Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.11 no.5 Texcoco Jun./Ago. 2020 Epub 03-Out-2021
https://doi.org/10.29312/remexca.v11i5.2280
Research notes
Quality and storage of papaya fruits from plants inoculated with Glomus mosseae
1Campo Experimental Cotaxtla-INIFAP. Carretera Veracruz-Córdoba km 34.5, Medellín de Bravo, Veracruz, México.
2Colegio de Postgraduados-Campus Montecillo. Carretera México-Texcoco km 36.5, Montecillo, Texcoco, México. CP. 56230.
3Escuela de Ciencias Agropecuarias de la Universidad Michoacana de San Nicolás de Hidalgo. Mariano Jiménez s/n, colonia el Varillero, Apatzingán, Michoacán, México. CP. 60660.
4Unidad Académica de Agricultura-Universidad Autónoma de Nayarit. Carretera Tepic-Compostela km 9, Xalisco, Nayarit, México.
The use of arbuscular mycorrhizal fungi (AMF) has proven to be efficient in increasing the yield of plants. Few studies show its effect on fruit quality, but none indicate its interaction with post-harvest treatments during its shelf life. Therefore, the objective of this work was to determine the quality of the ‘Maradol red’ papaya fruits from plants inoculated with Glomus mosseae (730 spores 100 g-1 inoculant) and their response to storage for 10 and 20 days at 10 ºC , treated with a ripening inhibitor (1-methylcyclopropene (1-MCP). The results showed that the inoculation of inoculated papaya plants significantly reduces the weight loss of the fruits. The application of 1-MCP maintained firmness in the fruits stored for 10 days and delayed their loss for three more days in those stored for 20 days. AMF inoculation did not significantly affect the response of papaya fruits to treatment with 1-MCP in both storage periods at 10 °C.
Keywords: Carica papaya L.; 1-methylcyclopropene; red Maradol; refrigerated storage; sugars.
El uso de hongos micorrízicos arbusculares (HMA) ha probado ser eficiente al incrementar el rendimiento de las plantas. Son pocos los estudios que muestran su efecto en la calidad del fruto, pero ninguno señala su interacción con tratamientos postcosecha durante su vida de anaquel. Por lo que el objetivo del presente trabajo fue determinar la calidad de los frutos de papaya ‘Maradol roja’ procedentes de plantas inoculadas con Glomus mosseae (730 esporas 100 g-1 inoculante) y su respuesta al almacenamiento por 10 y 20 días a 10 ºC, tratados con un inhibidor de la maduración (1-metilciclopropeno (1-MCP). Los resultados mostraron que la inoculación de plantas de papaya inoculadas reduce significativamente la pérdida de peso de los frutos. La aplicación de 1-MCP mantuvo la firmeza en los frutos almacenados por 10 días y retrasó su pérdida por tres días más en los almacenados por 20 días. La inoculación de HMA no afectó significativamente la respuesta de los frutos de papaya al tratamiento con 1-MCP en ambos periodos de almacenamiento a 10 °C.
Palabras clave: Carica papaya L.; 1-metilciclopropeno; almacenamiento refrigerado; azúcares; Maradol roja.
Papaya is one of the main tropical fruits after bananas, oranges, mangoes and pineapples, it represents around 16% of the total production of tropical fruits in the world (FAO, 2019). The cultivation requires continuous applications of fertilizer for its development and continuous production (Nakasone and Paull, 1998), but the use of inorganic fertilizers in agricultural agroecosystems has generated environmental problems such as the eutrophication of bodies of water. This has sparked interest in the use of beneficial microorganisms such as arbuscular mycorrhizal fungi (AMF) to improve crop productivity (Cuenca et al., 2007). AMF are a key component of the rhizosphere, as they form a mutualistic association with the roots of more than 80% of land plants (Smith and Read, 1997).
This symbiosis stimulates the biosynthesis of secondary metabolites, which help the plant to overcome different types of stress, it also facilitates the absorption of water, phosphorus and nitrogen to the plant, improves the structure of the soil, increases the area of exploration of the root (Camargo-Ricalde et al., 2012) also favor the diversity of beneficial micro-and macro-organisms in the soil and possible antagonisms of disease-causing pathogens (Téliz and Mora, 2007).
The use of AMF has a significant effect on the yield of different crops, some studies show its influence on fruit quality. For example, in fruits of different strawberry cultivars (Fragaria x ananassa), inoculation of plants with AMF resulted in an increase in the total soluble solids/acidity and phenolic compounds index (Cordeiro et al., 2019), in ancho pepper (Capsicum annuum L. cv San Luis) increased the size, color and content of pigments (Mena-Violante et al., 2006) and in papaya increased the content of sugars, firmness and color (Vázquez-Hernández et al., 2011).
However, there are no studies showing the post-harvest changes shown by the fruits from plants inoculated with AMF during refrigerated storage and the application of some retarder of ripening. 1-Methylcyclopropene (1-MCP) has been recommended to prolong post-harvest life and preserve the quality characteristics of papaya fruits (Jacomino et al., 2002; Osuna-García et al., 2005).
This product has been used to prolong the post-harvest life of numerous fruits, since it blocks the receptor sites of ethylene, preventing its action and effects related to ripening (tissue softening, pigment degradation and starch splitting) (Blankenship and Dole, 2003). Based on the above, the objective of this work was to evaluate the post-harvest characteristics of ‘Maradol red’ papaya fruits from plants inoculated with Glomus mosseae and stored cold for 10 and 20 days at 10 °C, with the application of 1 -methylcyclopropene (1-MCP).
The fruits were harvested in an experimental plantation located in Huamuxtitlan, Guerrero, Mexico (17º 41’-17º 54’ north latitude and 98º 26’-98º 40’ west longitude), at an altitude of 890 m. The climate is warm dry with an average temperature of 25.9 ºC and 803.3 mm of average annual precipitation (SMN-CONAGUA, 2019). In the nursery when the seedlings were 6 cm high, light root pruning was carried out and they were placed in bags with 1 kg of growth substrate (soil: river sand, 2: 1, sterilized) containing 100 g of Glomus mosseae inoculum (Nicol. & Gerd.) Gerd. & Trappe (730 spores 100 g-1 inoculant).
The plants were then transplanted when they reached 20 cm in height or 7 fully expanded true leaves. At the time of harvesting the fruits, samples were taken from the roots of the plants to verify mycorrhizal colonization (hyphae, vesicles, and arbusculi) (Rufykiri et al., 2000). Control plants and those inoculated with G. mosseae had 16.46% and 91.52% of mycorrhizal colonization, respectively.
Plants inoculated with G. mosseae produced a greater number of fruits (24.4) and larger fruits (2.07 kg), than control plants (17.2 and 1.43 kg) respectively (Figure 1). The fruits were harvested seven months after transplantation, when they reached 40% of epidermis color development, they were subsequently washed and treated with a Prochloraz fungicide (500 μl L-1, 2 min) and dried in the environment.
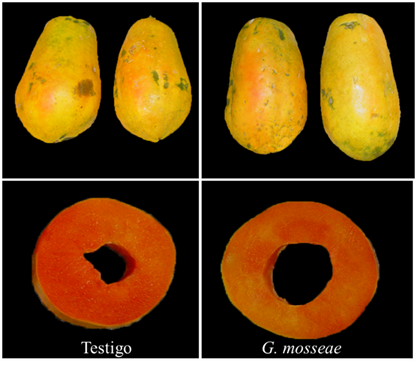
Figure 1 Color characteristics of the epidermis and pulp of ‘Maradol red’ papaya fruits from control plants inoculated with Glomus mosseae.
The fruits of plants with or without G. mosseae (GM) were randomly selected and classified into two groups of 60 fruits each. Subsequently they were placed in a hermetically sealed box to be treated with 1-MCP vapors (SmartFresh® 14% Rohm and Hass Co., Philadelphia, PA) in concentrations of 0 and 300 nl L-1 for 16 h in hermetically sealed containers, establishing the following treatments: 1) control; 2) 1-MCP; 3) GM; and 4) GM + 1-MCP. The total fruit was divided into two batches, one stored for 10 days and the other for 20 days at 10 ±1 ºC and 85% RH. Once the storage period ended, the fruits were transferred at 20 +1 ºC and 60% RH for ripening.
The sampling periods were at 0, 2, 4, 6 and 8 days after storage; each fruit was considered as an experimental unit with 3 repetitions. The variables evaluated were: firmness by puncture, with a 10 mm strut measured at 4 points of each fruit, without epidermis and evaluated with a texturometer (FDV-30, Wagner Instruments, USA), weight loss (%) due to the difference in weight with a digital scale (EY-2200-A, Alsep, Japan), total sugars per anthrone (Witham et al., 1971) and color that was measured with a colorimeter (D-25a PC2, Hunter Lab, USA) whose values a* and b* were used to calculate the Hue angle [H= arctan (b*/a*)] and chroma (C=√(a2 + b2)). The results showed that treatment with 1-methylcyclopropene (1-MCP) delayed the loss of firmness in the fruits, regardless of whether they came from plants inoculated or not with G. mosseae, reaching values between 38.8-39.9 N on the fourth day, significantly higher to those of the fruits that were not treated with 1-MCP (6.8-7.4 N) (Figure 2A).
The fruits of plants inoculated with G. mosseae without 1-MCP, stored for 10 days at 10 ºC had a delay in loss of firmness of two days in relation to the fruits of plants without inoculation and without 1-MCP (Figure 2A). On the second day after being transferred at 20 ºC, the fruits of plants with G. mosseae showed a firmness of 36.9 N, while in fruits of plants without G. mosseae it was 12.1 N, but from the sixth day, the fruits of both treatments did not show significant differences. Asmar et al. (2010) applied 1-MCP (270 nl L-1) in ‘Sunrise solo’ papaya fruits for different periods of time, observing that exposure for 12 or 24 h delayed the softening of the fruit with firmness values of around 20 N, 8 days after storage, while the control fruits considerably lost firmness (5 N) on the same day.
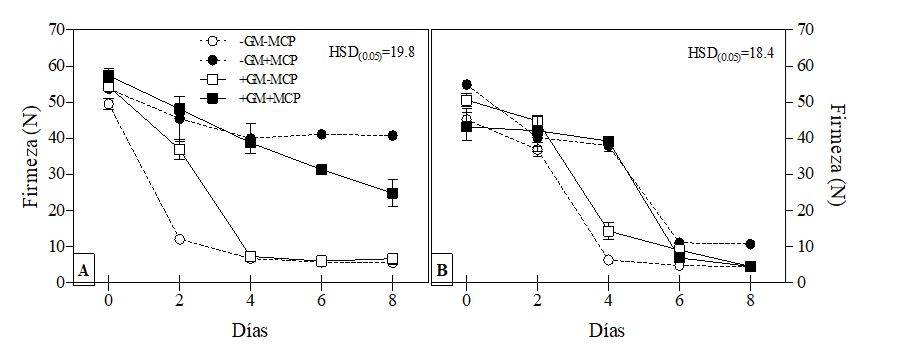
Figure 2 Firmness (N) of fruits of plants inoculated or not with Glomus mosseae (GM), treated or not with 1-methylcyclopropene (1-MCP) stored for 10 (a) and 20 (b) days at 10 ºC and transferred at 20 ±2 ºC for its maturation. HSD= Tukey’s significant honest difference (p< 0.05).
The loss of firmness of papaya fruits is attributed to the depolymerization of pectin in cell walls, a process catalyzed by enzymes such as polygalacturonase (PG), pectin methyl esterase (PME), glucanase, β-galactosidase and cellulase. Some of these enzymes have been reported to be sensitive to ethylene during fruit ripening, so the application of 1-MCP inhibits its action and as a consequence the cascade of events that lead to the degradation of the cell wall, since reduces the activity of these enzymes and delays the softening of the fruits (Sharma et al., 2012; Zerpa-Catanho et al., 2017).
The 1-MCP also delayed the loss of firmness in the fruits of plants with and without G. mosseae stored for 20 days at 10 ºC, presenting a higher firmness (37.9-39.2 N) on the fourth day compared to the fruits that were not treated with 1-MCP (6.4-14.4 N). However, from day 6 no significant differences were found between treatments. This fact confirms that the softening of papaya fruits is one of the most sensitive processes to the action of ethylene (Osuna et al., 2005).
The fruits presented a significant reduction in firmness when reaching a color development of 50-60% (4.8-11.1 N). Santamaría et al. (2009) report in papaya ‘Maradol’, a significant reduction in firmness (at least eight times) when the fruits reached around 60% of color development, which according to the degrees of maturity detected occurs between the stages 3 and 4 when most of the chlorophyll is degraded and gives way to the light orange color with firmness values of 16 N and finally when they develop their full color the firmness falls to 5 N.
The 1-MCP delayed the loss of firmness, with a longer effect on the fruits stored for 10 d, than on those stored for 20 d. The loss of the effect of 1-MCP that can be attributed to the fact that the molecules of this compound occupy the sites of action of ethylene, but in prolonged periods of storage new ethylene receptor sites are generated and will be occupied by the ethylene produced during storage already that the biosynthesis of this hormone is not completely inhibited, even increases after prolonged periods of storage, as has been shown in some climacteric fruits (Blankenship and Dole, 2003; Arévalo-Galarza et al., 2007).
Regarding the weight loss, the fruits of the control plants had greater weight losses in both storage periods, where the effect of 1-MCP was not significant (Figure 3). Weight losses in the fruits stored for 20 days fluctuated between 12.8 and 16.6% at the end of the ripening process (Figure 3B). The increase in fruit size and, consequently, less weight loss, was another beneficial effect of mycorrhizae, since the larger fruits have a lower surface/volume ratio than the smaller fruits produced by control plants (Vázquez -Hernández et al., 2011).

Figure 3 Weight loss (%) of fruits of plants inoculated or not with Glomus mosseae (GM), treated or not with 1-methylcyclopropene (1-MCP) stored for 10 (A) and 20 (B) days at 10 ºC and transferred at 20 ±2 ºC for maturation. HSD= Tukey’s significant honest difference (p< 0.05).
Total sugar content showed consistent increases after storage at 10 ºC; however, only significant differences were found in the total sugar content in the fruits stored for 20 days at the end of the ripening period. The fruits of plants with G. mosseae and without 1-MCP had a higher sugar content (8.53%) and those of plants with G. mosseae and with 1-MCP (6.28%).
The higher content of total soluble solids in the fruits after storage at 20 days compared to those stored for 10 days, could be due to dehydration, since papaya fruits do not have a significant amount of starch (approximately 0.5%) for hydrolysis during the ripening process, so there are no substantial changes during the post-harvest stage (Mosca and Durigan, 1995; Sivakumar and Wall, 2013).
In the color of the epidermis (ºHue), no significant differences were found between treatments, since on average the initial values of ºHue in the initial epidermis were 105.9-119.5º and at the end of the evaluation 84.5-87º. In fruits stored for 20 days, the ºHue value of the epidermis varied from its initial value (106.7-121.7º) to 82.9-101.4º at the end of the evaluation. It is possible that the differences between treatments were not evident, since the storage periods (10 and 20 d at 10 °C) were prolonged and the effect of 1-MCP was not noticeable; for example, in papaya ‘Sinta’ concentrations of 0.1 and 0.5 µl L-1 of 1-MCP delayed the development of color for 6 days in relation to the control fruits, but at 25-28 ºC (Esguerra et al., 2010).
Conclusions
The inoculation of G. mosseae did not affect the accumulation of sugars or the color of the fruits; however, the fruits from inoculated plants had lower weight losses during refrigerated storage, attributed to the fact that they were larger fruits and with a lower surface/volume ratio that allowed them to lose proportionally less weight than smaller fruits. The use of 1-MCP significantly delayed the loss of firmness of the fruits in four days and two days in the fruits stored for 10 and 20 days at 10 °C respectively, regardless of whether they came from inoculated plants or not.
Literatura citada
Arévalo-Galarza, L.; Bautista-Reyes, B.; Saucedo-Veloz, C. y Martínez-Damían, T. 2007. Almacenamiento refrigerado y aplicaciones de 1-metilciclopropeno (1-MCP) en frutos de chicozapote (Manilkara sapota (L.) P. Royen). Agrociencia. 41(4):469-477. http://www.colpos.mx/agrocien/Bimestral/2007/may-jun/art-10.pdf. [ Links ]
Asmar, A. S.; de Abreu, C. M. P.; Lima, R. A. Z.; Corrêa, A. D. and dos Santos, D. 2010. Firmness of papaya treated with 1-MCP in different exposition times. Ciênc. Agrotecnol. 34(2):440-444. http://dx.doi.org/10.1590/S1413-70542010000200024. [ Links ]
Blankenship, S. M. and Dole, J. M. 2003. 1-Methylcyclopropene: a review. Postharvest Biol. Technol. 28(1):1-25. https://doi.org/10.1016/S0925-5214(02)00246-6. [ Links ]
Camargo-Ricalde, S. L, N.; Manuel-Montaño, C. J.; De la Rosa-Mera, y Montaño-Arias, S. A. 2012. Micorrizas: Una gran unión debajo del suelo. Rev. Digital Universitaria. 13(7):1067-60710. http://www.revista.unam.mx/vol.13/num7/art72/art72.pdf. [ Links ]
Cordeiro, E. C.; Resende, J. T.; Córdova, K. R. V.; Nascimento, D. A.; Saggin, J.; Orivaldo, J.; Zeist, A. R. and Favaro, R. 2019. Arbuscular mycorrhizal fungi action on the quality of strawberry fruits. Hortic. Bras. 37(4):437-444. https://dx.doi.org/10.1590/s0102-053620190412. [ Links ]
Cuenca, G.; Cáceres, A.; Oirdobro, G.; Hasmy, Z. y Urdaneta, C. 2007. Las micorrizas arbusculares como una alternativa de agricultura sustentable en áreas tropicales. Interciencia. 32(1):23-29. [ Links ]
Esguerra, E. B., Marcaida, M. P. and Rosales, R. A. 2010. Effects of 1-methylcyclopropene on ripening of two papaya (Carica papaya L.) cultivars. Acta Hortic. 875(1):81-88. https://doi.org/10.17660/ActaHortic.2010.875.8 [ Links ]
FAO. 2019. Food and Agriculture Organization. Statistical database Internet http://www.fao.org/faostat/en/#data. [ Links ]
Jacomino, A. P.; Kluge R. A. and Brackmann, A. 2002. Ripening and senescence of papaya with 1-methylcyclopropene. Sci. Agric. 59(2):303-308. http://dx.doi.org/10.1590/S0103-90162002000200015. [ Links ]
Mena-Violante, H.; Ocampo-Jiménez, O.; Dendooven, L.; Martínez-Soto, G.; González-Castañeda, J.; Davies, T. F. and Olalde-Portugal, V. 2006. Arbuscular mycorrhizal fungi enhance fruit growth and quality of chile ancho (Capsicum annuum L. cv San Luis) plants exposed to drought. Mycorrhiza. 16(2):261-267. Doi: https://doi.org/10.10071~00572-006-0043-z. [ Links ]
Mosca, J. L. and Durigan, J. F. 1995. Post-harvesting conservation of papaya fruits Carica papaya L. ‘improved sunrise solo line 72/12’, with utilization of protector films and wax, associated with refrigeration. Acta Hortic. 370(1):217-221. https://doi.org/10.17660/ActaHortic.1995.370.34. [ Links ]
Nakasone, H. Y. and Paull, R. E. 1998. Tropical fruits. First (Ed.). CAB International, Wallingford. UK. 445 p. [ Links ]
Osuna-García, J. A.; Beltrán, J. A. y Pérez-Barraza, M. H. 2005. Mejoramiento de vida de anaquel y calidad de papaya ‘Maradol’ con 1-metilciclopropeno (1-MCP). Rev. Chapingo Ser. Hortic. 11(1):7-12. https://www.chapingo.mx/revistas/revistas/articulos/doc/rchshXI121.pdf. [ Links ]
Rufykiri, G.; Declerck S.; Dufey, J. E. and Delvaux B. 2000. Arbuscular mycorrhizal fungi might alleviate aluminium toxicity in banana plants. New Phytol. 148(2):343-352. http://dx.doi.org/10.1046/j.1469-8137.2000.00761.x. [ Links ]
Santamaría, B. F.; Sauri, D. E.; Espadas, G. F.; Díaz, P. R.; Larqué, S. A. and Santamaría, J. M. 2009. Postharvest ripening and maturity indices for Maradol papaya. Interciencia . 34(8):583-588. http://www.scopus.com/inward/record.url?scp=73849090632&partner ID=8YFLogxK. [ Links ]
Sharma, S.; Sharma, R. R.; Pal, R. K.; Paul, V. and Dahuja, A. 2012. 1-Methylcyclopropene influences biochemical attributes and fruit softening enzymes of ‘Santa Rosa’ Japanese plum (Prunus salicina Lindl.) J. Plant Biochem. Biotechnol. 21(2):295-299. Doi:https://doi.org/10.1007/s13562-011-0098-6. [ Links ]
Sivakumar, D. and Wall, M. M. 2013. Papaya fruit quality management during the postharvest supply chain. Food Reviews International. 29(1):24-48. Doi: https://doi.org/10.1080/87559129.2012.692138. [ Links ]
Smith, S. E. and Read, D. J. 1997. Mycorrhizal symbiosis. Second edition. Academic Press, London. eBook. ISBN: 9780080537191. 605 p. [ Links ]
SMN- CONAGUA . 2019. Servicio Meteorológico Nacional. Comisión Nacional del Agua. https://smn.conagua.gob.mx/es/informacion-climatologica-por-estado?estado=gro. [ Links ]
Téliz, O. D. y Mora A. A. 2007. El manejo integrado del aguacate. In: El aguacate y su manejo integrado. Segunda edición, Ediciones Mundiprensa, México, DF. 287-306 pp. [ Links ]
Vázquez-Hernández, M. V.; Arévalo-Galarza, L.; Jaen-Contreras, D.; Escamilla-García, J. L.; Mora-Aguilera, A.; Hernández-Castro, E.; Cibrián-Tovar, J. and Téliz-Ortiz, D. 2011. Effect of Glomus mosseae and Entrophospora colombiana on plant growth, production, and frui tquality of ‘Maradol’ papaya (Carica papaya L.). Sci. Hortic. 138(3):255-260. https://doi.org/10.1016/j.scienta.2011.01.031. [ Links ]
Witham, F. H.; Blaydes, D. F. and Devlin, R. M. 1971. Experiments in plant physiology. Van Nostrand Reinhold Company. New York. 245 p. [ Links ]
Zerpa-Catanho, D.; Esquivel, P.; Mora-Newcomer, E.; Sáenz, M. V.; Herrera, R. and Jiménez, V. M. 2017. Transcription analysis of softening-related genes during postharvest of papaya fruit (Carica papaya L. ‘Pococi’ hybrid). Postharvest Biol. Technol . 125(1):42-51. http://dx.doi.org/10.1016/j.postharvbio.2016.11.002. [ Links ]
Received: June 01, 2020; Accepted: July 01, 2020











 texto em
texto em 


