Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 spe 23 Texcoco sep./nov. 2019 Epub 20-Nov-2020
https://doi.org/10.29312/remexca.v0i23.2025
Articles
Identification of new secondary metabolites in Persea Americana Miller variety Drymifolia
1Unidad de Investigaciones Avanzadas en Agrobiotecnología-Facultad de Agrobiología ‘Presidente Juárez’. Paseo Gral. Lázaro Cárdenas y Berlín s/n, Col. Viveros, Uruapan, Michoacán, México. CP. 60170. Tel. 4525236474. Fax. 4525236474. (hguillenandrade@gmail.com).
2Centro de Investigaciones en Ecosistemas-Universidad Nacional Autónoma de México. Antigua carretera a Pátzcuaro núm. 8701, Col. ex-Hacienda de San José de la Huerta, Morelia, Michoacán, México. CP. 58190. Tel. 4433222777, ext. 42628. Fax. 4433222719. (pitagt@hotmail.com; ygarcia@cieco.unam.mx; espinosa@cieco.unam.mx).
3Campo Experimental Uruapan-INIFAP. Av. Latinoamericana núm. 1101, Col. Revolución, Uruapan, Michoacán. CP. 60150. Tel. 4525237392. (mariotv60@hotmail.com).
Plants have developed various defense strategies under conditions of biotic and abiotic stress, among the main ones, highlights the synthesis of secondary metabolites that act as herbicides, larvicides, repellents, attractants, fungicides, bactericides, insecticides, etc. In the present investigation, the chemical characterization of 54 collections of P. americana Miller variety Drymifolia from the avocado producer fringe of the state of Michoacán and conserved in the germplasm bank of the Faculty of Agrobiology ‘Presidente Juárez’ was raised. The study is part of a comprehensive strategy to evaluate the potential of collections as rootstocks with attributes of resistance to pests and diseases. When analyzing the chemical profile, a total of 47 secondary metabolites were identified, 16 of them, have not been reported in Persea leaf tissue. Some compounds such as β-pinene, caryophyllene, estragole, hexadecanoic acid, heptacosane and α-tocopherol were present in the 54 collections analyzed. Estragole with antifungal, larvicidal, insecticidal and genotoxic biological activity predominated with a concentration of 26.53%, with respect to the total. Additionally, significant statistical differences were determined for 12 secondary metabolites. In contrast, no relationship was found between the concentration of the metabolites and the origin of the collections.
Keywords: avocado; secondary metabolites; variability
Las plantas han desarrollado diversas estrategias de defensa bajo condiciones de estrés biótico y abiótico, entre las principales, destaca la síntesis de metabolitos secundarios que actúan como herbicidas, larvicidas, repelentes, atrayentes, fungicidas, bactericidas, insecticidas, etc. En la presente investigación, se planteó la caracterización química de 54 colecciones de P. americana Miller variedad Drymifolia procedentes de la franja aguacatera del estado de Michoacán y conservadas en el banco de germoplasma de la Facultad de Agrobiología ‘Presidente Juárez’. El estudio forma parte de una estrategia integral para evaluar el potencial de las colecciones como portainjertos con atributos de resistencia a plagas y enfermedades. Al analizar el perfil químico, se identificaron un total de 47 metabolitos secundarios, 16 de ellos, no han sido reportados en tejido foliar de Persea. Algunos compuestos como el β-pineno, cariofileno, estragol, ácido hexadecanoico, heptacosano y α-tocoferol estuvieron presentes en las 54 colecciones analizadas. El estragol con actividad biológica antifungica, larvicida, insecticida y genotóxica predominó con una concentración de 26.53%, respecto al total. Adicionalmente, se determinaron diferencias estadísticas significativas para 12 metabolitos secundarios. En contraste, no se identificó relación alguna entre la concentración de los metabolitos y el origen de las colecciones.
Palabras clave: aguacate; metabolitos secundarios; variabilidad
Introduction
Mexico ranks as the world’s leading producer of avocado, a place that obtains the 149 185 hectares planted, the state of Michoacán, is placed as the leading producer of this fruit with 195 042 t representing 94.9% of national production (SIAP, 2019). In Michoacán, avocado cultivation is established in a region known as the ‘avocado producer fringe of the state of Michoacán’ (Gutiérrez-Contreras et al., 2010). There is a wide genetic diversity of P. americana Miller variety Drymifolia it has been used as rootstock of the Hass variety (Cuiris-Pérez et al., 2009).
For being a source of genes for resistance to physical factors, pests and pathogens (Rincón-Hernández et al., 2011); however, the enormous phytogenetic wealth is threatened by the destruction of ecosystems and substitution of traditional cultivars by improved cultivars (Lorea-Hernández, 2002). Consequently, during the last years, efforts have been added for the conservation and characterization of these genetic resources (Gutiérrez-Diez et al., 2009).
In particular, in the state of Michoacán, work has been done on the characterization of the creole avocado germplasm; these works include genotypic information (Cuiris-Pérez et al., 2009) and phenotypic information (Guillén-Andrade et al., 2013). The importance of studying the chemistry of plants is that they have developed various defense strategies under conditions of biotic and abiotic stress; for example, the plant synthesizes secondary metabolites (MS) that cause plants to be unpleasant or toxic to some herbivores (Anaya-Lang and Espinoza-García, 2006).
Some with larvicidal biological activity (Senthilkumar et al., 2008), bactericidal and fungicidal insecticide (Khokra et al., 2008; Moreno et al., 2009; Hanamanthagouda et al., 2010), in addition to their ecological importance by participating in the plant adaptation processes, attraction of pollinating insects and seed dispersers (Sepúlveda-Jiménez et al., 2003).
The variation of the chemical profiles of the Persea genus has been documented in several studies (Quing-Yi et al., 2009; Torres-Gurrola et al., 2009; Rincón-Hernández et al., 2011) in the most (Torres- Gurrola et al., 2016) reported a total of 363 identified MS, corresponding to 13 chemical groups, of the total, 258 are related in different tissues of Persea americana: 125 have been reported in pericarp, 109 in leaf tissue, 17 in seed, three in bark, two in flower and two in root. Due to the importance of knowing the chemical profiles, as defense mechanisms, of the genetic resources of P. americana Miller variety Drymifolia.
With the objective of knowing the foliar chemical variability of the 54 collections of this species, established in the germplasm bank of the Faculty of Agrobiology, dependent on the Michoacán University of San Nicolás de Hidalgo, as an integral part of a strategy to evaluate its potential as rootstocks with characteristics of resistance to pests and diseases, among other attributes additionally, the need to establish the possible relationship of the MS present in the collections with their geographical origin was raised.
Materials and methods
The research was developed in the Plant Genetic Resources Laboratory (LAREFI) of the Advanced Research Unit in Agrobiotechnology (UIAA) of the ‘Presidente Juárez’ Faculty of Agrobiology, of the Michoacán University of San Nicolás de Hidalgo, located in the city of Uruapan, Michoacán, Mexico, at the coordinates 19° 25’ 10’’ north latitude and 102° 03’ 30’’ west longitude at 1 620 meters above sea level. The process of extraction, identification and quantification of MS was carried out at the Center for Research in Ecosystems (CIEco) of the National Autonomous University of Mexico-Campus Morelia.
The genetic material analyzed consisted of 54 accessions of P. americana Miller variety Drymifolia, with four individuals per accession, the accessions come from localities belonging to the municipalities of Ario, Los Reyes, Salvador Escalante, Tacambaro, Tingambato, Uruapan and Ziracuaretiro, all of them located in the Michoacán avocado strip between coordinates 18° 45’ and 20° 6’ north latitude and 101° 47’ and 103° 13’ west longitude (Guillén-Andrade et al., 2007; Gutiérrez-Contreras et al., 2010).
The chemical profile of each of the accessions was determined from leaf tissue of mature leaves and with the use of a gas chromatograph (Agilent HP6890®), provided with a mass detector (Agilent 6890®). The procedures for extracting extracts, the conditions of the gas chromatograph for injecting and analyzing the samples, the identification of MS based on the information contained in the mass spectrum library NIST 05 (National Institute of Standars and Technology) and the Quantification of MS were made based on what was described by Torres-Gurrola et al. (2009).
The content and concentration database (mg g-1 dry leaf) of each of the identified MS was subjected to a one-way analysis of variance, based on a completely randomized experimental design, with four repetitions, considering to each accession as a treatment. Each observation was represented in the statistical model corresponding to the experimental design, by means of the following expression: Yij= M +Ci + eij. Where: Yij= concentration of MS in collection i and repetition j; M= general mean of the experiment; Ci= effect of the collection i; Eij= experimental error in collection i and repetition j; i= 1, 2, 3, ..., 54 and j= 1, 2, 3, 4.
This analysis was done using the Proc Anova procedure of the statistical package SAS( version 9.4 (SAS Institute Inc., 2012). Based on the analysis of variance, the MS were selected for which there were statistically significant differences (p< 0.05). Later, on the list of those selected MS, a discriminant analysis of Stepwise was carried out (Romano and Wolf, 2005) to debug and maintain only that MS that contributed more information to the total variance.
Additionally, a correlation analysis of Pearson and Filom (1898) was carried out to eliminate highly correlated variables, and to avoid problems of collinearity in the matrices. Subsequently, a matrix of averages was obtained to carry out a principal components analysis (PCA), using the matrix of correlations; the Euclidean distance matrix (DE) between accessions was calculated and with it, the cluster analysis was done by means of the Genalex statistical package (Peakall and Smouse, 2006).
Finally, the corresponding dendogram was constructed using the Neighbor-Joinning grouping method. All analyzes were made with the statistical package SAS( version 9.4 (SAS Institute Inc. 2012). The geographical coordinates of the accession source sites were converted to decimals with the Federal Communications Commission program (http: // transition.fcc.gov/mb/audio/bickel/DDDMMSS-decimal.html), with this data was used to construct a Geographical Distances (DG) matrix, which was correlated with a matrix of Euclidean distances, using the Mantel test with the GenAlex statistical package (Peakall and Smouse, 2006; Flanagan, 2006).
Results and discussion
In the analysis of 54 accessions of established in the germplasm bank of the Faculty of Agrobiology, the results obtained indicate the presence of 47 MS of the total, there is a first group of 31 that have been reported in P. americana Miller and related species: α-pinene, sabinene, β-pinene, eucalyptus, β-cis-ocimene, estragole, caryophyllene oxide, limonene, α-cubebene, phytol, heptacosane, squalene, chavicol, β-cubebene, caryophyllene, α-humulene, germacrene D-4-ol, tetradecanoic acid, copaene, cubenol, decane, phenyl ethyl alcohol, methylenegolol, nerolidol (E), tetradecanal, (-elemene, ethyl linolenate, hexadecanoic acid, methyl hexadecanoate, myrcene and oleic acid (Quing-Yi et al., 2009; Torres-Gurrola et al., 2009; Rincón-Hernández et al., 2011; Torres-Gurrola et al., 2016).
A second group, consisting of 11 MS, have not been reported for P. americana and related species; however, for these there are reports of their presence in other plant species. 4,8,13-Duvatrien-1,3-diol has been identified in tobacco as growth inhibitor 8,11,14 eicosatrienoic acid (z,z,z)- in Melittis melissophyllum (Velasco-Negueruela et al., 2004), eugenol acetate, has been reported as the main component in the essential oil of Syzigium aromaticum and with antioxidant activity; cis, cis, 7,10-hexadecadienal in Euphorbia heterophylla with cytotoxic activity, antioxidant and antimicrobial cis,cis,cis-7,10,13-hexadecatrienal in Azadirachta indica and Allamanda cathartica with larvicidal activity.
The elemicin in Myristica fragrans and Daucus carota with toxic and antibacterial activity and in Syzigium aromaticum with antioxidant activity palmite in Annona diversifolia acting as an anticonvulsant (Cano-Europa et al., 2010), α-tocopherol in Elaeis oleifera and Vaccinium meridionale with antioxidant activity cycloartenol acetate in propolis with antimicrobial activity 2H-pyrene, 2- (7-heptadecinyloxy) tetrahydro- me was identified in the medicinal plant Andrographis paniculata 2-methilenecholestan-3-ol, was reported as a component of the essential oil of the flowers of Artemisia austro-yunnanensis with antioxidant activity (Chen-xing et al., 2014) and in Alstonia scholaris: plant used in herbal medicine for its medicinal properties (Islam et al., 2013).
Finally, a third group of five MS were identified for which there are no reports of their presence in plant species: undecane and tridecane have been reported as defensive compounds in Loxa deducta and Pellaea stictica methyl arachidonate as a growth factor in animals. α-glyceryl linolenate, is used as an industrial solvent, undecane 4-methyl has been identified in wastewater and crude oil.
Based on the works, which have been published on this topic, in this research a total of 16 new metabolites were present in foliar tissue of P. American Miller Drymifolia variety and belonging to nine chemical groups: esters (4), alcohols (2), phenolic compounds (1) acids; (1) ether; (1) alkanes; (3) aldehydes; (2) acetone; (1); and polyterpenes (1). In Table 1, the name of the metabolite and its chemical structure are presented.
Table 1 Chemical structures of 16 new secondary metabolites identified in foliar tissue of Persea americana Miller variety Drymifolia.
|
4,8,13-Duvatrien-1,3-diolc |
Elemicin3 |
Cicloartenol acetato1 |
Metil araquidonato1 |
|
8,11,14-ácido eicosatrienoico (z,z,z)-4 |
Palmitona8 |
2H-pireno, 2-(7-heptadeciniloxi) tetrahidro5 |
α-gliceril linolenato1 |
|
Acetato de eugenol1 |
Undecano6 |
2-metilencholestan-3-ol2 |
Undecano, 4-metil6 |
|
Cis,cis-7,10- hexadecadienal7 |
Cis,cis,cis-7,10,13-hexadecatrienal7 |
α-tocoferol9 |
Tridecano6 |
1= esters, 2= alcohols, 3= phenolic compounds, 4= acids, 5= ether, 6= alkanes, 7= aldehydes, 8= ketones and, 9= polyterpenes.
When analyzing the concentration and frequency at which the 47 MS were identified in the 54 accessions, it was determined that β-pinene, estragole, caryophyllene, hexadecanoic acid, heptacosane and α-tocopherol were identified in all the accessions and, of these last, estragole stood out for presenting the highest percentage values (26.5327 mg g-1 of concentration). In contrast, chavicol and eugenol acetate were present in only five of the 54 accessions analyzed. On the other hand, phenyl ethyl alcohol was the one that showed the lowest percentage (0.0164 mg g-1 of leaf) concentration in the collections analyzed. In Table 2, the secondary metabolite information, retention time, Kovats index, concentration (%) and frequency of each of the MS identified in the 54 accessions are presented.
Table 2 Secondary metabolite, retention time, Kovats index, total concentration (%) and frequency of the 47 secondary metabolites identified in 54 accessions of Persea americana Miller Drymifolia variety of the germplasm bank of the Faculty of Agrobiology.
| Metabolito secundario | TR1 | IK2 | [%]3 | F4 | Secondary metabolite | TR1 | IK2 | [%]3 | F4 |
| α-pineneA | 2.79 | 942 | 0.982 | 12 | Nerolidol (E)-E | 7.2 | 1 577 | 0.6543 | 49 |
| SabinenoA | 3.11 | 986 | 0.02 | 7 | Germacrene D-4-olE | 7.37 | 1 606 | 0.0403 | 17 |
| β-pineneA | 3.15 | 993 | 4.7539 | 54 | Caryophylene oxideE | 7.44 | 1 618 | 0.5922 | 46 |
| MyrceneA | 3.2 | 1 000 | 0.5881 | 49 | TetradecanalL | 8.1 | 1 732 | 1.73 | 49 |
| DecaneN | 3.26 | 1 007 | 0.0933 | 28 | Tetradecanoic acidF | 8.3 | 1 766 | 0.0583 | 26 |
| LimoneneA | 3.5 | 1 038 | 0.0213 | 8 | Cis,cis, 7, 10- hexadecadienalL | 9.09 | 1 903 | 0.1906 | 41 |
| EucalyptusA | 3.54 | 1 043 | 0.7361 | 53 | Cis,cis,cis-7,10,13-hexadecatrienalL | 9.12 | 1 909 | 4.4192 | 52 |
| β-cis-ocimenoA | 3.63 | 1 056 | 0.3887 | 39 | Methyl HexadecanoateG | 9.28 | 1 936 | 1.7413 | 40 |
| UndecaneN | 4.01 | 1 107 | 0.5654 | 44 | Hexadecanoic acidF | 9.46 | 1 967 | 4.6696 | 54 |
| Phenyl ethyl alcoholJ | 4.21 | 1 134 | 0.0164 | 9 | FitolB | 10.37 | 2 125 | 4.7608 | 53 |
| Undecane,4-metilN | 4.75 | 1 206 | 2.7795 | 43 | Oleic acidF | 10.43 | 2 137 | 3.6279 | 50 |
| EstragoleJ | 4.83 | 1 217 | 26.5327 | 54 | Ethyl LinoleateG | 10.72 | 2 189 | 0.8929 | 23 |
| ChavicolK | 5.14 | 1 262 | 0.077 | 5 | Methyl arachidonateG | 11.16 | 2 268 | 3.3274 | 48 |
| TridecanN | 5.45 | 1 306 | 0.09388 | 28 | 8,11,14-eicosatrienoic acid (z,z,z)-F | 11.86 | 2 398 | 0.6953 | 21 |
| τ-elemeneE | 5.81 | 1 359 | 0.0783 | 10 | 4,8,13- Duvatrien-1,3-diolM | 11.91 | 2 406 | 1.1932 | 28 |
| α-cubebenoE | 5.89 | 1 370 | 0.0389 | 15 | 2H-pyrene, 2-(7- heptadecyloxy) tetrahydro-I | 12.49 | 2 519 | 2.1822 | 46 |
| CopaenoE | 6.09 | 1 400 | 0.398 | 44 | 2-methylenenecholestan -3-olM | 13.02 | 2 626 | 1.5813 | 34 |
| β-cubebenoE | 6.17 | 1 414 | 0.251 | 20 | HeptacosanN | 13.4 | 2 705 | 3.9402 | 54 |
| MetileugenolK | 6.2 | 1 418 | 3.5294 | 49 | α- glyceryl linolenateG | 13.65 | 2 763 | 2.9127 | 45 |
| CaryophyleneE | 6.41 | 1 451 | 5.852 | 54 | Cycloartenol acetateG | 13.84 | 2 805 | 0.8669 | 30 |
| α-humuleneE | 6.62 | 1 484 | 0.6148 | 49 | SqualeneC | 14.03 | 2 848 | 3.5109 | 52 |
| Eugenol AcetateG | 6.98 | 1 541 | 0.0518 | 5 | α- tocopherolD | 15.76 | 3 170 | 5.6894 | 54 |
| CubenolE | 6.99 | 1 543 | 0.6123 | 48 | PalmitoneH | 16.72 | 3 301 | 1.5751 | 34 |
| ElemicinK | 7.14 | 1 568 | 0.0708 | 17 |
1= retention time; 2= Kovats index; 3= percent concentration in relation to the total; 4= frequency number of collections in which each MS was identified; A= monoterpene; B= diterpene; C= triterpene; D= polyterpene; E= sesquiterpene; F= acids; G= esters; H= acetone; I= ether; J= aromatic compounds; K= phenolic compounds; L= aldehydes; M= alcohols; N= alkanes.
Estragole was the secondary metabolite highlighted by being present in higher concentration and in the 54 collections analyzed. These results are similar to those reported in similar works in germplasm different Persea species from different regions of Mexico (Rincón-Hernández et al., 2011). The evaluation of the content of this MS in each of the collections is interesting, for example, the interaction avocado creole-Trioza anceps has been evaluated, the trees with the highest concentration of estragole showed less presence of leaf galls (Torres-Gurrola et al., 2009).
In addition, estragole has been identified as the main component in other species and, with important biological defense activities, as an insecticide (López et al., 2008) in Ocimum basilicum induced contact mortality to the rice weevil (Sitophilus oryzae) (Pascual-Villalobos et al., 2004), it has also been used against the red flour beetle (Tribolium castaneum Herbst) and the grain borer (Rhyzopertha dominica) in Pimpinella anisum, it was reported with bactericidal effect, it has been related to antifungal activity (Fontenelle et al., 2008), larvicide (Senthilkumar et al., 2008) and genotoxic (Zani et al., 1991).
In the present study, three previous collections of the municipality of Tingambato, Michoacan, Mexico, were those that presented the highest concentration of estragole, so it would be expected that these collections could present better defense attributes to be used as selected rootstocks.
In the Figure 1 shows the variation determined in the content of estragole in the 54 collections analyzed.
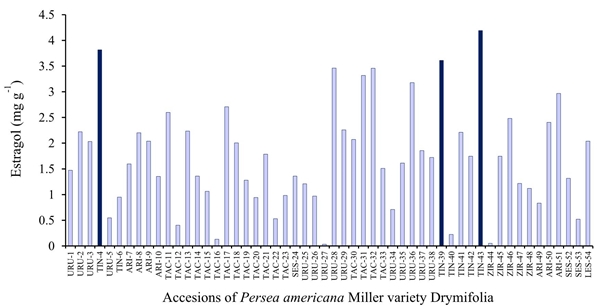
Figure 1 Histogram for concentration of estragole in 54 accessions of Persea americana Miller variety Drymifolia collected in the municipalities of Ario (ARI), Los Reyes (LES), Salvador Escalante (SES), Tacámbaro (TAC), Tingambato (TIN), Uruapan (URU) and Ziracuaretiro (ZIR) established in the germplasm bank of the Faculty of Agrobiology.
The values of the coefficient of determination (R2) oscillated between 0.33 and 0.46, in relation to the coefficients of variation (CV), the values fluctuated between 98.13 and 896.43. The highest CV value was obtained for the eugenol acetate in contrast to the Cis, cis, cis 7, 10, 13-hexadecatrienal. Likewise, the average values ranged from 0.00108 to 0.28877 in percentage of total concentration.
Later, in the Stepwise discriminant analysis, the tridecane was identified as one of the MS that did not provide information to explain the variation in MS concentration and when reviewing the Pearson correlation values, the dean was highly correlated (<0.0001) with the tridecane.
These results are explained by the fact that the collections present differences in their gene expression and the chemical profile is subject to strong genetic control, as it is in other tree species (Langenheim and Stubblebine 1983; Gershenzon et al., 2000; McConkey et al., 2000).
The results obtained from the one-way analysis of variance, performed for 47 MS content identified by analyzing 216 individuals from 54 collections of P. americana Miller variety Drymifolia, indicated significant statistical differences (p< 0.05) for concentration for ten of them and differences highly significant (p< 0.01) for phenyl ethyl alcohol and 8,11,14 -eicosatrienoic acid (z,z,z)-. Table 3 shows the simple statistics obtained for the 12 secondary metabolites that presented significant statistical differences.
Table 3 Simple statistics mean squares of error and probability of 12 MS with significant statistical differences obtained from the analysis of variance practiced for concentration of 47 MS identified in 54 collections of Persea americana Miller Drymifolia variety of the germplasm bank of the Faculty of Agrobiology.
| Secondary metabolites | R2* | CV1 | DE2 | Mean | CME3 | Pr>F |
| Decane | 0.34 | 196.54 | 0.01199 | 0.0061 | 0.00014 | 0.0131 |
| Phenyl ethyl alcohol | 0.46 | 473.28 | 0.00509 | 0.00108 | 0.00003 | <0.0001 |
| Undecane 4 methyl | 0.41 | 128.08 | 0.23263 | 0.18162 | 0.05411 | 0.0002 |
| Tridecan | 0.38 | 198.86 | 0.0122 | 0.00613 | 0.00015 | 0.0019 |
| β-cubebeno | 0.41 | 341.69 | 0.05605 | 0.0164 | 0.00314 | 0.0002 |
| Eugenol Acetate | 0.4 | 896.43 | 0.03039 | 0.00339 | 0.00092 | 0.0004 |
| Caryophylene Oxide | 0.33 | 170.22 | 0.06588 | 0.0387 | 0.00434 | 0.028 |
| Cis,cis7,10 hexadecadienal | 0.33 | 271.52 | 0.03382 | 0.01246 | 0.00114 | 0.0341 |
| Cis,cis,cis-7,10,13 hexadecatrienal | 0.34 | 98.13 | 0.28338 | 0.28877 | 0.0803 | 0.019 |
| 8,11,14-eicosatrienoic acid (z,z,z)- | 0.43 | 299.19 | 0.13595 | 0.04544 | 0.01848 | <0.0001 |
| 4,8,13-duvatrien 1,3-diol | 0.34 | 222.07 | 0.17315 | 0.07797 | 0.02998 | 0.0184 |
| 2-methylenecholestan-3-ol | 0.39 | 175.88 | 0.18174 | 0.10333 | 0.03303 | 0.0009 |
*= coefficient of determination; 1= coefficient of variation; 2= standard deviation; 3= Mean square of the error.
To reduce the number of variables to be considered for the explanation of the variability in relation to the concentration of secondary metabolites, a principal component analysis (PCA) was carried out. The results indicated that the first three main components (CP), explain together 29.64% of the variance that exists between the accessions, while with 17 it is explained 80%.
Similar results have been obtained in works of in situ morphological characterization of Persea and other fruit species, since it has been observed that in this type of work is not achieved more than 80% of the variance explained with the first three components (Tofiño et al., 2012; López-Gúzman et al., 2012; Guillén-Andrade et al., 2013; Medina-Torres et al., 2015; Montes-Hernández et al., 2017).
In Figure 2, the distribution of the 54 collections is shown according to three main components. When analyzing the eigenvectors with values above 0.3, it was determined that the CP1 is a function of the presence of the eucalyptus, caryophyllene and α-humulene, the CP2 was determined in terms of the decane, undecane, 4-methyl and tridecan. finally, CP3 is a function of chavicol and τ-elemeno. It is important to note that the tridecane and the undecane, 4-methyl with the highest weight in CP2 are reported for the first time in Persea americana Miller variety Drymifolia.

Figure 2 Dispersion of 54 collections of P. americana Miller variety Drymifolia from the germplasm bank of the Faculty of Agrobiology, based on three main components.
On the other hand, the dendrogram presented in Figure 3, generated from the cluster analysis based on Ward’s grouping method, showed that accessions are not associated in a logical way in relation to their geographical origin. These results were corroborated when performing the Mantel test, the correlation value obtained was r= -0.064 and a significance of 0.069, which confirmed the non-relationship between the geographic origin of the collections and the MS content identified in 54 collections.

Figure 3 Complete clustering dendrogram of 54 collections of Persea americana Miller variety Drymifolia, based on data on the concentration of 47 secondary metabolites. The colored circles indicate the place of origin of the germplasm.
These results may be masked because the leaf tissue samples were isolated from the collections established in the germplasm bank; that is, they are in a single environment.
The results of the aforementioned test are presented in Figure 4.
Conclusions
The 54 collections established in the germplasm bank of the Faculty of Agrobiology of Persea americana Miller variety Drymifolia, are contrasting in the type and concentration of 47 identified secondary metabolites. Estragole was a constant in terms of content and greater concentration in all the germplasm analyzed. The identification of 16 new secondary metabolites in the evaluated germplasm is an important contribution of the present work.
Finally, no relationship was determined between the chemical profile and the geographical origin of origin of the collections analyzed.
Acknowledgments
To CONACYT, CIECO-UNAM and UIAA-Faculty of Agrobiology-UMSNH, for the scholarship for master’s degree studies and for the facilities respectively, for the execution of research activities. Also, to the doctors who contributed ideas and collaborated with their knowledge, especially Dr. Pedro Antonio Lopez for his enormous advice in the statistical part.
REFERENCES
Anaya-Lang, A. L. y Espinoza-García, F. J. 2006. La química que entreteje a los seres vivos. Ciencias. 83(1):4-13. [ Links ]
Cano-Europa, E; González-Trujano, M. E.; Reyes-Ramírez, A.; Hernández-García, A.; Blas-Valdivía, V. and Ortiz-Butrón, R. 2010. Palmitone prevents pentylenetetrazole-caused neuronal damage in the CA3 hippocampal region of prepubertal rats. Neuroscience Letters 470(2):111-114. [ Links ]
Chen-Xing, Z.; Zhang, Mi.; Jing, He.; Ya-Fang, and Bao-Cai, Li. 2014. Chemical composition and antioxidant activity of the essential oil from the flowers of Artemisia austro-yunnanensis. J. Chem. Pharmaceutical Res. 6(7):1583-1587. [ Links ]
Cuiris-Pérez, H.; Guillén-Andrade, H.; Pedraza-Santos, M. E.; López-Medina, J. and Vidales-Fernández, I. 2009. Genetic variability withing mexican race avocado (Persea americana Miller.) germoplasm collections determined by ISSRs. Rev. Chapingo Ser. Hortic. 15(2):169-175. [ Links ]
Flanagan, N. S. 2006. A guide to GenAlex 6. Genetic analysis in Excel. Australian National University. [ Links ]
Fontenelle, R. O. S.; Orais, S. M.; Rito, E. H. S.; Rilhante, R. S. N.; Ordeiro, R. A.; Nascimento, N. R. F.; Erntopf, M. R.; Idrim, J. J. C. and Ocha, M. F. G. 2008. Antifungal activity of essential oils of Croton species from the Brazilian Caatinga biome. J. Appl. Microbiol. 104(5):1383-1390. [ Links ]
Gershenzon, J.; McConkey, M. and Croteau, R. B. 2000. Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol. 122(1):205-213. [ Links ]
Guillén-Andrade, H.; Lara-Chávez, M. B. N.; Gutiérrez-Contreras, M.; Ortiz-Catón, M. y Angel-Palomares, M. E. 2007. Cartografía agroecologica del cultivo del aguacate en Michoacán. Morevallado (Ed.). ISBN 9789707035669. 140 p. [ Links ]
Guillén-Andrade, H.; Lara-Chávez, M. B. N.; Torres-Gurrola, G., Escalera-Ordaz, A. K. y Tapia-Vargas, L. M. 2013. Caracterización fenotípica de germoplasma de aguacate (Persea americana Miller) criollo de Michoacán. Rev. Inter. Contam. Amb. 29(4):212-212. [ Links ]
Gutiérrez-Contreras, M.; Lara-Chávez, M. B. N.; Guillén-Andrade, H. y Chávez-Barcenas, A. T. 2010. Agroecología de la franja aguacatera en Michoacán, México. Interciencia. 35(9):647-653. [ Links ]
Gutiérrez-Diez, A.; Martínez-de la Cerda, J.; García-Zambrano, E. A.; Iracheta-Donjuan, L.; Ocampo-Morales, J. D. y Cerda-Hurtado, I. M. 2009. Estudio de la diversidad genética del aguacate nativo en Nuevo León, México. Rev. Fitotec. Mex. 32(1):9-18. [ Links ]
Hanamanthagouda, M. S.; Kakkalameli, S. B.; Naik, P. M.; Nagella, P.; Seetharamareddy, H. R. and Murthy, H. N. 2010. Essential oils of Lavandula bipinnata and their antimicrobial activities. Food Chem. 118(3):836-839. [ Links ]
Islam, F.; Islam, S.; Nandi, N. C. and Satter, M. A. 2013. Essential oil composition from the flowers of alstonia scholaris of Bangladesh. Inter. Food Res. J. 20(6):3185-3188. [ Links ]
Khokra, S. L.; Prakash, O.; Jain, S.; Aneja, K. R. and Dhingra, Y. 2008. Essential oil composition and antibacterial studies of Vitex negundo linn. extracts. Indian J. Pharmaceutical Sci. 70(4):522-526. [ Links ]
Langenheim, J. H. and Stubblebine, W. H. 1983. Variation in leaf resin composition between parent tree and progeny in Hymenaea: Implications for herbivory in the humid tropics. Bio. Systematic Ecol. 11(2):97-106. [ Links ]
López, M. D.; Jordan, M. J. and Pascual-Villalobos, M. J. 2008. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J. Stored Products Res. 44(3):273-278. [ Links ]
López-Guzmán, G.; Medina, T. R.; Guillén-Andrade, H.; Ramírez, G. L.; Aguilar, C. J. y Valdivía, R. M. 2012. Características fenotípicas de hoja y fruto en selecciones de aguacate criollo de clima subtropical en el estado de Nayarit. Revista Fuente. 4(10):56-62. [ Links ]
Lorea-Hernández, F. G. 2002. La familia Lauraceae en el sur de México: diversidad, distribución y estado de conservasión. Boletin de la Sociedad Botánica de México. 71(1):59-70. [ Links ]
McConkey, M. E.; Gershenzon, J. and Croteau, R. B. 2000. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 122(1):215-223. [ Links ]
Medina-Torres, R.; Juárez-López, P.; Salazar-García, S.; López-Guzmán, G. G.; Ibarra-Sanchez, L. S.; Arrieta-Ramos, B. G. y Martínez-Moreno, E. 2015. Evaluación de calidad en frutos de 41 genotipos de nanche (Byrsonima crassifolia L. HBK) de Nayarit , México. Rev. Mex. Cienc. Agríc. 16(2):253-264. [ Links ]
Montes-Hernández, S.; de la Torre-Vizcaino, J. D.; Heredia-García, E.; Hernández-Martínez, M. y Camarena-Hernández, M. G. 2017. Caracterización morfológica de germoplasma de aguacate mexicano (Persea americana var. Drymifolia, lauraceae). Interciencia. 42(3):175-180. [ Links ]
Moreno, H. P. R.; Leite-Lima, M. E.; Rossi-Caruzo, M. B.; Carneiro-Torres, D. S.; Cordeiro, I. and Marx, M. C. 2009. Chemical composition and antimicrobial activity of the essential oil from croton heterocalyx Baill. (Euphorbiaceae s.s.) Leave. J. Essential Oil Res. 21(2):190-192. [ Links ]
Peakall, R. and Smouse, P. E. 2006. Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes. 6(1):288-295. [ Links ]
Pearson, K. and Filon, L. N. G. 1898. Contributions to the mathematical theory of evolution, IV: on the probable errors of the frecuency constants and on the influence of random selection on variation and correlation. Philosophical Transactions of the Royal Society of London A. (191):229-311. [ Links ]
Quing-Yi, L.; Zang, Y.; Wang, Y.; Wang, D.; Ru-Po, L.; Gao, K.; Birns, R. and Heber, D. 2009. California Hass avocado: profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. J. Agric. Food Chem. 57(21):10408-10413. [ Links ]
Rincón-Hernández, C. A.; Sánchez-Pérez, J. y Espinoza-García, F. J. 2011. Caracterización química foliar de los árboles de aguacate criollo (Persea americana variedad Drymifolia) en los bancos de germoplasma de Michoacán, México. Rev. Mex. Biod. 82(2):395-412. [ Links ]
Romano, J. P. and Wolf, M. 2005. Stepwise multiple testing as formalized data snooping. Econometrica. 73(4):1237-1282. [ Links ]
SAS Institute Inc. 2012. Introduction to bayesian analysis procedures. In: SAS/STAT 9.2 User’s Guide, Ch. 7. SAS Institute, Inc., Cary, NC. 141-179 pp. [ Links ]
Senthilkumar, A.; Kannathasan, K. and Venkatesalu, K. 2008. Chemical constituents and larvicidal property of the essential oil of Blumea mollis (D. Don) Merr. against Culex quinquefasciatus. Parasitol. Res. 103(4):959-962. [ Links ]
Sepúlveda-Jiménez, G.; Porta-Ducoing, H. y Rocha-Sosa, M. 2003. La participación de los metabolitos secundarios en la defensa de las plantas. Rev. Mex. Fitopatol. 21(3):355-363. [ Links ]
SIAP. 2019. Servicio de Información Agroalimentaría y Pesquera, México. Boletin mensual de producción de aguacate. https://www.gob.mx/cms/uploads/attachment/file/438986/Bolet-n-mensual-de-la-producci-n-de-aguacate-enero-2019.pdf. [ Links ]
Tofiño, A.; Cabal, D. y Gil, L. F. 2012. Análisis de componentes del sistema productivo de aguacate, con incidencia probable de Phytophthora en Cesar, Colombia. Avances en Investigación Agropecuaria. 06(2):69-90. [ Links ]
Torres-Gurrola, G.; Montes-Hernández, S. y Espinoza-García, F. J. 2009. Patrones de variación y distribución geográfica en fenotipos químicos foliares de Persea americana variedad Drymifolia. Rev. Fitotec. Mex. 32(1):19-30. [ Links ]
Torres-Gurrola, G.; García-Rodríguez, Y. M.; Lara-Chávez, Ma. B. N.; Guillén-Andrade, H.; Delgado, G. y Espinosa-García, F. J. 2016. Ecología química y alelopatía: avances y perspectivas. Capítulo 5: análisis de la riqueza de metabolitos secundarios de Persea spp. Bajo algunas hipótesis que proponen explicar la función de la diversidad fitoquímica. Universidad Nacional Autónoma de México (UNAM). Primera edición. ISBN: 978-607-402-912-3. 195-292 pp. [ Links ]
Velasco-Negueruela, A.; Sanz, J.; Pérez-Alonso, M. J. and Palá-Paúl, J. 2004. The volatile components of the aerial parts of Melittis melissophyllum L. subsp. melissophyllum gathered in Spain. Botanica Complutensis. 28(1):133-136. [ Links ]
Zani, F.; Massimo, G.; Benvenuti, S.; Bianchi, A.; Albacini, A.; Melegari, M.; Vampa, G.; Belloti, A. and Mazza, P. 1991. Studies on the genotoxic properties of essential oils with Bacillus subtilis rec-assay and Salmonella/microsome reversion assay. Planta Medica 57(03):237-241. [ Links ]
Received: January 01, 2019; Accepted: April 01, 2019











 texto en
texto en