Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.11 no.4 Texcoco may./jun. 2020 Epub 13-Sep-2021
https://doi.org/10.29312/remexca.v11i4.2466
Articles
Vegetation and habitat structure that determines the diet of insectivorous birds in agroforestry systems
1Maestría en Ciencias en Agroforestería para el Desarrollo Sostenible-Universidad Autónoma Chapingo. Carretera Federal México-Texcoco km 38.5. Texcoco, México. CP. 56230. (blondynunez@gmail.com; favia-rd@hotmail.com).
2Departamento de Suelos-Área de Recursos Naturales Renovables-Universidad Autónoma Chapingo. Carretera Federal México-Texcoco km 38.5, CP. 56230. Texcoco, México.
3Centro Regional Universitario Oriente-Universidad Autónoma Chapingo. Carretera Huatusco-Jalapa km 6, Huatusco, Veracruz. CP. 94100. (tinoco@correo.chapingo.mx).
To define the vegetation and habitat structure in relation to the presence of birds and prey from August (2018) to January (2019), the monitoring of birds, vegetation and insects was carried out, using counting at points with a fixed radius; intensive search; cut and shake of branches, Canfield lines, quadrants with center point and closest neighbor. Observation frequency (FO) and relative abundance index (IAR) were determined. Kruskal wallis and X2 analyzes were carried out to determine differences and determine if what was recorded is expected. To assess association between habitat and vegetation in relation to the presence of birds and insects, a Poisson regression analysis (ARP) was applied. To know the degree of inertia between habitat and vegetation in response to the presence of birds and insects, canonical correspondence analysis (ACC) was developed. The FO indicates high values for: Birds Melanerpes aurifrons, Cyanocorax morio and Empidonax sp.; Poaceae, Selaginellaceae and Verbenaceae vegetation; insects Plecia nearctica and Chauliognathus pensylvanicus. The IAR indicates high values for: Poaceae, Selaginellaceae and Verbenaceae vegetation; insects Plecia nearctica and Chauliognathus pensylvanicus. Kruskal wallis without differences and X2 that what is registered is expected. The ARP suggests an AIC= 283.65; 219.4; 240.38. The ACC shows an inertia of 66.26%; 78.54%; 93.89% and 84.62%. Vegetation and habitat structure are determining factors in the abundance of birds, they have a food stock that guarantees the conservation and ecological balance of agroforestry systems.
Keywords: birdlife; conservation; feeding; tree cover; systematic sampling
Para definir la vegetación y estructura del hábitat con relación a la presencia de aves y presas de agosto 2018 a enero 2019 se llevó acabo el seguimiento de aves, vegetación e insectos, empleando recuento en puntos con radio fijo; búsqueda intensiva; corte y sacudida de ramas, líneas de Canfield, cuadrantes con punto central y el vecino más cercano. Se determinó frecuencia de observación (FO) e índice de abundancia relativa (IAR). Para determinar diferencias y conocer si lo registrado es lo esperado se desarrollaron análisis de kruskal wallis y X2. Para evaluar asociación entre el hábitat y vegetación en relación con la presencia de aves e insectos se aplicó un análisis de regresión Poisson (ARP). Para conocer el grado de inercia entre hábitat y vegetación en respuesta a la presencia de aves e insectos se desarrollaron análisis de correspondencia canónica (ACC). La FO señala valores altos para: Aves Melanerpes aurifrons, Cyanocorax morio y Empidonax sp.; vegetación Poaceae, Selaginellaceae y Verbenaceae; insectos Plecia nearctica y Chauliognathus pensylvanicus. El IAR señala valores altos para: vegetación Poaceae, Selaginellaceae y Verbenaceae; insectos Plecia nearctica y Chauliognathus pensylvanicus. Kruskal wallis sin diferencias y X2 que lo registrado es lo esperado. El ARP sugiere un AIC= 283.65; 219.4; 240.38. El ACC evidencia una inercia de: 66.26%; 78.54%; 93.89% y 84.62%. La vegetación y estructura del hábitat son factores determinantes en la abundancia de aves, disponen del stock alimenticio que garantiza la conservación y equilibrio ecológico de los sistemas agroforestales.
Palabras clave: alimentación; avifauna; cobertura-arbórea; conservación; muestreo-sistemático
Introduction
Globally, a total of 10 500 species of birds are recorded (Navarro-Sigüenza et al., 2014). However, due to a series of anthropic actions, there are increasing negative effects that put the survival of ecosystems and the habitat of various organisms at risk (Ramírez-Albores, 2009; Vázquez-Pérez et al., 2009). Such actions have caused the extinction of several bird species and many of them are threatened (Cruz and Lauro, 2006).
Mexico registers a total of 1076 bird species, of which 102 are endemic (Valencia-Trejo et al., 2014). Likewise, the cause of a series of anthropic events has dramatically deteriorated their natural environment, putting their survival at a critical point (Almazan-Núñez et al., 2009). The abundance and distribution of birds is a reflection of the historical and ecological components. Among the ecological factors, the structure of the habitat and food resource stands out (Cuento, 2006).
The vegetation that defines birdlife diversity is transformed based on a spatial scale integrated at the habitat and landscape level and a time scale that determines the food heritage (Buitrón-Jurado and Tobar, 2007). However, the perception of how said temporal space disturbance afflicts different organisms is still incipient (Naranjo and Ulloa, 1997).
Despite having studies on the horizontal movement of some birds in search of better feeding scenarios and vertical movements of certain strata, studies of these fluctuations are very limited (Rangel-Salazar et al., 2009). The number of species that inhabit a certain region will depend on three important factors: food availability, nesting areas and predation to which they could be exposed (Serial and Grigera, 2005).
For their part, agroforestry systems are an alternative that favors the availability of habitat for different species (Abouhamad et al., 2017). Having a conservationist principle, under a sustainable approach (Tzuc-Martínez et al., 2017; García, 2018). The structure of these systems is integrated by vertical and horizontal components (herbaceous, arboreal and shrubby) that simulate a natural environment conducive to the conservation of fauna species.
Where birds could find a trophic niche that guarantees their survival (Castillo and Calderón, 2017; López-Ferrer et al., 2017). An agroforestry system of coffee immersed in mountain mesophilic forest (BMM), is located in the Municipality of Huatusco, Veracruz. This system is made up of different tree species in which a great diversity of birds could inhabit.
However, despite the importance of this system in terms of the conservation of species, so far there are no studies conducted where this topic is addressed. The objective of this work was to define the relationship in the presence of prey birds and insects with the vegetation and habitat structure.
Materials and methods
Study area. It is located between the geographical coordinates of 19º 09’ north latitude and 96º 57’ west longitude at an altitude of 1 933 meters above sea level, belonging to the municipality of Huatusco, Veracruz, Mexico. For this study, three conditions to be evaluated were considered: traditional coffee (CT); pasture (PT) and mountain mesophyll forest (BMM) in a total area of 32.42 ha.
Sampling and data collection. In each evaluated condition, a systematic sampling was applied to convenience with linear distances of 150 m between each point. Bird monitoring was performed monthly from August 2018 to January 2019 using counting at points with fixed radius and intensive search (Ponce et al., 2012; Alonso et al., 2017).
At the same time, Canfield lines were applied to determine the habitat variables (Bueno et al., 2015) quadrants with a central point for herbaceous vegetation and the closest neighbor for tree vegetation (Mora et al., 2019). In addition to the counting at points with a fixed radius, cutting and shaking of branches was used, this methodology consisted of the researcher locating a feeding bird, placing a plastic bag on the branch on which the bird was located and shaking to collect the insects that this bird could potentially be feeding on.
Analysis of data. Observation frequency (FO) was determined for birds, vegetation and insects. Relative abundance index for vegetation and insects. In order to evaluate significant differences in vegetation and insects, Kruskal wallis and X2 analysis were applied to determine if what was recorded is potentially present. To determine the possible association between habitat variables that determine the presence of birds and insects.
Poisson regression analysis (ARP) were applied, using the Stepwise polynomial variable selection procedure, the adjustment of the models was performed with the minimum Akaike criterion in R.13.0 (Akaike, 1969). In order to determine the degree of association between the abundance of birds and insects determined by the variables of the habitat and vegetation, association analyzes were applied using canonical correspondence in the statistical software XLSTAT version 2018.7.
Results and discussion
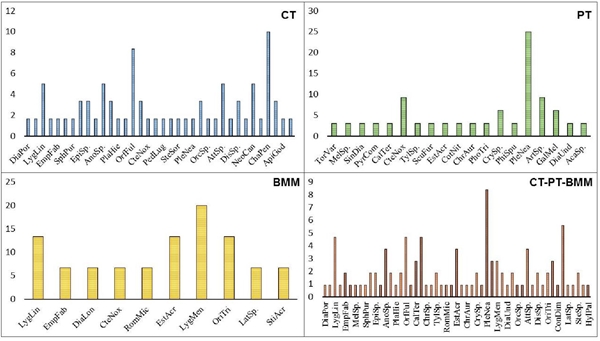
A total of 495 bird specimens distributed in 10 orders, 19 families, 42 genera and 51 species, 1 741 vegetation specimens grouped into 38 families (Figure 1) and 165 insects, distributed in 9 orders, 40 families, 51 genera and 53 species were described (Figure 2).
The FO establishes high values for: Melanerpes aurifrons (8.31%); Cyanocorax morio (7.7%; Figure 3); Poaceae (61.49%; Figure 4) and Plecia nearctica (8.41%; Figure 5).
The IAR indicates that the vegetation with the highest abundance was Poaceae (0.61). For their part, the most abundant insects were: CT= Chauliognathus pensylvanicus (0.1); PT= Plecia nearctica (0.25); BMM= Lygaeidae mendax (0.2) and CT-PT-BMM= Plecia nearctica (0.084) (Figure 6).
Kruskal wallis shows similarity in the evaluated vegetation (p= 0.7961) and differences in the insect record (p= 0.0321*). X2 establishes that the vegetation (p= 0.8452) and insects (p= 0.2392) registered are those that predominate in the evaluated conditions. The Poisson regression shows that only seven variables have an effect on the abundance of birds and 10 on the presence of insects (Table 1).
Table 1 Poisson regression for the variables of habitat and vegetation that determine the abundance of birds and insects.
Coefficients |
Estimate |
Standard error |
Z Value |
Pr(>|z|) |
Significance |
Variables that determine the abundance of birds | |||||
(Intercept) |
1.318439 |
0.04456 |
29.588 |
< 2e-16 |
|
Water body |
-0.004905 |
0.255097 |
-2.53 |
0.01195 |
|
Tree cover |
-0.004905 |
0.001631 |
-3.008 |
0.00287 |
|
Herbaceous cover |
-0.006758 |
0.002766 |
-2.444 |
0.01515 |
|
Distance |
0.039078 |
0.013872 |
2.817 |
0.00519 |
|
Melastomataceae |
-0.208431 |
0.103539 |
-2.013 |
0.04505 |
|
Poaceae |
0.006529 |
0.003104 |
2.103 |
0.03631 |
|
Solanaceae |
-0.260134 |
0.126041 |
-2.064 |
0.03993 |
|
Variables that determine the abundance of insects | |||||
(Intercept) |
11.3399659 |
2.2316807 |
5.081 |
0.0000222 |
|
Clean stem height |
0.3241767 |
0.1332116 |
2.434 |
0.021588 |
|
Altingiaceae |
4.2039426 |
2.00778 |
2.094 |
0.045452 |
|
Height |
-0.0016319 |
0.0006404 |
-2.548 |
0.016596 |
|
Height (cm) |
0.0206169 |
0.0049136 |
4.196 |
0.000248 |
|
Tree height |
-0.5102171 |
0.1612225 |
-3.165 |
0.003723 |
|
Average height |
0.0419453 |
0.0097174 |
4.316 |
0.000179 |
|
Tree cover |
0.0484761 |
0.0175949 |
2.755 |
0.010197 |
|
Arboreal plant coverage |
-0.0855678 |
0.0226578 |
-3.777 |
0.000763 |
|
Shrub vegetation coverage |
-0.0801637 |
0.0221586 |
-3.618 |
0.001159 |
|
Herbaceous vegetation cover |
-0.0847409 |
0.0215242 |
-3.937 |
0.000497 |
|
Significance codes= 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1.
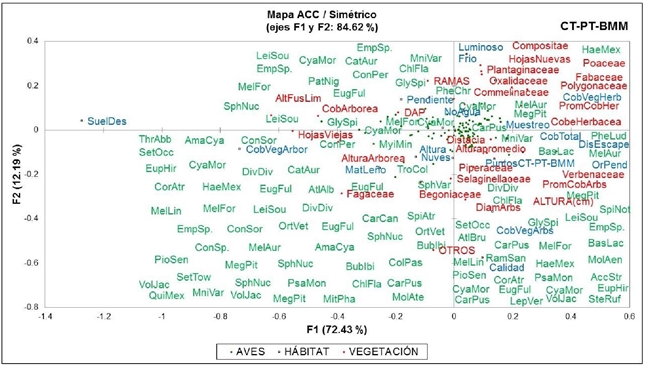
The ACC establishes an accumulated inertia in its first two axes of 84.62% for birds (Figure 7) and 87.98% for insects (Figure 8). In the figures, it is evident how the species of birds and insects have a preference for certain variables of habitat and vegetation.
The trends recorded for the habitat and vegetation variables that determine the abundance of birds and insects are consistent with what was indicated by Sánchez et al. (2016) who evaluated the tree structure of an agroforestry system (SAF) in Tabasco, Mexico; in this study they point out that the most representative vegetation families are Fabaceae, Verbenaceae, Moraceae, among others, as recorded in the present in which birds associated with the tree stratum were detected (with families of this type) feeding on insects, mostly of the order Coleoptera and Araneae (some considered pests), agrees with Caprio et al. (2015); Figueroa-Sandoval et al. (2019) who point out how the tree structure is a key element in the abundance of insects (particularly carabids and spiders) that fix the food stock of birds, according to Saavedra et al. (2015) this stratum has a large number of Coleoptera (particularly in the Scarabaeidae family).
The above could be related to the solar incidence on the canopy, this because the insects have the characteristic of being ectotherms, which is why they require heat to cover their minimum requirements; however, patterns of segregation, adaptation and use of niches have caused certain insects, as well as some birds, to settle in the lower stratum, exhibiting trophic coexistence patterns that allow an adaptation in the distribution of resources.
It was observed how migratory species of small size fed on the vegetation cover of the lower stratum (herbaceous and shrubby) collecting insects of the order araneae, coleoptera and hymenoptera; in this way, it can be inferred how the habitat structure determines the avifauna food supply, as described by Schaub et al. (2010); Bosco et al. (2019); Bucher et al. (2019) who point out how the lower stratum has beetles and spiders that feed the birds, allowing a balance in the abundance of insects.
In the same tenor Morales et al. (2008); Tarjuelo et al. (2017) show patterns of ecological segregation based on plant structure, making certain birds use the lowest stratum to acquire food, favoring trophic coexistence between species; as it is exposed in the regression analysis, visualizing individuals of Molothrus aeneus who fed on the herbaceous cover of the PT consuming insects of the order lepidoptera and coleoptera.
It coincides with Ortega-Rivera et al. (2019) who point out how the opening of the canopy in disturbed areas allow more radiation that increases the incidence of insects that could become pests, but are regulated by birds and other taxonomic groups (reptiles and amphibians) that maintain the plant health of ecosystems.
However, this balance is not only reflected among the birds but also by the presence of parasitic insects that control the incidence of organisms harmful to agricultural production; In this way, four parasitic insects were recorded in CT and one in PT that fed on insects that could be harmful to certain forest species, fulfilling the role of biological control; agrees with Thomson and Hoffmann (2009) who point out that uncultivated areas.
They favor a greater diversity of arthropods that feed different taxonomic groups (birds, reptiles, amphibians, parasitic insects, among others) giving a balance in the incidence of insects for the different strata; thus Traba et al. (2008); Puig-Montserrat et al. (2017) point out how the multi-layer plant structure in organic crops, like the SAFs, allows an adequate distribution of resources, exhibiting patterns of coexistence. Where birds have different niches for use in horizontal and vertical substrates and strata creating conditions conducive to a greater diversity of arthropods that fulfill the role of pollinators and serve as food for avifauna and other taxonomic groups.
With this in mind, we can infer how well-designed SAFs contribute to bird conservation by providing feeding niches, refuge, nesting sites, rest areas, among others; likewise, they reward such benefits by developing environmental functions that maintain environmental balance under optimal conditions, exposing better production rates for producers. The differences in the evaluated plant structure (CT, PT, BMM) have an effect on the abundance of some bird species, this agrees with Arteaga (2018) who points out that the plant structure and composition determines the abundance of birds.
In a pine agroforestry system; thus, plant heterogeneity is a determining factor in the abundance and distribution of species, therefore the condition of the CT with more strata hosted a greater number of individuals per bird species compared to PT and BMM, the latter is due to the fact that the present This study only considered the evaluation of birds from the lower and middle strata, considering that the largest number of species in mature forests are found in the upper stratum.
Certain authors point out that the abundance of species is greater in disturbed areas compared to conserved areas, however, the fact that a disturbed forest supports a greater number of individuals does not imply that it is of greater importance for conservation; thus Van Horne (1983); McArthur et al. (2019); Roberts and King (2019) point out that the density of individuals alone should not be positively linked to the quality of the habitat; it is necessary to consider other factors such as the composition, structure and population dynamics; points out that between the diversity of habitats and fauna diversity, there is not always a positive relationship, since it depends on the number of generalist and specialist species, it points out the probability that more conserved habitats originate a greater number of individuals and that due to territorial effects (competition and segregation for resources).
The excess of these is available in lower quality habitats and edge effect sites, although the abundance and density alone do not determine the environmental quality, when they are related to the characteristics of the vegetation, they allow generating response projections on the populations of birds becoming an important instrument for habitat management and the populations that use it (be they specialists or generalists). In this way Durán and Kattan (2005) point out that disturbed forests with secondary vegetation such as TC and PT surrounded by mature forest (BMM) do not necessarily have a negative effect on bird communities and their ecological processes (competition, segregation, among others); therefore, generalist species should also be subject to conservation, since they contribute to general diversity and have a greater capacity to respond to disturbance factors compared to rare and specialist species.
From the ecological point of view, the three evaluated conditions (CT, PT, BMM) are important in the conservation of this taxonomic group. In this context, the abundances of birds registered in each condition should not be considered as an indicator of environmental quality, their ecological interpretation must be associated with different population and habitat factors to infer the quality of these production systems.
This study provides relevant information on SAFs and conserved forest (BMM) in relation to the abundance of birds and their food resource (insects) for this particular region of Huatusco, Veracruz, Mexico; however, in order to design better agroforestry management and habitat improvement plans, more specific studies are needed to understand the implications of these systems for bird population parameters as indicators of environmental quality that allow the conservation of this and other taxonomic groups.
Conclusions
It was possible to define the vegetation and habitat structure in relation to the presence of prey birds and insects. These factors are determining in the abundance of birds and have the food stock guaranteeing the conservation and environmental balance of the agroforestry systems. This work is one of the first antecedents for future work where the subject on vegetation and habitat use that determines the food acquis and birdlife coexistence address.
Acknowledgments
To the National Council of Science and Technology (CONACYT) for financing for the development of this work. To the Master of Science in Agroforestry for Sustainable Development and to the Regional University Center (CRUO) for allowing to develop this project in their experimental fields.
REFERENCES
Abouhamad, S. de L.; Ramírez, M.; Ramírez, J.; Céspedes, K. y Alpízar, A. 2017. Servicios ecosistémicos de regulación que benefician a la sociedad y su relación con la restauración ecológica. Biocenosis. 31(1-2):80-92. [ Links ]
Akaike, H. 1969. Fitting autoregressive models for prediction. Annals of the Institute of Statistical Mathematics. 21(1):243-217. [ Links ]
Almazán-Núñez, R. C.; Puebla-Olivares, F. y Almazán-Juárez, Á. 2009. Diversidad de aves en bosques de pino-encino del centro de guerrero, México. Acta Zoológica Mexicana. 25(1):123-142. [ Links ]
Alonso, Y.; Hernández, F. R. y Barrero, H. 2017. Diversidad de aves residentes y permanentes asociadas a un pinar natural de Pinus tropicalis y su relación con la estructura vertical de la vegetación. Revista Cubana de Ciencias Forestales. 6(1):31-44. [ Links ]
Arteaga, M. 2018. Estructura y composición de la vegetación de dos pinares de Pinus caribaea Morelet y su relación con la diversidad de las aves asociadas. Revista Científico estudiantil Ciencias Forestales y Ambientales. 3(2):193-206. [ Links ]
Bosco, L.; Arlettaz, R. and Jacot, A. 2019. Ground greening in vineyards promotes the Woodlark Lullula arborea and their invertebrate prey. Journal of Ornithology. 160(3):799-811. [ Links ]
Bucher, R.; Nickel, H.; Kaib, S.; Will, M.; Carchi, J. and Farwig, N. 2019. Birds and plants as indicators of arthropod species richness in temperate farmland. Ecological Indicators. 103(1):272-279. [ Links ]
Bueno, P.; Sánchez, I.; Velásquez, M. A.; Esquivel, G. y Palomo, M. 2015. Caracterización de la vegetación de una microcuenca ubicada en la parte media de RH36. Agrofaz. 15(2):143-149. [ Links ]
Buitrón-Jurado, G. y Tobar, M. 2007. Posible asociación de la ardilla enana Microsciurus flaviventer (rodentia: sciuridae) y bandadas mixtas de aves en la amazonia ecuatoriana. Mastozoología Neotropical. 14(2):235-240. [ Links ]
Caprio, E.; Nervo, B.; Isaia, M.; Allegro, G. and Rolando, A. 2015. Organic versus conventional systems in viticulture: Comparative effects on spiders and carabids in vineyards and adjacent forests. Agricultural Systems. 136(1):61-69. [ Links ]
Castillo, Y. y Calderón, J. 2017. Plantas usadas por aves en paisajes cafeteros de Nariño, Colombia. Revista de Ciencias Agrícolas. 34(2):3-18. [ Links ]
Cruz, B. B. J. y Lauro, L. M. 2006. Asociación de la riqueza y diversidad de especies de aves y estructura de la vegetación en una selva mediana subperennifolia en el centro de Veracruz, México. Revista Mexicana de Biodiversidad. 77(2):235-249. [ Links ]
Cuento, V. R. 2006. Escalas en ecología: su importancia para el estudio de la selección de hábitat en aves. Hornero. 21(1):1-13. [ Links ]
Durán, S. M. and Kattan, G. H. 2005. A test of the utility of exotic tree plantations for understory birds and food resources in the Colombian Andes. Biotropica. 37(1):129-135. [ Links ]
Figueroa-Sandoval, B.; Pimentel-López, J.; Ugalde-Lezama, S.; Figueroa-Rodríguez, O. L.; Figueroa-Rodríguez, K. A. y Tarango-Arámbula, L. A. 2019. Aves en sistemas agrícolas con labranza de conservación en el centro-norte de México. Revista Mexicana de Ciencias Agrícolas. 22(Especial):31-42. [ Links ]
García, A. 2018. Fauna silvestre alimentaria de la reserva sierra de Montenegro, Morelos, México. Ethnoscientia. 3(1-2):715. [ Links ]
López-Ferrer, U. del C.; Brito-Vega, H.; López-Morales, D.; Salaya-Domínguez, J. M. and Gómez-Méndez, E. 2017. Papel de Trichoderma en los sistemas agroforestales cacaotal como un agente antagónico. Tropical and Subtropical Agroecosystems. 20(1):91-100. [ Links ]
McArthur, N.; Boulton, R.; Richard, Y. and Armstrong, D. 2019. The role of pine plantations in source-sink dynamics of North Island robins. New Zealand Journal of Ecology. 43(1):33-62. [ Links ]
Mora, J. M.; Batista, A. E. y López, U. L. I. 2019. Regeneración natural en sitios impactados por incendios en la Reserva Biologíca Uyuca, Honduras. CEIBA A Scientific Technical Journal. 842(1):1-10. [ Links ]
Morales, M.; Carriles, T.; Delgado, M. and García de la Morena, E. 2008. Sexual differences in microhabitat selection of breeding little bustards Tetrax tetrax: Ecological segregation based on vegetation structure. Acta oecologica. 34(3):345-353. [ Links ]
Naranjo, L. G. y Ulloa, P. C. 1997. Diversidad de insectos y aves insectivoras de sotobosque en habitats perturbados de selva lluviosa tropical. Caldasia. 19(3):507-520. [ Links ]
Navarro-Sigüenza, A. G.; Rebón-Gallardo, M. F.; Gordillo-Martínez, A.; Peterson, A. T.; Berlanga-García, H. y Sánchez-González, L. A. 2014. Biodiversidad de aves en México. Revista Mexicana de Biodiversidad. 85(12):S476-S495. [ Links ]
Ortega-Rivera, K.; Flores-Hernández, N.; Zarza, H. y Chávez, C. 2019. Caracterización del estado fitosanitario de Quercus obtusata Bonpl., en bosque mesófilo de montaña, Xicotepec, Puebla Phytosanitary characterizaction of Quercus obtusata Bonpl., in a mountain cloud forest, Xicotepec, Puebla. Revista Mexicana de Ciencias Forestales. 10(53):64-85. [ Links ]
Ponce, L. P.; Aguilar, V. B.; Rodríguez, T. D.; López P. E. y Santillán, P. J. 2012. Influencia del fuego sobre la riqueza y diversidad de aves en un bosque templado en Puebla. Revista Mexicana de Ciencias Forestales. 3(10):65-76. [ Links ]
Puig-Montserrat, X.; Stefanescu, C.; Torre, I.; Palet, J.; Fàbregas, E.; Dantart, J.; Arrizabalaga, A. and Flaquer, C. 2017. Effects of organic and conventional crop management on vineyard biodiversity. Agriculture, Ecosystems and Environment. 243(1):19-26. [ Links ]
Ramírez-Albores, J. E. 2009. Diversidad de aves de hábitats naturales y modificados en un paisaje de la Depresión Central de Chiapas, México. Revista de Biologia Tropical. 58(1):511-528. [ Links ]
Rangel-Salazar, J. L.; Enríquez, P. L. y Sántiz López, E. C. 2009. Variación de la diversidad de Aaes de sotobosque en el parque nacional lagos de Montebello, Chiapas, México. Acta Zoológica Mexicana. 25(3):479-495. [ Links ]
Roberts, P. and King, D. 2019. Variation in plumage reflects avian habitat associations not revealed by abundance. The Wilson Journal of Ornithology. 131(2):339-347. [ Links ]
Saavedra, A. D.; Vaz de Mello, F.; Ugaz, A. y Pacherre, T. C. 2015. Coleópteros (Coleóptera: Scarabaeidae) de los bosques de niebla, Ramos y Chin Chin, Ayabaca-Huancabamba, Piura-Perú. Indes. 3(1):108-116. [ Links ]
Sánchez, G. F.; Pérez-Flores, J.; Obrador, O. J.; Sol, S. Á. y Ruiz-Rosado, O. 2016. Estructura arbórea del sistema agroforestal cacao en Cárdenas, Tabasco, México. Revista Mexicana de Ciencias Agrícolas. 7(14):2695-2709. [ Links ]
Schaub, M.; Martinez, N.; Tagmann-Ioset, A.; Weisshaupt, N.; Maurer, M. L.; Reichlin, T. S. Abadi, F.; Zbinden, N.; Jenni, L. and Arlettaz, R. 2010. Patches of Bare Ground as a Staple Commodity for Declining Ground-Foraging Insectivorous Farmland Birds. PlosOne. 5(10):1-5. [ Links ]
Serial, R. M. B. y Grigera, D. 2005. Dinámica estacional del ensamble de aves de un bosque norpatagónico de lenga (Nothofagus pumilio) y su relación con la disponibilidad de sustratos de alimentación. Hornero. 20(2):131139. [ Links ]
Tarjuelo, R.; Traba, J.; Morales, M. and Morris, D. 2017. Isodars unveil asymmetric effects on habitat use caused by competition between two endangered species. Oikos. 126(1):73-81. [ Links ]
Thomson, L. and Hoffmann, A. 2009. Vegetation increases the abundance of natural enemies in vineyards. Biological Control. 40(3):259-269. [ Links ]
Traba, J.; Morales, M.; García de la Morena, E. and Delgado, M. P. 2008. Selection of breeding territory by little bustard (Tetrax tetrax) males in Central Spain: the role of arthropod availability. Ecological Research. 23(3):615-622. [ Links ]
Tzuc-Martínez, R.; Casanova-Lugo, F.; Caamal-Maldonado, A.; Tun-Garrido, J.; González-Váldivia, N. y Cetzal-Ix, W. 2017. Influencia de las especies leñosas en la dinámica de arvenses en sistemas agroforestales en Yucatán, México. Agrociencia. 51(3):315-328. [ Links ]
Valencia-Trejo, G. M.; Ugalde-Lezama, S.; Pineda-Pérez, F. E.; Tarango-Arámbula, L. A.; Lozano-Osornio, A. y Cruz-Miranda, Y. 2014. Diversidad de aves en el Campus Central de la Universidad Autónoma Chapingo, México. Agropoductividad. 7(5):37-44. [ Links ]
Van Horne, B. 1983. Density as a misleading indicator of habitat quality. Journal of Wildlife Management. 47(4):893-901. [ Links ]
Vazquez-Perez, J. R.; Enríquez-Rocha, P. L. y Rangel-Salazar, J. L. 2009. Diversidad de aves rapaces diurnas en la reserva de la biosfera Selva El Ocote, Chiapas, México. Revista Mexicana de Biodiversidad. 80(1):203-209. [ Links ]
Received: March 01, 2020; Accepted: May 01, 2020











 texto en
texto en