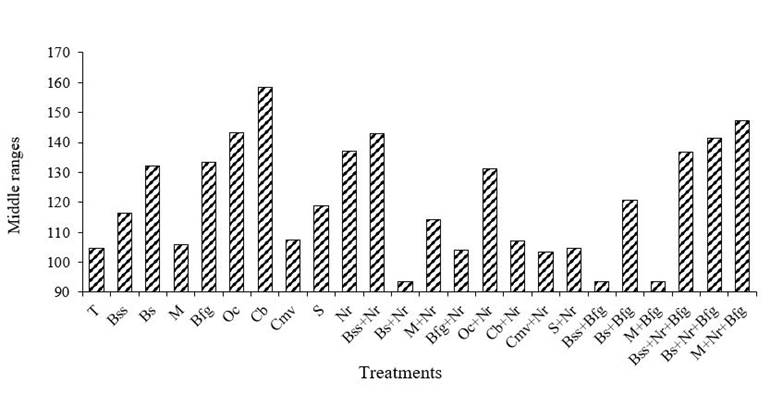
Figure 3a Response of biofungicides on the visual severity of H. vastatrix in seedlings of C. arabica L. var. Geisha, at 150 dda. Low values media ranges equivalent to less severe.

Figure 4 Response of biofungicides on digitized severity of H. vastatrix in seedlings of C. arabica L. var. Geisha, at 150 dda. Low values media ranges equivalent to less severe.
Infante et al. (2009) indicate that, during the infection process, Trichoderma spp., deactivates the enzymes of the phytopathogenic fungi. El-Sharkawy et al. (2015) found that Trichoderma harzianum and Streptomyces viridosporus had the best effects for controlling the severity of wheat leaf rust, compared to chemical fungicides. Evangelista-Martínez et al. (2015) mention that bacteria of the genus Streptomyces are producers of secondary metabolites and enzymes that act in the control of diseases caused by fungi in plants.
Conclusions
The logarithmic-diagrammatic scale facilitated the measurement of the severity in leaves in the field; however, although this activity requires time to evaluate each of the leaves with rust, since a thorough revision of the leaves is required when very small lesions appear and resemble the images proposed on the scale. The bacterium B. subtilis is used for the biological control of coffee rust, but its effects also influence the growth and development of seedlings. B. subtilis in combination with Streptomyces sp. and bat guano, the latter rich in nutrients that help strengthen seedlings.
Which is also reflected when applying the Trichoderma harzianum fungus that helps the seedling to inhibit rust and to capture nutrients found in the soil. B. subtilis has proven to be an important option in biological control, especially of foliar diseases. Bacteria belonging to the genus Bacillus have been used in the biological control of phytopathogenic fungi. The use of B. subtilis bacteria is an alternative for the biological control of coffee rust, and it has also been used for the development of successful coffee seedlings.
Literatura citada
Avelino, J.; Willocquet, L. and Savary, S. 2004. Effects of crop management patterns on coffee rust epidemics. Plant Pathol. 53(5):541-547. https://doi.org/10.1111/j.1365-3059.2004.01067.x. [ Links ]
Barka, G. D.; Caixeta, E. T.; de Almeida, R. F.; Alvarenga, S. M. and Zambolim, L. 2017. Differential expression of molecular rust resistance components have distinctive profiles in Coffea arabica - Hemileia vastatrix interactions. Eur. J. Plant Pathol. 149(3):543-561. https://doi.org/10.1007/s10658-017-1202-0. [ Links ]
Barros, S. T.; Oliveira, T. N.; Bastos, T. G. and Maia, C. L. 1999. Hyperparasitism of Cladosporium uredinicola over Puccinia puta on the host Ipomoea fistulosa. Mycologist. 13(1):23-24. https://doi.org/10.1016/S0269-915X(99)80071-8. [ Links ]
Bonilla, A. 2018. Desarrollan sistema de vigilancia epidemiológica para cultivo de café́. Consejo Nacional de Ciencia y Tecnología. México, CDMX. http://conacytprensa.mx/index.php/ ciencia/ambiente/19135-sistema-vigilancia-epidemiologica-cafe. [ Links ]
Boosalis, M. G. 1964. Hyperparasitism. Annual Review of Phytopathology 2(1):363-376. https://doi.org/10.1146/annurev.py.02.090164.002051. [ Links ]
Brown, J. K. M. and Hovmøller. M. S. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science. 297(5581):537-541. https://doi.org/10.1126/science.1072678. [ Links ]
Cacefo, V.; de Araújo, F. F. and Pacheco, A. C. 2016. Biological control of Hemileia vastatrix Berk. & Broome with Bacillus subtilis Cohn and biochemical changes in the coffee. Coffee Sci. 11(4):567-574. [ Links ]
Capucho, A. S.; Zambolim, L.; Lopes, U. N. and Milagres, N. S. 2013. Chemical control of coffee leaf rust in Coffea canephora cv. conilon. Australasian Plant Pathol. 42:667-673. https://doi.org/10.1007/s13313-013-0242-y. [ Links ]
CEPAL. 2014. Comisión Económica para América Latina y el Caribe y el Consejo Agropecuario Centroamericano del Sistema de la Integración Centroamericano (CAC/SICA). Impactos potenciales del cambio climático sobre el café en Centroamérica. México, DF. 131 p. [ Links ]
Chavarria, G.; Pelisser, W.; Hoffmann, L. e Durigon. M. R. 2015. Regulador de crescimento em plantas de trigo: reflexos sobre o desenvolvimento vegetative, rendimento e qualidade de grãos. Revista Ceres. 62(6):583-588. http://dx.doi.org/10.1590/0034-737X201562060011. [ Links ]
Chemura, A.; Mutanga, O.; Sibanda, M. and Chidoko, P. 2017. Machine learning prediction of coffee rust severity on leaves using spectroradiometer data. . Trop. Plant Pathol. 43(2):117-127. https://doi.org/10.1007/s40858-017-0187-8. [ Links ]
Cisneros-Rojas, C. A.; Sánchez-de Prager, M. y Menjivar-Flores, J. C. 2017. Efecto de bacterias solubilizadoras de fosfatos sobre el desarrollo de plántulas de café. Agron. Mesoam. 28(1):149-158. doi:10.15517/am.v28i1.22021. [ Links ]
Dorighello, D. V. 2013. Controle da ferrugem asiática da soja (Phakopsora packyrhizi) com óleo de café e Bacillus spp. Dissertação (Mestrado em Agronomia). Faculdade de Ciências Agronômicas, Universidade Estadual Paulista, Botucatu. 45 p. [ Links ]
Duicela, G. L. A. y Ponce, V. L. A. 2015. Uso de fungicidas sistémicos en el control de la roya del cafeto (Hemileia vastatrix Berk. & Br.) en la Provincia de Manabí. La Técnica. 15:6-17. [ Links ]
El-Sharkawy, H. H. A.; Tohamey, S. and Khalil. A. A. 2015. Combined effects of Streptomyces viridosporus and Trichoderma harzianum on controlling wheat leaf rust caused by Puccinia triticina. Plant Pathol. J. 14(4):182-188. doi: 10.3923/ppj.2015.182.188. [ Links ]
Evangelista-Martínez, Z.; Quiñones-Aguilar, E. y Rincón-Enríquez, G. 2015. Actividad inhibitoria de especies de Streptomyces del suelo contra Phytophthora capsici, Fusarium oxysporum y Rhizoctonia solani. Sup. Rev. Mex. Fitopatol. 33(1):128-129. [ Links ]
Flores, V. F. 2015. La producción de café en México: ventana de oportunidad para el sector agrícola de Chiapas. Universidad Autónoma de Nuevo León. Espacio I+D, Innovación más Desarrollo. 4(7):174-194. [ Links ]
Gómez-De La Cruz, I.; Pérez-Portilla, E.; Escamilla-Prado, E.; Martínez-Bolaños, M.; Carrión-Villarnovo, G. L. L. y Hernández-Leal, T. I. 2017. Selección in vitro de microparásitos con potencial de control biológico sobre roya del café (Hemileia vastatrix). Rev. Mex. Fitopatol. 36(1):172-183. https://doi.org/10.18781/R.MEX.FIT.1708-1. [ Links ]
Gonza, C. K.; López, E.; Zavaleta, C.; De La Cruz, J. y Mendoza, W. 2013. Efecto biofungicida de Trichoderma harzianum y de extractos de Eucalyptus globulus, Rosmarinus officinalis y Ricinus communis sobre Rhizoctonia solani. REBIOLEST. Rev. Científ. Estudiantes. 1(1):43-48. [ Links ]
Haddad, F.; Saraiva, R. M.; Mizubuti, E. S. G.; Romeiro, R. S. and Maffia. L. A. 2014. Isolation and selection of Hemileia Vastatrix antagonists. Eur. J. Plant Pathol. 139(4):763-772. https://doi.org/10.1007/s10658-014-0430-9. [ Links ]
Hernández, L. y Sandoval, J. S. 2015. Escala diagramática de severidad para el complejo mancha de asfalto del maíz. Rev. Mex. Fitopatol . 33(1):95-103. [ Links ]
Hernández-Martínez, G. y Velázquez-Premio, T. 2016. Análisis integral sobre la roya del café y su control. Rev. Inter. Des. Reg. Sustent. 1(1):92-96. [ Links ]
INEGI. 2019.Instituto Nacional de Estadística y Geografía SIATL. Simulador de flujos de agua de cuencas hidrográficas. http://antares.inegi.org.mx/analisis/red-hidro/siatl/#. [ Links ]
Infante, D.; Martínez, B.; González, N. y Reyes, Y. 2009. Mecanismos de acción de Trichoderma frente a hongos fitopatógenos. Rev. Protec. Veg. 24(1):14-21. [ Links ]
Leonel, A. H. and Barros, B. H. R. 2013. Utilização de preparados homeopáticos para controle da ferrugem do café (Hemileia vastatrix) na região da Alta Mogiana. Resumos do VIII Congresso Brasileiro de Agroecologia - Porto Alegre/RS. Cuadernos de Agroecología. 8(2):1-5. [ Links ]
Li, H.; Zhao, J.; Feng, H.; Huang, L. and Kang, Z. 2013. Biological control of wheat stripe rust by an endophytic Bacillus subtilis strain E1R-j in greenhouse and field trials. Crop Protec. 43:201-206. http://dx.doi.org/10.1016/j.cropro.2012.09.008. [ Links ]
McCook, S. 2009. La roya del café en Costa Rica: epidemias, innovación y medio ambiente, 1950-1995. Rev. Historia. 59-60:99-117. [ Links ]
Melchor, R. L. A.; Rosales, V. G.; Pérez, M. C. G.; Fernández, S. P.; Álvarez, G. O. and Mastache, J. M. N. 2018. Effectiveness of carboxylic acids from Pichia membranifaciens against coffee rust. Ciência e Agrotecnologia. 42(1):42-50. http://dx.doi.org/10.1590/1413-70542018421018817. [ Links ]
Nascimento, A. R. P.; Michereff, S. J.; Mariano, R. de L. R. and Gomes, A. M. A. 2005. Elaboração e validação de escala diagramática para cancro bacteriano da videira. Summa Phytopathologica. 31(1):59-64. [ Links ]
Obando, N. V.; Mestanza, C. A. y Oliva, S. M. 2013. Efecto del manejo cultural y caldo bordelés sobre la roya del café (Hemileia vastatrix) en la provincia de Rodríguez de Mendoza, Amazonas. Rev. Indes. 1(2):51-58. doi:10.25127/indes.201302.006. [ Links ]
Oliveira, C. M.; Ferreira, J. A. M.; Oliveira, R. M.; Santos, F. O. and Pallini, A. 2014. Ricoseius loxocheles, a phytoseiid mite that feeds on coffee leaf rust. Exp. Appl. Acarol. 64:223-233. https://doi.org/10.1007/s10493-014-9814-y. [ Links ]
Pérez, L.; Belmonte, J. R.; Núñez, H. G.; Guzmán, R. y Mendoza, B. 2015. Sensibilidad in vitro de dos especies de Sclerotinia spp. y Sclerotium cepivorum a agentes de control biológico y fungicidas. Rev. Mex. Fitopatol . 33(2):256-267. [ Links ]
Rincón, N.; Olarte, M. A. y Pérez, J. C. 2012. Determinación del área foliar en fotografías tomadas con una cámara Web, un teléfono celular o una cámara semiprofesional. Rev. Fac. Nac. Agron. Medellín. 65(1):6399-6405. [ Links ]
Rissato, B. B.; Stangarlin, J. R.; Coltro-Roncato, S.; Forlin, D. O. D.; Valentina, G. E. D.; Broetto, L.; Kuhn, O. J.; Lorenzetti, E.; Muriel, T.; Pereira, F. E. P.; Barrientos, W. T. F. and Urbanski L. J. C. 2016. Control of white mold in bean plants by homeopathic medicines. Afr. J. Agric. Res. 11(24):2174-2178. doi: 10.5897/AJAR2016.10988. [ Links ]
Romero, G. A. 2010. Efecto de los sistemas agroforestales del café y del contexto del paisaje sobre la roya, (Hemileia vastatrix), broca (Hypothenemus hampei (Ferrari) y los nemátodos Meloidogyne spp.), con diferentes certificaciones en la provincia de Cartago Costa Rica. Tesis de grado de Magister Scientiae en Agricultura Ecológica. Escuela de Posgrado del Centro Agronómico Tropical de Investigación y Enseñanza. Turrialba, Costa Rica. 85 p. [ Links ]
Samayoa-Juárez, J. O. y Sánchez-Garita, V. 2000. Enfermedades foliares en café orgánico y convencional. Manejo Integrado de Plagas. 58:9-19. [ Links ]
SARH. 1993. Secretaría de Agricultura y Recursos Hidráulicos. Enfermedades del cafeto y su control en México. División Agrícola. Instituto Nacional de Investigaciones Forestales y Agropecuarias (INIFAP)-Centro de Investigación Regional del Golfo Centro-Campo Experimental Xalapa. Veracruz, Ver., México. Folleto técnico núm. 4. 19 p. [ Links ]
SAS. Institute Inc. 2011 SAS/QC 9.3 User’s Guide. Cary, NC: SAS Institute Inc. https://support.sas.com/documentation/cdl/en/qcug/63964/PDF/default/qcug.pdf. [ Links ]
SEMARNAT. 2002. Secretaría de Medio Ambiente y Recursos Naturales. Norma Oficial Mexicana NOM-021-RECNAT-2000, que establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreo y análisis. http://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2009/DO2280n.pdf. [ Links ]
SENASICA. 2016. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroaliemntaria. Roya del cafeto Hemileia vastatrix Berkeley & Broome. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA). Dirección General de Sanidad Vegetal. México, D.F. Ficha técnica núm. 40. 23 p. [ Links ]
Sussel, A. A. B.; Pozza, E. A. and Castro, H. A. 2009. Elaboração e validação de escala diagramática para avaliação da severidade do mofo cinzento em mamoneira. Tropical Plant Pathol. 34(3):186-191. [ Links ]
Waller, J. M. 1982. Coffee rust-epidemiology and control. Crop Protec. 1(4):385-404. https://doi.org/10.1016/0261-2194(82)90022-9. [ Links ]
Zambolim, L. 2016. Current status and management of coffee leaf rust in Brazil. Tropical Plant Pathol. 41:1-8. doi:10.1007/s40858-016-0065-9. [ Links ]
Received: July 01, 2020; Accepted: August 01, 2020











 text in
text in 



