Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.11 no.2 Texcoco Fev./Mar. 2020 Epub 15-Mar-2021
https://doi.org/10.29312/remexca.v11i2.1742
Articles
Incidence of Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) and defense mechanisms in Gerbera hybrida
1Autonomous University of the State of Mexico-UAEM Tenancingo University Center. Ex-Hacienda of Santa Ana, Tenancingo-Villa Guerrero highway km 1.5, Tenancingo, State of Mexico. CP. 52400. Tel. 714 1407724. (ing.alcantar@yahoo.com.mx; marthaelenam@gmail.com; soteromex@hotmail.com).
The existence of natural variation at different levels of whitefly infestation (Trialeurodes vaporariorum) in gerbera genotypes (Gerbera hybrida) allows the existence of different levels of resistance or susceptibility to the insect. In this investigation the natural variation of gerbera was evaluated at the incidence of T. vaporariorum. In a randomized complete block design, ten gerbera genotypes were analyzed in different phenological stages without incidence (SI) and with incidence (CI) of whitefly. Nymphs were counted, in a cm2 at the center of the underside of the leaves and total adults per leaf. The relative content of chlorophyll (USPAD), the content of phenolic compounds (Cf), the enzymatic activity of peroxidases (AePOX) and the antioxidant capacity DPPH (Caox) were measured. Gerbera genotypes showed significant differences in the incidence of whiteflies (p≤ 0.01), as well as in Cf, AePOX and Caox, both in SI and CI (p≤ 0.01), which suggests differences in insect preference a its host and the ability of genotypes to respond to whiteflies. In CI with respect to SI, they increased the content of Cf (1.4 times), AePOX (4 times) and Caox (12 times). USPAD decreased in the genotype with the highest incidence of the insect (-4%). The significant correlations between the number of insects (nymphs) with Cf (-0.71**) and AePOX (-0.65**) and Caox with Cf (-0.73**) and AePOX (-0.76**), showed that the feeding of the insect interferes with the metabolism of its hosts and triggers integral responses in the defense mechanism of plants, with differences in resistance that can be used in genetic improvement.
Keywords: enzymatic activity of peroxidases; genetic variability; phenols
La existencia de variación natural a diferentes niveles de infestación de mosca blanca (Trialeurodes vaporariorum) en genotipos de gerbera (Gerbera hybrida) posibilita la existencia de diferentes niveles de resistencia o susceptibilidad al insecto. En esta investigación se evaluó la variación natural de gerbera a la incidencia de T. vaporariorum. En un diseño de bloques completos al azar, se analizaron diez genotipos de gerbera en diferentes etapas fenológicas sin incidencia (SI) y con incidencia (CI) de mosca blanca. Se contabilizaron ninfas, en un cm2 al centro del envés de las hojas y total de adultos por hoja. Se midieron el contenido relativo de clorofila (USPAD), el contenido de compuestos fenólicos (Cf), la actividad enzimática de las peroxidasas (AePOX) y la capacidad antioxidante DPPH (Caox). Los genotipos de gerbera mostraron diferencias significativas en la incidencia de la mosca blanca (p≤ 0.01), así como en Cf, AePOX y Caox, tanto en SI como CI (p≤ 0.01), lo cual sugiere diferencias en la preferencia del insecto a su hospedero y en la capacidad de respuesta de los genotipos a la mosca blanca. En CI respecto a SI, incrementaron el contenido de Cf (1.4 veces), la AePOX (4 veces) y la Caox (12 veces). USPAD disminuyó en el genotipo con la mayor incidencia del insecto (-4%). Las correlaciones significativas entre número de insectos (ninfas) con Cf (-0.71**) y AePOX (-0.65**) y Caox con Cf (-0.73**) y AePOX (-0.76**), mostraron que la alimentación del insecto interfiere en el metabolismo de sus hospederos y desencadena respuestas integrales en el mecanismo de defensa de las plantas, con diferencias en resistencia aprovechables en mejoramiento genético.
Palabras clave: actividad enzimática de las peroxidasas; fenoles; variabilidad genética
Introduction
Gerbera (Gerbera hybrida) is one of the most commercially important ornamentals in the State of Mexico (Andrade and Castro, 2018). The main producers in the floricultural region of the south of the state are the municipalities of Villa Guerrero, Tenancingo, Coatepec Harinas and Zumpahuacán, which generate a production of 1 108 384 t per crop cycle (SIAP, 2016). One of the pests that affects the gerbera crop is the whitefly Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) (Parrella et al., 2014; Reddy, 2016).
For its control, insecticides are commonly applied whose improper use has led to the development of hemiptera resistance (Pérez-Sandoval et al., 2011; Bass et al., 2015). The use of genotypes resistant to this pest, detectable in genetic variability, can be an easily adoptable, economical, environmentally safe control method and can be an alternative to reduce the use of insecticides (Sulistyo and Inayati, 2016; Hoshino et al., 2017).
Conventional genetic improvement has received great benefits from the genetic variation of gerbera and has resulted in the development of genotypes with aesthetic and performance characteristics (Senapati et al., 2013; Pradhan et al., 2017; Mangroliya et al., 2018). According to the perception of producers and marketers of the aforementioned floricultural region, approximately 200 varieties of gerbera are cultivated that, in addition to being morphologically different, also show differences in susceptibility to pests and can be used as a genetic resource. In this sense, variations between gerbera and chrysanthemum genotypes have been reported in the synthesis of secondary metabolites and antioxidant enzymes as defense mechanisms in response to pest attack (Krisps et al., 2001; He et al., 2011).
For example, Sierra et al. (2014) mention that the presence of certain secondary metabolites in plants causes insects, such as whiteflies, to have preference for certain plants and repellency for others due to variations in compounds such as coumarins, terpenes and steroids. In plant species, the stress caused by insects induces antioxidant enzymes such as peroxidases, which participate in the defense mechanism with the synthesis of cell wall polymers such as lignin and suberine, natural physical barriers (Dicko et al., 2005; Zhao et al., 2016).
In gerbera, evidence of variation between genotypes could be exploited in the search for materials resistant to whiteflies, which would imply a decrease in production costs, particularly in the use of insecticides for the control of this pest. That is why the objective of this research was to evaluate the variation of 10 genotypes of G. hybrida in the incidence of T. vaporariurum, content of phenolic compounds, enzymatic activity of peroxidases, antioxidant capacity and relative chlorophyll content.
Materials and methods
Experiment location
The research was conducted from September 2016 to December 2017, at the facilities of the University Center Tenancingo of the Autonomous University of the State of Mexico, which are located at km 1.5 of the Tenancingo-Villa Guerrero road, Tenancingo, State of Mexico a 18º 97’ 03’’ north latitude and 99º 61’ 17’’ west longitude and at an altitude of 2 200 m.
Vegetative material
Ten genotypes of G. hybrida obtained by in vitro culture were used. Seven of them developed at the University Center UAEM Tenancingo and the company Comprehensive Services for Horticulture and identified as: Sofia (Sof), Estrella (Est), Andrea (And), Magda (Mag), Lisieka (Lis), Carmin (Car ) and Morelia (Mor), the remaining three are commercial varieties of names Dino (Din), Opera (Ope) and Completa (Com).
Establishment of the experiment
Seedlings acclimated for six to eight weeks with averages of 10 cm in height and 6 leaves were established under greenhouse in 20 L plastic pots with previously disinfected substrate, composed of a mixture of peat and perlite expanded in a 2:1 ratio (v/v), commonly used for macetry in the region. The plants were established in an experimental design of randomized complete blocks with 10 repetitions. The experimental unit was a pot with a plant.
Fertilization of the crop
It was according to Surin (2011) with modifications in micronutrients. The pH was adjusted in a range of 5.5 to 6 and electrical conductivity of 2 to 2.7 mS m-1.
Incidence of the whitefly Trialeurodes vaporariorum
The gerbera infestation was by natural invasion of the insect at the beginning of the emission of the floral chapter, during a period of 30 days in which no insecticide applications were made. Nymphs were quantified, without considering urging, in a square centimeter to the center of the underside of mature leaves and the total of adults per complete mature leaf. Measurements were made 30 and 60 days after the infestation (Morales and Cermeli, 2001).
Evaluation of biochemical variables
They were determined in mature leaves with a higher relative chlorophyll content, recorded in 58 ±3.5 SPAD units, (SPAD-502, Minolta Camera Co., Osaka Japan). They were measured in conditions of no incidence (SI), in the vegetative stage of the plants, prior to the infestation of the insect and with incidence of the insect (CI) at the beginning of the chapter emission and in flowering. The following variables were measured by spectrophotometry (Genesys 10S UV-Vis Thermo Scientific).
Phenolic compounds (Cf). They were determined by the folin-ciocalteu (FC) method described by Waterman and Mole (1994). Samples of 100 mg of leaf were macerated in 5 mL of methanol (50%) and incubated at 100 °C for five min, centrifuged at 5 400 rpm for 5 minutes to separate and preserve the supernatant at 4 °C. At 0.15 mL of supernatant the sample was added the same amount of FC reagent and 0.5 mL of 20% Na2CO3, they were added to 4.5 mL with distilled water and allowed to stand in the dark for 30 min. The calibration curve was made with gallic acid (10 mg in 10 mL of 50% methanol) as a standard at concentration intervals of 0.005 mL. The results were expressed in mg of gallic acid equivalents per gram of the analyzed sample.
Enzymatic activity of peroxidases (AePOX). It was determined according to the method described by Anderson et al. (1995). Samples of ± 50 mg of mature leaf were macerated in 200 μL of 50 mM extraction buffer of pH 7.2 potassium phosphate, 1 mM of ethylene diamine tetraacetic acid (Edta) and 1% of polyvinylpyrrolidone (PVP). The extract was centrifuged at 6 000 rpm for 5 min and the 0.020 mL volume supernatant was used to quantify AePOX in a reaction mixture buffer with 50 mM sodium phosphate pH 7, 3.33 mM guaiacol, 4 mM H2O2, at 25 ° C, in a final volume of 3 mL. As a blank, reaction buffer without supernatant was used.
The enzymatic activity was determined by the oxidation of the guaiac substrate in the presence of H2O2 at 470 nm for three min in 30 s intervals (extinction coefficient of guaiacol ε= 26.6 Mm-1 cm-1, in the nmol equation min-1 mg-1). Enzymatic activity Pox = (Abs) (ε) (final test volume/sample volume) (mg of protein).
DPPH antioxidant capacity (Caox). It was determined by the 1,1-diphenyl-2-picryl hydracil (DPPH) method according to Abe et al. (1998). The sample extraction methodology was the same as for Cf. A solution of DPPH at 250 μM in 80% methanol (98.58 mg in 1000 mL of methanol) was used. To 2 750 mL of the DPPH solution, 0.25 mL of the gerbera extract supernatant was added and allowed to stand for one hour. Readings were made at 517 nm absorbance. The spectrophotometer blank was 80% methanol. The calibration curve was performed with ascorbic acid (10 mg in 10 mL of 80% methanol), with concentration ranges of 0.005 mL. Each sample was analyzed in triplicate. The results were expressed in mg of equivalents of ascorbic acid per gram of the analyzed sample.
Statistical analysis
The data obtained were analyzed with the statistical package Info Stat (Di Rienzo et al., 2016) and submitted to analysis of variance (α= 0.05), where there were significant differences, a comparison of means with the Duncan test was applied (α= 0.05). Multiple correlation analysis was also done.
Results and discussion
Trialeurodes vaporariorum incidence
In the occurrence of adults plus whitefly nymphs, highly significant differences (p≤ 0.01) were found among the ten gerbera genotypes evaluated. The variation of genotypes with extreme values was 1 to 43 between Com and Lis, respectively (Figure 1). The comparison of means grouped the genotypes into four categories, defined in this study, according to their incidence in: low (A), Com, Din, Ope, Sof, And and Car, moderate low (AB), Est and Mor , moderate high (B), Mag and high (C), Lis.
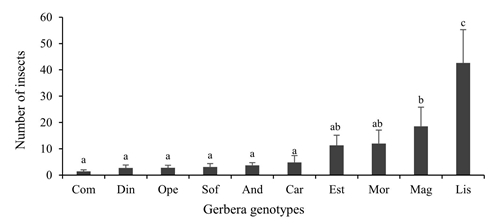
Figure 1 Incidence of trialeurodes vaporariorum (adults plus nymphs) in Gerbera hybrida in the phenological stages of the beginning of floral chapter emission and flowering. Error bars= standard error. Values with the same letter are statistically equal according to the Duncan test (p≤ 0.05).
The presence of T. vaporariorum in gerbera has been documented by several authors (Berndt and Meyhöfer, 2008; Parrella et al., 2014; Rojas et al., 2018). The variation in the response between genotypes could respond to differences in morphological characteristics of plants, which can act as physical barriers to insects (Taggar and Gill, 2012; Belete, 2018) and factors related to plant metabolism and mechanisms Antioxidants such as the participation of secondary metabolites (Zhang et al., 2017) and the activity of certain enzymes (Taggar et al., 2014; Zhao et al., 2016).
In this regard, studies in gerbera, highlight the variation between genotypes in the synthesis of secondary metabolites in response to the attack of pests (Krisps et al., 2001), which constitute an important element for their use in the development of resistant varieties (Broekgaarden et al., 2011; Mitchell et al., 2016; Hoshino et al., 2017).
In studies conducted by Lucatti et al. (2010)) with genotypes of Solanum lycopersicum L., suggested that the variation between their populations to the incidence of whitefly allows the selection of genotypes with some resistance to the insect for genetic improvement and obtaining resistant varieties. Those genotypes that showed lower incidence of T. vaporariorum could be used in the genetic improvement of gerbera to develop resistant or tolerant varieties to this pest, which would imply a decrease in the use of insecticides destined for its control.
Cf Content
Highly significant differences (p≤ 0.01) were found in the Cf content in both SI and CI between the ten genotypes evaluated (Figure 2). In SI, the difference between extreme values was almost double between Ope and Mor, which suggests that those genotypes with higher content could have an advantage in insect repellency over those that presented lower values of this metabolite, by synthesizing them as permanent defense strategy. However, defense chemicals such as phenolic compounds are considered expensive for plants due to resources consumed in their biosynthesis (induced) or the ecological consequences of their accumulation as constitutive expression (Lattanzio, 2013).

Figure 2 Content of phenolic compounds (cf) in mature leaves of ten genotypes of Gerbera hybrida without incidence (SI) in vegetative stage and with incidence (CI) of T. vaporariorum at the beginning of emission of floral chapter and flowering. Error bars correspond to the standard error. Lowercase letters= comparison of SI means; uppercase letters= comparison of CI means. Values with the same letter are statistically equal according to the Duncan test (p ≤ 0.05).
In CI, an average increase of 40% in the Cf content with respect to SI was observed, and in particular, there was a variation in response between genotypes from 5 (Ope) to 108% (Mor). Genotypes such as Ope and Car, even though their increase from SI to CI was low, remained among the genotypes with the highest Cf values, showing high values from SI.
The fact that those genotypes that had a significant increase from SI to CI such as Mor and Com, suggests that they induce the synthesis of this metabolite as a defense mechanism towards T. vaporariorum and according to the incidence of the Com insect could present some resistance to The whitefly has a lower incidence of the insect and a high concentration of Cf in CI. Lis, Est and Mag genotypes had the lowest concentrations of Cf in both SI and CI, suggesting that they may be more susceptible to the insect not having other defense mechanisms other than these compounds.
Different studies report the participation of phenolic compounds such as chlorogenic acid, caffeic acid, cinnamic acid (Sandhyarani and Usha, 2013), ferulic acid, catechin (Zhang et al., 2017), coumarins (Rehman et al., 2012), tannins and lignin (War et al., 2012: Vaca-Sánchez et al., 2016), to counteract insect stress as a defense mechanism. Tannins, for example, exhibit some toxicity to pests and can significantly reduce the growth, development and survival of insects (War et al., 2012), others such as lignin strengthen the cell wall of plants, and act as a barrier physics towards insects (Lattanzio, 2013; Sandhyarani and Usha, 2013).
Sierra et al. (2014) mentioned that the presence of secondary metabolites including phenolic compounds in plants, causes insects, such as whiteflies to have preference for certain plants and repellency for others due to variations in the concentration of secondary metabolites. Likewise, the increase in the content of phenols in plants is considered a common response to insect damage (Sandhyarani and Usha, 2013).
In this investigation, the results indicate a significant increase in the Cf content with the presence of the insect, which indicates that this metabolite has an important participation in the defense mechanisms of the plants possibly to counteract the stress of the plant generated by T. vaporariorum Similar results were reported by Zhang et al. (2017) who indicate an increase in the content of phenolic compounds in genotypes of Nicotiana tabacum after the incidence of T. vaporariorum and Bemisia tabaci. In soybeans, aphid-resistant genotypes showed a greater increase in phenolic compounds than those susceptible (Jiang et al., 2009). The variation in Cf synthesis between gerbera genotypes in both SI (Ope and Car), and CI (Com), suggests differences in the type of response of plants to the insect, according to a metabolic priority by the plant (Strauss et al., 2002).
Enzymatic activity of peroxidases (AePOX)
There were highly significant differences (p≤ 0.01) between the genotypes studied for AePOX in both SI and CI measurements (Figure 3). In SI the difference between extreme values of enzyme activity was 20 times between Din and Mor. From SI to CI, a 4 times average increase in AePOX was observed. The variation between extreme CI values was 1 to 3 times between Car and Lis, respectively. Those genotypes with higher AePOX in SI (Com and Din), showed a lower increase in enzyme activity compared to other genotypes in CI, however, their previous values (in SI) allowed them to be on par with those genotypes with high AePOX in IC (Figure 1). In contrast, the genotypes that had a significant proportional increase from SI to CI were Mor and Lis, although their values remained low compared to other genotypes.
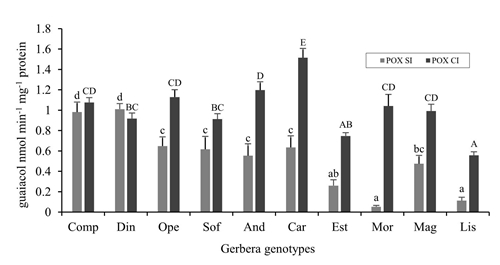
Figure 3 Enzymatic activity of peroxidases (AePOX) in mature Gerbera hybrida leaves; no incidence (SI), in vegetative stage; and with incidence (CI) of T. vaporariorum at the beginning of emission of floral and flowering chapter. Error bars correspond to the standard error. Lowercase letters= comparison of SI means; uppercase letters= comparison of CI means. Values with the same letter are statistically equal according to the Duncan test (p≤ 0.05).
In the plant-insect interaction, the participation of antioxidant enzymes such as POX and polyphenol oxidases (PPO) has also been reported (Jiang et al., 2009; Sandhyarani and Usha, 2013), which catalyze the oxidation of a wide variety of phenols for the synthesis of lignin and suberine that accumulate in the cell wall or for the formation of quinones, toxic to insects (Lattanzio et al., 2006; Lopez-Nicolas and García Carmona, 2010; Shigeto and Tsutsumi, 2016).
In this study, the differences in AePOX between gerbera genotypes evaluated, both in SI and in CI, indicate natural variation between genotypes in the synthesis of this enzyme possibly regulated at the level of transcription or translation (Cosio and Dunand, 2009; Shigeto and Tsutsumi, 2016). In genotypes of other ornamentals of the same family as Crysanthemum grandiflorum, variation in the expression of antioxidant enzymes (super oxide dismutase and phenyl ammonium lyase) has also been reported, including POXs, both without incidence and with incidence of aphids and suggest the participation of these enzymes in insect resistance (He et al., 2011).
Those genotypes that more induced the enzyme from SI to CI, such as Lis, Mor and Car, guided the exercise of their energy resources differently (Strauss et al., 2002). Similar results to this research on the increase in the enzymatic activity of POX with the incidence of whitefly were obtained in black lentil genotypes (Vigna mungo L.) affected by Bemisia tabaci, where those more resistant to insect stress showed a greater increase in AePOX than those susceptible (Taggar et al., 2014). Zhao et al. (2016) reported an increase in the enzymatic activity of POX and catalase in tobacco plants after the presence of B. tabaci, in comparison to the control plants. On the other hand, Mor's ability to promote AePOX with the high incidence of T. vaporariorum suggests the participation of the enzyme as a defense mechanism.
DPPH antioxidant capacity (Caox)
Highly significant differences (p≤ 0.01) were found among the ten gerbera genotypes evaluated in both SI and CI (Figure 4). In SI, although there was variation between almost 2:1 genotype, their values were less than 0.l mg of ascorbic acid per gram of fresh weight in all genotypes evaluated. In contrast, in CI the proportional increase in each genotype varied from six to 20 times with respect to SI. Lis that stood out with intermediate values of antioxidant capacity, showed low values of Cf and AePOX content, which may explain the higher incidence value of T. vaporariorum, otherwise Com that presented low values of antioxidant capacity and high values of Cf and AePOX, in addition to the lower incidence values of the insect.

Figure 4 Antioxidant capacity DPPH (Caox) of Gerbera hybrida without incidence (SI), in vegetative stage and with incidence (CI) of T. vaporariorum at the beginning of the emission of floral chapter and flowering. Lowercase letters= comparison of SI means, capital letters= comparison of CI means. Error bars correspond to the standard error. Values with the same letter are statistically equal according to the Duncan test (p≤ 0.05).
The higher antioxidant capacity values suggest a higher production of reactive oxygen species (EROS) as a result of different types of stress in the plant such as the incidence of insects or infection by pathogens, mechanical damage, wounds by herbivores or abiotic factors (Mittler, 2002; Benezer-Benezer et al., 2008). In fact, only egg oviposition promotes an increase in EROS, such as the hypersensitive response induced by pathogens, which lead to the desiccation of the egg and its subsequent detachment of plants (Hilker and Fatouros, 2016).
In other species such as tobacco (Nicotiana tabacum L.), whitefly infestation increased H2O2 levels by 77.3% more than in control plants (Zhao et al., 2016). H2O2, in addition to being a signaling molecule under stress conditions and in the physiological processes of plants (Sharma et al., 2012), is used as a substrate by peroxidases for both lignification and suberization of the cell wall, such as for the oxidation of phenolic compounds during insect injuries, which indirectly converts it into an antioxidant enzyme (He et al., 2011).
Relative content of chlorophyll (USPAD)
Highly significant statistical differences (p≤ 0.01) were presented in both SI and CI evaluations (Figure 5), but with an average discrete variation of 15% between extreme values between genotypes of both measurements. Likewise, there was an average increase of SI to CI of 8%; however, in two genotypes their values decreased, and they corresponded to those that showed the highest insect incidence. These results suggest that the variation in the relative chlorophyll content between genotypes is due to different factors such as the variability of the species, the phenological stage of the plant in which it was evaluated, and the same incidence of pests as the whitefly.
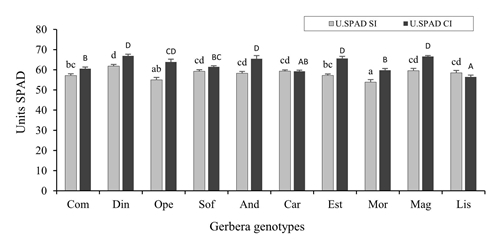
Figure 5 Relative content of chlorophyll (USPAD) in Gerbera hybrida of photosynthetically active leaves without incidence (SI), in vegetative stage and with incidence (CI) of T. vaporariorum at the beginning of the emission of floral chapter and flowering. Lowercase letters= comparison of SI means, capital letters= comparison of CI means. Error bars correspond to the standard error. Values with the same letter are statistically equal according to the Duncan test (p≤ 0.05).
Goławska et al. (2010) cites that the relative content of chlorophyll is one of the most important parameters in the relationship between plants and insects. A significant reduction in the relative chlorophyll content due to insect infestation has been reported in different plant species (Goławska et al., 2010; Huang et al., 2014). Kerchev et al. (2012) suggested that insect herbivory far from stimulating photosynthesis reduces carbon fixation and this response occurs by reprogramming the gene expression of the primary metabolism.
Correlation analysis
The negative and highly significant correlations of the incidence of insects, particularly nymphs, with phenols (r= -0.71) and activity of the POX enzyme (r= -0.65) (Table 1), suggest an influence of these biochemical variables on protection of plants, as already mentioned by different authors (Sandhyarani and Usha 2013; Taggar et al., 2014). Also, the phenolic content was positively correlated and highly significant with AePOX (r= 0.62).
Table 1 Pearson correlation matrix of six variables measured in Gerbera hybrida.
|
Adults (A) |
Nymphs (n) |
AePOX |
||||
Adults (A) |
1 |
|
|
|
|
|
|
Nymphs (n) |
0.84** |
1 |
|
|
|
|
|
0.99** |
0.89** |
1 |
|
|
|
|
|
-0.55 |
-0.71** |
-0.6 |
1 |
|
|
|
|
AePOX |
-0.66** |
-0.52 |
-0.65** |
0.62** |
1 |
|
|
-0.51 |
-0.13 |
-0.45 |
-0.14 |
0.04 |
1 |
|
|
0.32 |
0.27 |
0.32 |
-0.73** |
-0.76** |
0.15 |
1 |
A + n= adults plus nymphs; Cf= Phenolic compounds; POX= enzymatic activity of peroxidases; U SPAD= SPAD units; Caox= antioxidant capacity; **= highly significant values.
In this association between variables it is important to highlight that peroxidases, in addition to catalyzing the lignification of the cell wall and detoxifying peroxides produced due to oxidative stress, produce phenoxy and other oxidative radicals that in association with phenols that act as deterrents for feeding and they produce toxins that reduce the digestibility of plant tissue to insects (Dicko et al., 2005; Shigeto and Tsutsumi, 2016).
The antioxidant capacity was negatively and highly significant correlated with Cf (r= -0.73) and AePOX (r= -0.76), which suggests that the phenols and the activity of the enzyme in its action to minimize damage by the insect, neutralize free radicals indirectly represented by antioxidant capacity. The relative chlorophyll content was not significantly correlated with any variable; however, the trend of negative correlation with number of insects was similar to that reported by Huang et al. (2014).
Conclusions
Gerbera genotypes showed variation in the incidence of T. vaporariorum, as well as in the content of phenolic compounds and in the AePOX, which indicates genetic variation of this species, and preferences of the insect towards certain hosts. Those genotypes with lower incidence of the insect can be used to develop resistant or tolerant varieties as alternatives in floricultural production. The ability of those genotypes both without incidence and with incidence, in the synthesis of enzymatic antioxidant compounds (AePOX) and non-enzymatic (phenolic compounds), to counteract the oxidative stress caused by the insect, probably gives them some bioprotection as a defense mechanism against T. vaporariorum infestation.
Acknowledgments
The authors thank the National Council of Science and Technology (CONACYT) for a scholarship granted to Santa Mayra Alcántar-Acosta for master’s studies period 2016-2018.
REFERENCES
Abe, N.; Murata, T. and Hirota, A. 1998. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Bio. Biotechnol. Biochem. 62(4):661-666. Doi: 10.1271/bbb.62.661. [ Links ]
Anderson, M. D.; Prasad, T. K. and Stewart, C. R. 1995. Changes in isoenzyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 109(4): 1247-1257. Doi: 10.1104/pp.109.4.1247. [ Links ]
Andrade, G. J. A. y Castro, D. P. 2018. Redes migratorias en el mercado de trabajo de la floricultura en el Estado de México (México). Rev. Antropología Social. 27(1):145-168. Doi: 10.5209/RASO.59436. [ Links ]
Bass, C.; Denholm, I.; Williamson, S. and Nauen, R. 2015. The global status of insect resistance to neonicotinoid insecticides. Pesticide Biochem. Phisiol. 121:78-87. Doi: 10.1016/j.pestbp.2015.04.004. [ Links ]
Belete, T. 2018. Defense mechanisms of plants to insects’ pests: from morphological to biochemical approach. J. Natural Sci. Res. 8(9):1-8. [ Links ]
Benezer-Benezer, M.; Castro-Mercado E. y García-Pineda E. 2008. La producción de especies reactivas de oxígeno durante la expresión de la resistencia a enfermedades en plantas. Rev. Mex. Fitopatol. 26(1):56-61. [ Links ]
Berndt, O. and Meyhöfer, R. 2008. Whitefly control in cut gerbera: is it possible to control Trialeurodes vaporariorum with Encarsia formosa? BioControl. 53:751-762. Doi: 10.1007/s10526-007. [ Links ]
Broekgaarden, C.; Snoeren, T. A.; Dicke, M. and Vosman, B. 2011. Exploiting natural variation to identify insect‐resistance genes. Plant Biotechnol. J. 9(8):819-825. Doi: 10.1111/j.1467-7652.2011.00635.x. [ Links ]
Cosio, C. and Dunand, C. 2009. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 60(2): 391-408. Doi: 10.1093/jxb/ern318. [ Links ]
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; González, L.; Tablada, M. y Robledo, C. W. 2016. InfoStat versión 2016. Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar. [ Links ]
Dicko, M. H.; Gruppen, H., Barro, C.; Traoré, A. S.; Van Berkel, W. J. and Voragen, A. G. 2005. Impact of phenolic compounds and related enzymes in sorghum varieties for resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 31(11):2671-2688. Doi: 10.1007/s10886-005-7619-5. [ Links ]
Goławska, S.; Krzyżanowski, R. and Łukasik, I. 2010. Relationship between aphid infestation and chlorophyll content in Fabaceae species. Acta Biológica Cracoviensia Series Botánica. 52(2):76-80. Doi: 10.2478/v10182-010-0026-4. [ Links ]
He, J.; Chen, F.; Chen, S.; Lv, G.; Deng, Y.; Fang, W. 2011. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Phisiol. 168: 687-693. doi: 10.1016/j.jplph.2010.10.009. [ Links ]
Hilker, M. and Fatouros, N. E. 2016. Resisting the onset of hervibore attack: plants perceive and respond to insect eggs. Current Opinion in Plant Biology. 32:9-16. Doi: 10.1016/j.pbi.2016.05.003. [ Links ]
Hoshino, A. T.; Buratto, J. S.; Dias, B. F.; Luski, P. G. G.and Androcioli, H. G. 2017. Resistance of different common bean genotypes (Phaseolus vulgaris L.) to whitefly (Bemisia tabaci Gennadius, 1889) b biotype (Hemiptera: Aleyrodidae). Annual Report of the bean improvement cooperative. Published by USDA-ARS / UNL Faculty. 1736. 60:84-86. [ Links ]
Huang, T. I.; Reed, D. A.; Perring, T. M. and Palumbo, J. C. 2014. Feeding damage by Bagrada hilaris (Hemiptera: Pentatomidae) and impact on growth and chlorophyll content of Brassicaceous plant species. Arthropod-Plant Interactions. 8(2):89-100. Doi: 10.1007/s11829-014-9289-0. [ Links ]
Jiang, Y. N.; Wang, B. and Wu, T. L. 2009. Response of enzyme activity and secondary metabolites of different soybean genotypes to Aphis glycines Matsmura invasion. Soybean Science. 1:104-107. http://en.cnki.com.cn/Article-en/CJFDTotal-DDKX200901025.htm. [ Links ]
Kerchev, P. I.; Fenton, B.; Foyer, C. H. and Hancock, R. D. 2012. Plant responses to insect herbivory: interactions between photosynthesis, reactive oxygen species and hormonal signalling pathways. Plant, Cell Enviro. 35(2):441-453. Doi: 10.1111/j.1365-3040.2011.02399.x. [ Links ]
Krisps, O. E.; Willems, P. E. L.; Gols, R.; Posthumus, M. A.; Gort, G. and Dicke, M. 2001. Comparison of cultivars of ornamental crop Gerbera jamesonii on production of spider mite-induced volatiles, and their attractiveness to the predator, Phytoseiulus persimilis. J. Chem. Ecol. 27(7):1355-1372. Doi: 10.1023/A:1010313209119. [ Links ]
Lattanzio, V. 2013. Phenolic compounds: introduction. phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. In: natural products. Ramawat, K. and Merillon, J. M. (Eds.). Berlin Heidelberg. 1543-1580. Doi: 10.1007/978-3-642-22144-6-57. [ Links ]
Lattanzio, V.; Lattanzio, V. M. and Cardinali, A. 2006. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry. Adv. Res. 661(2):23-67. [ Links ]
López-Nicolás, J. M.; and García-Carmona, F. 2010. Enzymatic and Nonenzymatic degradation of polyphenols. In: fruit and vegetable phytochemicals: chemistry, nutritional value and stability. De la Rosa, L. A.; Álvarez-Parrilla, E. and González-Aguilar, G. A. (Eds.). 1ª (Ed.). Wiley-Black well. USA. 101-130 pp. [ Links ]
Lucatti, A. F.; Álvarez, A. E.; Machado, C. R. and Gilardón, E. 2010. Resistance of tomato genotypes to the greenhouse whitefly Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae). Neotropical Entomol. 39(5):792-798. Doi: 10.1590/S1519-566X2010000500019. [ Links ]
Mangroliya, G. S.; Viridia, R. R.; Hirani, A. B. and Senjaliya, H. J. 2018. Varietal assessment and variability study of gerbera (Gerbera jamesonii Bolus.) in controlled condition. Inter. J. Chem. Studies. 6(1):1240-1244. [ Links ]
Mitchell, C.; Brennan, R. M.; Graham, J. and Karley, A. J. 2016. Plant defense against herbivorous pests: exploiting resistance and tolerance traits for sustainable crop protection. Frontiers in Plant Sci. 7(1132):1-8. Doi: 10.3389/fpls.2016.01132. [ Links ]
Mittler, R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Sci. 7(9):405-410. Doi: 10.1016/S1360-1385(02)02312-9. [ Links ]
Morales, P. y Cermeli, M. 2001. Evaluación de la preferencia de la mosca blanca Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) en cinco cultivos agrícolas. Entomotropica. 16(2):73-78. [ Links ]
Parrella, D.; Melicharek, A. and Murdock, M. 2014. Evaluation of greenhouse whitefly control in gerbera Daisy, 2012. Arthropod Management Tests. 39(1). Doi: 10.4182/amt.2014.G1. [ Links ]
Pérez-Sandoval, I.; Aguilar-Medel, S.; Rodríguez-Maciel, C. y Vásquez, G. L. M. 2011. Respuesta de la mosquita blanca Trialeurodes vaporariorum Westwood a insecticidas usados en cultivos ornamentales del Estado de México. Entomología Mexicana. 10(1):667-776. [ Links ]
Pradhan, S.; Thapa, B. and Rai, P. 2017. Assessment studies on genetic variability traits in different genotypes of gerbera in hill zone of West Bengal. Indian Hortic. J. 7(1):76-78. [ Links ]
Reddy, P. P. 2016. Sustainable crop protection under protected cultivation. Singapore. Springer. Doi: 10.1007/978-981-287-952-3. [ Links ]
Rehman, F.; Khan, F. A. and Badruddin, S. M. A. 2012. Role of phenolics in plant defense against insect herbivory. In: chemistry of phytopotentials: health, energy and environmental perspectives. Khemani, L.; Srivastava, M. and Srivastava, S. (Eds.). Springer Heidelberg. Berlin. 309-313 pp. [ Links ]
Rojas, Y. S.; Morales, J. y Sánchez, E. A. 2018. Registro de insectos y ácaros asociados a cultivos de gerbera Gerbera jamesonii Bolus ex Hook. f. (Compositae) en Venezuela. SABER. 30:238-243. [ Links ]
Sandhyarani, U. and Usha, P. R. 2013. Insect herbivory induced foliar oxidative stress: changes in primary compounds, secondary metabolites and reactive oxygen species in sweet potato Ipomoea batata (L). Allelopathy J. 31(1):157-168. [ Links ]
Senapati, A. K.; Prajapati, P. and Singh, A. 2013. Genetic variability and heretability studies in Gerbera jamesonii Bolus. Afr. J. Agric. Res. 8(41):5090-5092. Doi: 10.5897/AJAR2013.8038. [ Links ]
Sharma, P.; Jha, A. B.; Dubey, R. S. and Pessarakli, M. 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 1-26 pp. Doi: 10.1155/ 2012/217037. [ Links ]
Shigeto, J. and Tsutsumi, Y. 2016. Diverse functions and reactions of class III peroxidases. New Phytologist. 209(4):1395-1402. Doi: 10.1111/nph.13738. [ Links ]
SIAP. 2016. Servicio de Información Agroalimentaria y Pesquera. Cierre de la producción agrícola. Consultado 22-05-18, en: Consultado 22-05-18, en: http://nube.siap.gob.mx/cierre-agricola/ . [ Links ]
Sierra, V. P.; Quiroga, L. F. y Varón, E. H. 2014. Preferencia de mosca blanca (Paraleyrodes sp.) por cultivares de aguacate (Persea americana Mill.) en fresno, Tolima. Corpoica Ciencia y Tecnología Agropecuaria. 15(2):197-206. [ Links ]
Strauss, S. Y.; Rudgers, J. A.; Lau, J. A. and Irwin, R. E. 2002. Direct and ecological costs of resistance to herbivory. Trends in Ecol. Evol. 17(6):278-285. Doi: 10.1016/S0169-5347(02)02483-7. [ Links ]
Sulistyo, A. e Inayati, A. 2016. Mechanisms of antixenosis, antibiosis, and tolerance of fourteen soybean genotypes in response to whiteflies (Bemisia tabaci). Biodiversitas. 17(2):447-453. Doi: 10.13057/biodiv/d170207. [ Links ]
Surin, U. 2011. Effects of different nutrient solution formulations on yield and cut flower quality of gerbera (Gerbera jamesonii) grown in soilless culture system. Afr. J. Agric. Res. 6(21):4910-4919. [ Links ]
Taggar, G. K. and Gill, R. S. 2012. Preference of whitefly, Bemisia tabaci, towards black gram genotypes: Role of morphological leaf characteristics. Phytoparasitica. 40(5):461-474. Doi: 10.1007/s12600-012-0247-z. [ Links ]
Taggar, G. K.; Gill, R. S.; Gupta, A. K. and Singh, S. 2014. Induced changes in the antioxidative compounds of Vigna mungo genotypes due to infestation by Bemisia tabaci (Gennadius). J. Environ. Biol. 35(6):1037-1045. [ Links ]
Vaca-Sánchez, M. S.; González-Rodríguez, A.; Maldonado-López, Y.; Fernándes, W. G. y Cuevas-Reyes, P. 2016. Importancia de los taninos en especies del género Quercus como metabolitos secundarios asociados a defensa contra insectos herbívoros. Biológicas Revista de la DES Ciencias Biológico Agropecuarias. 18(1):10-20. [ Links ]
War, A. R.; Paulraj, M. G.; Ahmad, T.; Buhroo, A. A.; Hussain, B.; Ignacimuthu, S. and Sharma, H. C. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior. 7(10):1306-1320. doi: 10.4161/ psb.21663. [ Links ]
Waterman, P. G. and Mole, S. 1994. Analysis of phenolic plant metabolites. Blackwell Scientific Publication, Oxford. 238 p. [ Links ]
Zhang, X.; Sun, X.; Zhao, H.; Xue, M. and Wang, D. 2017. Phenolic compounds induced by Bemisia tabaci and Trialeurodes vaporariorum in Nicotiana tabacum L. and their relationship with the salicylic acid signaling pathway. Arthropod Plant Interactions. 11(5):659-67. Doi:10.1007/s11829-017-9508-6. [ Links ]
Zhao, H.; Sun, X.; Xue, M.; Zhang, X. and Li, Q. 2016. Antioxidant Enzyme responses induced by whiteflies in tobacco plants in defense against aphids: catalase may play a dominant role. PLoS ONE. 11(10):e0165454. https://doi.org/10.1371/journal.pone.0165454. [ Links ]
Received: December 01, 2019; Accepted: March 01, 2020











 texto em
texto em 


