Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 spe 22 Texcoco Mar./Abr. 2019
https://doi.org/10.29312/remexca.v0i22.1865
Articles
Trophic spectrum of Turdidae birds in a Pinus cembroides forest with two canopy openings
1Departamento de Suelos-Universidad Autónoma Chapingo. Carretera Federal México-Texcoco, km 38.5, Chapingo, Estado de México, México. CP 56230. (biologo-ugalde@hotmail.com; yessenia.cruzm@gmail.com).
2Campus San Luis Potosí-Colegio de Postgraduados. Maestría en Ciencias en Innovación en Manejo de Recursos Naturales. Iturbide No. 73, Salinas de Hidalgo, San Luis Potosí. CP 78600. (olmosg@colpos.mx; fmontoya@colpos.mx).
3División de Ciencias Forestales-Universidad Autónoma Chapingo. Carretera Federal México-Texcoco km 38.5, Chapingo, Estado de México, México. CP. 56230. (urimr-93@hotmail.com).
During January to October 2014, variables of insectivorous birds Turdidae were recorded in order to determine patterns of their trophic spectrum considering their feeding techniques and diets in a Pinus cembroides forest under two conditions of apparent disturbance in their canopy opening: semi-preserved (BPS) and disturbed (BPP) in the high protected natural area (ANPPA). It is used Canfield line methods, quadrants with central point and embedded frames, cut and shake of branches, count in points of 25 m with intensive search, capture with fog nets by constant effort; as well as indices of relative abundance (IAR), frequency of observation (Fo); Kruskal-Wallis, multiple correspondences (ACM); indexes of Jacknife1, Shannon-Wiener, Jaccard; χ2 tests, Cluster, Poisson regression (ARP). The IAR was similar, Fo show analogy; Kruskal-Wallis there are no differences, ACM formed defined groups; the average richness, diversity and similarity of the entomological orders were relatively low; χ2 the proportion of registered individuals are different, cluster graphically there are different groups, ARP there is effect of some plant variables on the abundances of insects and birds. Some patterns of the trophic amplitude of this type of birds were understood; finding that they contribute to the biological control of certain entomological components that could become harmful and forest pests of this type of forest; generating basic knowledge about the role played by the opening of the canopy in the presence of prey; however, it seems not to have it on avifaunal abundances in this particular region of Mexico.
Keywords: insectivore avifauna; trophic coexistence; determination of diets; hunting techniques
Durante enero a octubre de 2014 se registraron variables de aves insectívoras Turdidae con el objeto de determinar patrones de su espectro trófico considerando sus técnicas de alimentación y dietas en un bosque de Pinus cembroides bajo dos condiciones de perturbación aparente en su apertura de dosel: semiconservado (BPS) y perturbado (BPP) en el área natural protegida peña alta (ANPPA). Se emplearon métodos de línea de Canfield, cuadrantes con punto central y cuadros empotrados, corte y sacudida de ramas, recuento en puntos de 25 m con búsqueda intensiva, captura con redes de niebla mediante esfuerzo constante; así como índices de abundancia relativa (IAR), frecuencia de observación (Fo); Kruskal-Wallis, correspondencias múltiples (ACM); índices de Jacknife1, Shannon-Wiener, Jaccard; pruebas de χ2, clúster, regresión Poisson (ARP). El IAR fue similar, Fo muestran analogía; Kruskal-Wallis no existen diferencias, ACM conformaron grupos definidos; la riqueza, diversidad y similitud promedio de los órdenes entomológicos fueron relativamente bajas; χ2 la proporción de individuos registrados son diferentes, clúster gráficamente existen diversos grupos, ARP existe efecto de algunas variables vegetales sobre las abundancias de insectos y aves. Se lograron comprender algunos patrones de la amplitud trófica de este tipo de aves; encontrando que coadyuvan en el control biológico de ciertos componentes entomológicos que pudieran tornarse perjudiciales y plagas forestales de estos bosques; generando conocimiento básico sobre el papel que juega la apertura del dosel en la presencia de presas; sin embargo, parece no tenerlo sobre las abundancias avifaunísticas en esta región particular de México.
Palabras clave: avifauna insectívora; coexistencia trófica; dietas; técnicas de cacería
Introduction
The Turdidae family, belonging to the Passeriform order, is made up of more than 300 species of birds, of homogeneous, omnivorous characteristics, predominantly diurnal between residents and migrants, with quasi-cosmopolitan distribution, being Africa and Eurasia the sites with the greatest diversity followed by the South America (Meller, 2013). Trophic resources, as well as their capture and consumption, normalize the coexistence of organisms (Bo et al., 2007). In birds, the morphology seems to be evolutionarily associated with the selection of habitat, allowing in some cases to know the mechanisms of segregation between different guilds (Hernandez et al., 2017).
In some species of insectivorous birds, the variations in the availability of food seem to be related to the migration phenological patterns they make towards the tropics, so the structure, conformation, distribution and trophic ecology of bird communities fluctuate seasonally (Michalski et al., 2011). The vertical and horizontal distribution of some entomological communities are influenced by the existing plant physiognomy (Wen et al., 2016).
Insects are the food of multiple predators, including birds, which have acquired hunting techniques ranging from flight, to excavation using the beak (Norris and Martin, 2012) to optimize their feeding, some species have used the partition of resources to be able to coexist (Lovette and Hochachka, 2006). Studies related to trophic aspects and diets in birds such as Quilarque et al. (2010); however, they did not relate hunting techniques with morphological patterns to explain the consumption of prey.
Mexico is home to seven genera and 26 species of birds of the Turdidae Family (Navarro and Gordillo, 2006) distributed from forests to arid and semi-arid ecosystems, particularly temperate forests; nevertheless, their forests present diverse processes of ecological degradation, which alter the patterns in the distribution, abundance and composition of species (Almazán-Núñez et al., 2009).
An important forest remnant of Pinus cembroides (Zucc.) is distributed in the high natural protected peña alta area (ANPPA), which is not free of disturbances of various kinds that influence the dynamics and physiognomy of their forest stands, eliminating natural enemies of some insects causing their proliferation, even turning them into harmful species and pests, altering with it, patterns at various levels trophic system (Badii and Abreu, 2006).
The ANPPA supports 134 bird species (IEEG, 2002) of which the Turdidae family is represented by four species: Catharus guttatus, Sialia mexicana, Sialia sialis and Turdus migratorius, which are of vital importance as they directly and indirectly influence the maintenance of the ecological balance, incorporating and regulating various energy flows of the system; however, these species acquire relevance because they are frugivorous-insectivorous food habits (Flores and Galindo-González, 2004), since they help the dispersal of seeds and vegetative propagation, as well as the biological control of insects.
Despite its commercial, recreational, ethical, aesthetic, scientific value, contribution to the structure of ecosystems, efficiency as biological indicators and specifically the ecological role they play in the study area associated with its potential trophic amplitude, it is still unknown in forests that exhibit different canopy opening conditions as a result of various disturbance events. The objective of the research was to determine patterns of the trophic spectrum of Turdidae insectivorous birds considering feeding techniques and entomological components registered in the diets associated with their morphological characteristics (body fat, structure, skull and peak) in a P. cembroides forest under two conditions of apparent disturbance in its canopy opening: semi-preserved (BPS, in 85%) and disturbed (BPP, with 55%) of the ANPPA.
Materials and methods
The forest of P. cembroides (Zucc; Rzedowski, 2006) under study is located in the southern part of the ANPPA, at coordinates 21º 27’ 30.6” North latitude and 100º 59’ 6.5” West latitude, between the elevations of 2 147 at 2 332 masl (IEEG, 2002) where two apparent disturbance conditions were chosen in its canopy opening: BPS with 35.48 and BPP with 82.01 ha.
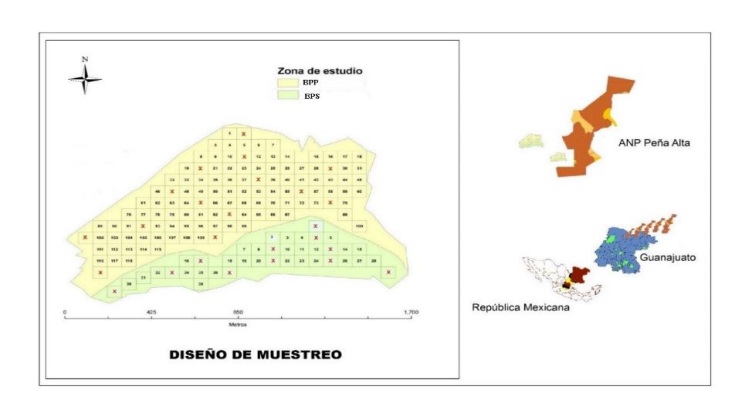
The sampling design implemented was the Systematic with random election of the units of choice (UEl’s); Cochran (1977), establishing 11 (BPS) and 14 (BPP) UEL’s of 0.5 ha each, in order to obtain the average number of birds and insects’ prey in each of them (Figure 1).
The variables were recorded monthly during January to October 2014, except vegetation only in: January, May and September; the tracking schemes were established based on the sampling design and in periods of one day for each condition, running parallel to each other. Habitat variables were recorded with the Canfield line according to their percentage occupation length on each line (Canfield, 1941).
The monitoring of the arboreal vegetation using quadrants with central point (Cottam and Curtis, 1956) and shrubs with Embedded Panels (Oosting, 1956). The identification of plant species was carried out in the 'CHAP' herbarium of the Autonomous University Chapingo (UACH). The registration of insects with cutting and shaking of branches (Schowalter, 1994), obtaining entomological samples of the foliage where the birds exhibited some feeding technique (Johnson et al., 2005) that were identified in the College of Postgraduates and the UACH, where a reference collection of prey was obtained (Gámez-Virués et al., 2007).
Feeding techniques (Remsen and Robinson, 1990) and entomological components in the diets (Rosenberg and Cooper, 1990) of Turdidae in both conditions (BPS and BPP) were obtained by: A) Counting in points with fixed radius of 25 m with intensive search (mixed, Ralph et al., 1996), using 25 x 50 m binoculars (Bushnell) and B). Capture by fog networks by constant effort (Hutto et al., 1986), physically containing the bird to record its morphometry and obtain its excreta by physiological stress, which was placed in 70% alcohol (Whitaker, 1988). In both cases, the identification was made with field guides for birds from Mexico and North America.
They were implemented in a parallel manner because they are complementary and increase the probability of detection in birds that are quiet, silent and conspicuous, reducing the bias in their identification through singing, since they could be searched and located visually (Karr, 1981).
The preference with which the birds occurred in both conditions for: feeding techniques, use and structures of insect substrates that make up their diets (excreta, at the level of order, family) was estimated with the index of frequency of observation (Fo), Antúnez-Ruiz et al. (2016), modified for the present in each case of analysis, graphing comparatively in Microsoft Excel (2016).
Differences in bird frequencies recorded for both conditions by: feeding techniques, use and substrate structures, morphology of the birds, registered entomological components with cut and shake branches (sizes) and insects that make up their diets (excreta, type), were determined with Kruskal-Wallis (Zar, 1999) in JMP IN v.14.0.1 (2018). The graphic association, in both conditions, between birds and feeding substrates, types and families of insects consumed, their morphology, was established by multiple correspondence analysis for categorical data (ACM, Greenacre, 2002) in Xlstat v.2018.5 (Addinsoft, 2018).
The average entomological richness, diversity and similarity registered in the diets of the birds, for both conditions, was estimated by analyzing the orders (assumed as species) of insects recorded by sampling by Jacknife of the first order (Jacknife1), Shannon-Wiener, Jaccard, respectively (Magurran, 2004) using EstimateS 9.1.0 (2018) and Microsoft Excel (2016).
To infer whether the proportion of birds is the same in both conditions for: feeding techniques, use and structures of substrates, their morphology, entomological components (sizes, types) that make up their diets, χ2 tests were developed (Agresti et al., 1990) in JMP IN v.14.0.1 (2018).
Graphic differences in entomological richness (considering families as species) recorded in bird diets, for both conditions, were determined by Cluster analysis considering the Euclidean distance (Hair et al., 1999) in Xlstat v.2018.5 (Addinsoft, 2018).
The association, in both conditions, between the variables (Y): birds (observed), insects (cut and shake of branches), captured birds and the vegetation variables (xi) were inferred with Poisson regression, using generalized linear models, procedure for the selection of variables and the Akaike criterion (AIC) González-Oreja (2003) in R v.3.5.1 (R, 2018). In all the analyzes, (= 0.05 was used.
Results and discussion
The index of frequency of observation (Fo) graphically display the trends in the percentage preferences with which Turdidae birds occurred in each of the variables of interest for both conditions (Figure 2).
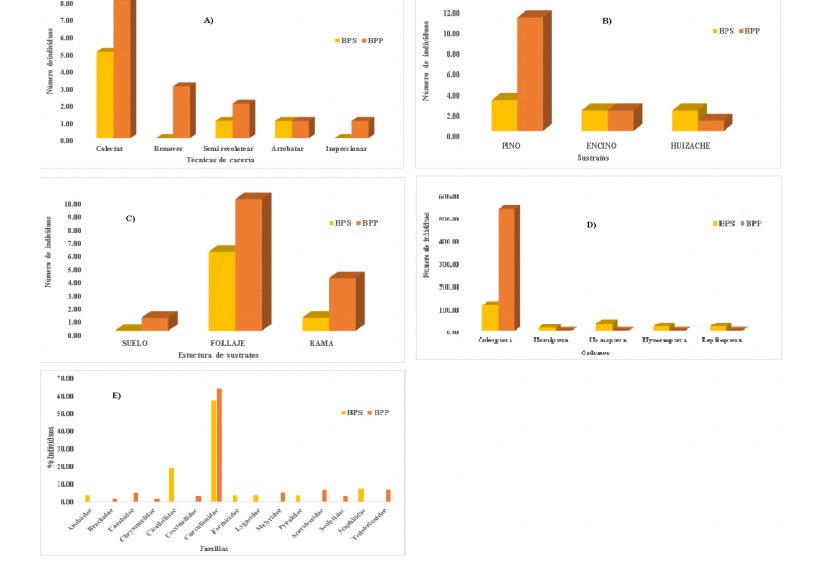
Figure 2 Fo of: A) feeding techniques; B) use; C) substrate structures, insects that make up their diets excreta; D) order; and E) family, for both conditions of the ANPPA.
The Kruskal-Wallis allow us to show that there are no significant differences in each of the variables of interest, therefore, Ho: B1= B2, at a level of significance of (= 0.05 (Table 1), is not rejected. concludes that these are similar in each case for both conditions.
Table 1 Kruskal-Wallis results for both conditions of the ANPPA.
| Analysis | Chi square | Degrees of freedom | Prob> Chi square |
| Feeding techniques | 0.9136 | 1 | 0.3392 |
| Use | 0.0119 | 1 | 0.0931 |
| Substrate structures | 0.3923 | 1 | 0.5311 |
| Bird morphology | 0.0528 | 1 | 0.8182 |
| Insect sizes | 0 | 1 | 1 |
| Types of insects | 0.75 | 1 | 0.3865 |
The ACMs show graphical association between the variables under study (the first two dimensions, give an account of the total inertia), it can be evidenced the conformation of well-defined groups in each the variables of interest for each case of analysis in both conditions (Figure 3).
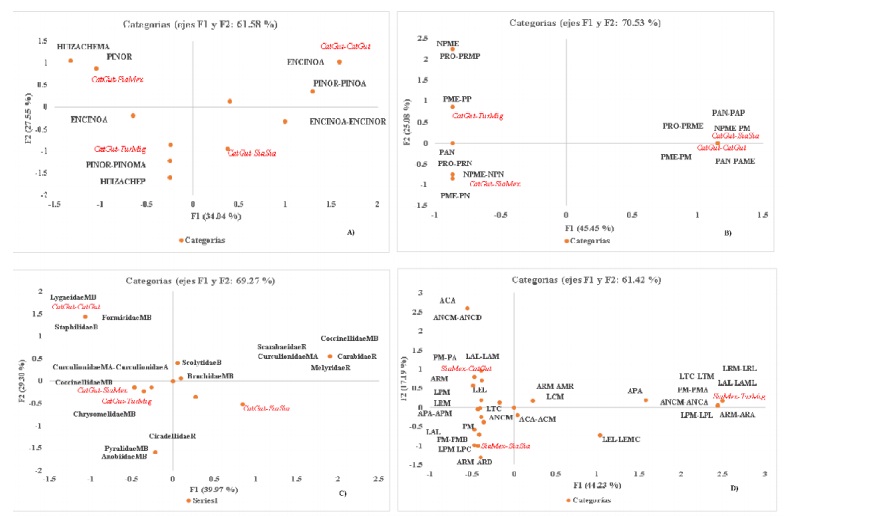
Figure 3 Graphic representation of the ACM shows the association between birds. A) feeding substrates; B) types and C) families of insects consumed; D) its morphology, for both conditions of the ANPPA. Codes for substrates: little (P); regular (R); high (A); very high (MA); types of insects= nothing (N); very little (MP); medium (Me); a lot (M); medium. Acronyms= plague (P); no plague (NP); predator (Pr); parasite (PA); insect families: null (N); very low (MB); low (B); regular (R); high (A); very high (MA); morphology: weight (P); wing length (LA); span length (LE); total length (LT); body width (AC); peak length (LP); peak width (AP). Birds: Catharus guttatus (CatGut), Sialia mexicana (SiaMex), Sialia sialis (SiaSia) and Turdus migratorius (TurMig). There are combinations of categories.
The Jacknife1 estimator for both conditions showed that the average number of species (entomological orders) exhibited an increase in the rarefaction curve. Shannon-Wiener presented an average entomological diversity value (insect orders) of H’= 1.8, differences between BPS and BPP can be seen. Jaccard shows a similarity of 36% (Figure 4).

Figure 4 Results A) Jacknife1; B) Shannon-Wiener; and C) Jaccard for entomological orders (assumed as species) recorded by sampling for both conditions of the ANPPA.
The χ2 suggest that the values obtained were lower than the contrast values, which suggests that the proportion of individuals registered for Turdidae birds are statistically different; that is, the observed values are different from those expected for each variable of interest (Table 2).
Table 2 Results of χ2 for both conditions of the ANPPA.
| Analysis | N | gl | R2 (U) | Ji2 (Pearson) | Prov> ji2 (Pearson) | χ2 of tables (Pearson) |
| Feeding techniques | 22 | 5 | 0.1143 | 4.268 | 0.5091 | 11.0705 |
| Use | 10 | 4 | 0.1586 | 3.333 | 0.5037 | 9.4877 |
| Substrate structures | 12 | 4 | 0.1452 | 3.143 | 0.5342 | 9.4877 |
| Morphology | 22 | 21 | 0.2242 | 22 | 0.3995 | 32.6706 |
| Insect sizes | 8 | 7 | 0.3333 | 8 | 0.3326 | 14.0671 |
| Types of insects | 8 | 7 | 0.3333 | 8 | 0.3326 | 14.0671 |
The Clusters show the dendogram resulting from the classification for families of insects (conceived as species) registered in bird diets, which shows the conformation of several amalgamations, note the break of scale in the axis of dissimilarity (Figure 5).

Figure 5 Classification for families (species) of insects registered in the Turdidae diets, carried out on the matrix of similarity of their incidence for both conditions of the ANPPA.
The ARPs (Table 3) show some variables (xi) of the vegetation that had some effect (in terms of statistically significant coefficients) on each (Y) of interest, for each case of analysis; with its respective AIC, which denoted the best adjustment of the GLM in each of them.
Table 3 Results of the ARP using GLM models for both conditions of the ANPPA.
| Coefficients | Estimate | Std. Error | z value | Pr(>|z|) |
Frequencies of Turdidae birds (observation) | ||||
| (Intercept) | 0.77259 | 0.20268 | 3.812 | 0.000138 |
| Alth | 1.56871 | 0.58951 | 2.661 | 0.00779 |
| Coba1 | 2.54733 | 0.49874 | 5.108 | 3.26E-07 |
| Coba2 | -1.22492 | 0.45211 | -2.709 | 0.006742 |
| Quadrant | -0.23797 | 0.07795 | -3.053 | 0.002267 |
No. of insect individuals (cutting and shaking of branches) | ||||
| (Intercept) | -2.186819 | 1.007184 | -2.171 | 0.02991 |
| Altfustelimpio | 0.055407 | 0.026445 | 2.095 | 0.03616 |
| Coba2 | 26.206683 | 2.929844 | 8.945 | < 2e-16 |
| Cobar1 | -0.109207 | 0.024039 | -4.543 | 5.55E-06 |
| Cobar2 | 0.192335 | 0.021393 | 8.99 | < 2e-16 |
| Cobh1 | -25.088683 | 4.809564 | -5.216 | 1.82E-07 |
| Cobh2 | 57.820814 | 5.635687 | 10.26 | < 2e-16 |
| Diama | -55.573266 | 4.779572 | -11.627 | < 2e-16 |
| Dist | 0.096708 | 0.014878 | 6.5 | 8.02E-11 |
| Date | -0.258311 | 0.051035 | -5.061 | 4.16E-07 |
| N. indveg | -1.600049 | 0.136182 | -11.749 | < 2e-16 |
| No. branches | 0.052769 | 0.010491 | 5.03 | 4.91E-07 |
| Point | -0.007282 | 0.001678 | -4.341 | 1.42E-05 |
| (%) hojasnuevasxrama | 0.034107 | 0.012639 | 2.699 | 0.00696 |
| (%) hojasviejasxrama | 0.055981 | 0.014076 | 3.977 | 6.98E-05 |
No. of individuals of Turdidae birds by sex | ||||
| (Intercept) | 1.827685 | 0.146741 | 12.455 | < 2e-16 |
| Coba2 | 0.896276 | 0.281814 | 3.18 | 0.00147 |
| (%) hojasnuevasxrama | -0.018168 | 0.002475 | -7.342 | 2.11E-13 |
| (%) hojasviejasxrama | -0.018109 | 0.003359 | -5.392 | 6.97E-08 |
No. of individuals of Turdidae birds by age | ||||
| (Intercept) | 1.482553 | 0.142001 | 10.44 | < 2e-16 |
| Coba2 | 1.041351 | 0.220541 | 4.722 | 2.3376E-06 |
| Point | 0.005679 | 0.001869 | 3.039 | 0.00238 |
| (%) hojasnuevasxrama | 0.019117 | 0.002121 | -9.012 | < 2e-16 |
| (%) hojasviejasxrama | 0.016094 | 0.002746 | -5.86 | 4.62E-09 |
No. of individuals of Turdidae birds by body condition or fat | ||||
| (Intercept) | 1.77935 | 0.160919 | 11.057 | < 2e-16 |
| High | 0.963289 | 0.370774 | 2.598 | 0.00938 |
| (%) hojasnuevasxrama | -0.018845 | 0.00268 | -7.032 | 2.04E-12 |
| (%) hojasviejasxrama | -0.019644 | 0.002969 | -6.617 | 3.66E-11 |
Nota= coefficients (p< 0.05) of ARPs, obtained by means of a logarithm as a link function between Y and xi; with AIC= 364.14, 1894.2, 305.45, 507.46, 319.12, respectively.
Trends in abundance may be associated with the availability of food resources and plant physiognomy, specifically in the vertical and horizontal planes, which apparently are different (at the canopy level) under the conditions evaluated, to which MacArthur and Levins (1964) ) point out that these factors condition the establishment of diverse faunal trophic communities and guilds due to the resources they provide; particularly insectivorous birds; even Murdoch et al. (1972) have shown a close relationship between the diversity of Homoptera, the number of strata and floristic composition.
However, the opening condition in the canopy did not seem to have an effect on them, according to Arguedas and Cespedes (2014), these birds can be established in this type of systems depending on the dams available for their consumption, despite anthropogenic pressure. Certain forests receive, as suggested by bird records for the present, whose trends are similar to those recorded in adverse environments, as reported by Almazán-Núñez et al. (2009) for pine-oak forest at different ecological succession conditions, this supports the idea put forward by Platt et al. (1984) who point out that abundance is often used to make ecological interpretations of the state that ecosystems keep since it is more sensitive to environmental distortions, even than species richness alone, so that this could potentially be positively affected or negatively, depending on the degree of disturbance or disturbance that said system exhibits, thus in the present, the synonyms in said estimator could be due to what was reported by Stiles (1978) who mentions that the structural complexity and the floristic composition determine the foraging substrates , as well as the availability of food, specifically in areas with different disturbance gradients, which according to Lambert (1992) are more preferred by opportunistic and generalist species.
The registers for feeding behaviors showed that the evaluated birds can coexist, because in a gregarious or solitary way, during their feeding they use several prey capture techniques on the different vegetable substrates, in this way, Fandiño et al. (2010) point out that said taxonomic group performs an adequate allocation of resources, which allows them to reduce trophic segregation; thus, strategies for their conservation under the conditions analyzed should focus on habitat improvement, promoting a greater number of feeding niches.
Sainz-Borgo (2015) suggests contemplating the feeding behavior of avifauna and its occurrence on vegetation. This is imperative, since the availability of plant strata influences the presence of certain larvae and prey insects and therefore on the trophic ethology of the birds. In this sense, the height at which these Turdidae were fed determined the distribution of their prey and consequently the deployment of their feeding techniques, as mentioned by Latta and Wunderle (1998) finding that some species of avifauna feed on transitions of certain strata and substrates, so these factors allow the separation of ecological niches, as reported by Nocedal (1984) who states that such use in temperate forests is selective to different structures of various substrates, including the canopy (foliage), place from where the species studied located their prey and defined which feeding technique to exhibit.
In this context, Ostrand (1999) supports that these eventually form feeding groups that function as catalytic indicators; that is, they show other species the location of areas with high density of prey, which attract different predators that interact with each other, establishing feeding strategies that allow them to ensure their permanence and trophically displace others that are not able to compete for the same resource. This agrees with Mills (1998) who mentions that this behavior explains the visual recruitment by means of which, individuals who use prolonged feeding techniques attract others for what said gregariousness, according to Guariguata and Kattan (2002) promotes the establishment of guilds that they demand similar resources, this explains part of the trophic coexistence in the birds evaluated.
The feeding techniques shown seem to be associated with the morphology of the birds analyzed, which is explained by Chávez et al. (2012) who suggest that the morphometry of the beak and legs are determinants for the acquisition of prey, in the same way Pyke et al. (1977) point out that predators avoid excessive energy expenditure by obtaining nearby prey that will provide them with sufficient energy for their vital physiological functions. In this way, the feeding techniques registered here are similar to that reported by Adamík and Korňan (2004) who point out that in some birds, the most common trophic behavior is collecting.
In contrast, Somasundaram and Vijayan (2008) suggest that others not necessarily insectivorous, support more versatile diets, which explains a lesser use of other techniques for more specific trophic niches, which allow them to be established almost all year round, due to the fact that seasonally and phenologically, they can obtain various larvae and insects, above and below the substrates, using different feeding techniques, which diminish the negative ecological interactions, promoting their food coexistence.
The substrates and their structures most used in the present by the birds showed that they coexist because they have an association with various feeding substrates; however, this seems to respond to the dominance of pinnaceae, feeding behavior and morphometry of birds, which provide some advantages to take advantage of specific trophic niches, this is supported by Becerra and Grigera (2005) who report the use of diverse substrates based on their availability, which provide them with food throughout the year. The trophic plasticity exhibited by these birds, showed how they optimized their trophic ecology in function of the use of different substrates and structures of them food carriers, adjusting their hunting techniques; nevertheless, the substrate most used seems to respond in a similar way to that reported by Somasundaram and Vijayan (2008) who assure that this trophic niche not only provides them with food, but also provides protection and coverage.
However, Lešo and Kropil (2007) point out that in other regions there are substrates and structures that can provide entomological resources in greater quantity and better energy quality, the registered substrates play a seasonally preponderant role over the registered feeding techniques; because it provided them, in a gregarious manner, a greater number of energy-efficient dams of different types as suggested by Albrecht and Gotelli (2001), which explains the presence of specialized and generalist birds in the use of disturbed habitats.
Trends in insect consumption by size and type coincide partially with that published by Rosas-Espinoza et al. (2008) who point out that some bird species consume insects that are detrimental or possibly considered forest pests, so they contribute to their biological control via depredation; it is imperative to point out that the records of insects in the Turdidae diets for the present, could be explained considering the physiological processes of digestion of each species and the time of consumption prior to obtaining the excreta and its relationship with the type and size of prey consumed, so the presence of these birds in the ANPPA has a controlling effect on certain pest insect populations as suggested by Gámez-Virués et al. (2007).
The condition of the birds showed a differential use in the procurement of food resources, feeding techniques, consumption and use of insects between gender; that is, their morphological structures were adapted to the taxa and sizes of the insects they consumed, so evolutionarily the peak is the key adaptation in the consumption of prey, slight variations in its dimensions influence the rate of ingestion, as well as the type and length of the legs, determine the consumption of various food resources on various substrates as suggested by Montaldo (2005) on the morphometry of birds and their relationship with food consumption.
These patterns have been demonstrated in frugivorous birds; in which the morphology of the peak and other structures depend on the size, shape, texture and type of food consumed and vice versa, therefore, the results of the present support the point made by Levey and Stiles (1994) who point out that these structures are adaptations key, so its size, shape and strength will affect your diet, as happened in this study between the morphology of birds and the consumption of insects of various sizes.
The physiological stress of the plants could increase the presence of amino acids and therefore the proliferation of insect pests, which progressively decimate the leaf density, favoring the opening of the foliage and the penetration of light, causing an increase in the vegetable substrates available for phytophagous insects, coupled with this, diurnal fluctuations in temperature exert an effect on insect dynamics (they are poikilotherms), therefore, in insectivorous birds. The insects in the present depended on various ANPPA events determined by their structure and floristic composition, as indicated by Medianero et al. (2003) based on principles on diversity, structure and microclimates for insects.
Conclusions
The techniques of feeding, use and structures of substrates used by Turdidae were differential. The entomological components that make up their diets and the morphometry were similar. There is an effect of some vegetation variables on insects and birds. Patterns of trophic coexistence were determined in two canopy opening conditions of the ANPP, specifically the role of the disturbance on the canopy in the presence of prey insects, said avifauna is relevant since within its trophic spectrum they consume harmful insects and pests, contributing in the biological control certain forestry undesirable entomological groups.
Literatura citada
Adamík, P. and Korňan, M. 2004. Foraging ecology of two bark foraging passerine birds in an old-growth temperate forest. Ornis Fennica 81:13-22. [ Links ]
Addinsoft, S. A. R. L. 2018. XLSTAT software, versión 2018.5. Addinsoft, Paris, France. https://www.xlstat.com/en/training/xlstat-version-2018-5. [ Links ]
Agresti, A.; Mehta, C. R. and Patel, N. R. 1990. Exact inference for contingency tables with ordered categories. J. Am. Statistical Association. 85(410):453-458. [ Links ]
Albrecht, M. and Gotelli, N. J. 2001. Spatial and temporal niche partitioning in grassland ants. Oecología, 126:134-141. [ Links ]
Almazán-Núñez, R. C.; Puebla-Olivares, F. y Almazán-Juárez, Á. 2009. Diversidad de aves en bosques de pino-encino del centro de Guerrero, México. Acta Zoológica Mexicana. 25(1):123-142. [ Links ]
Antúnez-Ruiz, G.; Ugalde-Lezama, S.; Tarango-Arámbula, L. A.; Lozano-Cavazos, E. A.; Cruz-Miranda, Y. y Rafael-Valdez, J. 2016. Abundancia y densidad de zorros chilla (Pseudalopex griseus Gray, 1837) y culpeo (Pseudalopex culpaeus Molina, 1782) en una formación xerófita. Agroproductividad. 9(9):77-83. [ Links ]
Arguedas, J. C. V. y Céspedes, J. V. 2014. Avifauna del Caribe sur asociada a ecosistemas alterados en Limón, Costa Rica. UNED Res. J. 6(2):187-196. [ Links ]
Badii, M. H. y Abreu, J. L. 2006. Control biológico una forma sustentable de control de plagas (biological control a sustainable way of pest control). Daena. Inter. J. Good Consc. 1(1):82-89. [ Links ]
Becerra, S. R. M. y Grigera, D. 2005. Dinámica estacional del ensamblaje de aves de un bosque Norpatagónico de Lenga (Nothofagus pumilio) y su relación con la disponibilidad de sustratos de alimentación. Hornero. 20(2):131-139. [ Links ]
Bó, M. S.; Baladrón, A. V. y Biondi, L. M. 2007. Ecología trófica de falconiformes y strigiformes: tiempo de síntesis. El Hornero. 22(2):97-115. [ Links ]
Canfield, R. H. 1941. Application of the line interception method in sampling range vegetation. J. Forestry. 39(4):388-394. [ Links ]
Chávez‐Villavicencio, C.; Sáenz‐Bolaños, C. y Spínola‐Parallada, M. 2012. Segregación en aves insectívoras con base en morfometría del pico y la longitud total. Huaraz, Perú. 5(1):60-67. [ Links ]
Cochran, W. G. 1977. Sampling techniques. New York, USA. John Wiley and Sons. 448 p. [ Links ]
Cottam, G. and Curtis, J. T. 1956. The use of distance measures in phytosociological sampling. Ecology. 37(3):451-460. [ Links ]
EstimateS for Windows runs under Windows 8. 2018. Versión 9.1.0. [ Links ]
Fandiño, B.; Berduc, A. J. y Beltzer, A. H. 2010. Ensambles de aves de bosques nativos y exóticos en la estación reproductiva de un área protegida en el espinal de Entre Ríos, Argentina. Ornitología Neotropical. 21:1-16. [ Links ]
Flores, P. R. y Galindo-González, J. 2004. Abundancia y diversidad de aves depredadoras de semillas de Pinus teocote Schl. et Cham. In: hábitats contrastantes de Veracruz, México. Foresta Veracruzana. 6(2):47-53. [ Links ]
Gámez-Virués, S.; Ronald, S. B.; Geoff, M. G.; Cilla, K.; Anantanarayanan, R. and Helen, I. N. 2007. Arthropod prey of shelterbelt-associated birds: linking faecal samples with biological control of agricultural pests. Australian J. Entomol. 46(4):325-331. [ Links ]
González-Oreja, J. A. 2003. Aplicación de análisis multivariantes al estudio de las relaciones entre las aves y sus hábitats: un ejemplo con passeriformes montanos no forestales. Ardeola. 50(1):47-58. [ Links ]
Greenacre, M. J. 2002. Correspondence analysis of the Spanish National Healt Survey. Gaceta Sanitaria. 16(2):160-170. [ Links ]
Guariguata, M. R. y Kattan, G. H. 2002. Ecología y conservación de bosques neotropicales. Ediciones LUR. Cartago, Costa Rica. 619 p. [ Links ]
Hair, J. F.; Anderson, R. E.; Tatham, R. I. y Black, W. 1999. Análisis Multivariante. 5 (Ed.). Editorial Prentice Hall. Madrid. 768 p. [ Links ]
Hernández, C. R.; Zerrato, J. J. G.; Ospina, J. F. C. y García, O. E. M. 2017. Variación morfométrica en el ensamblaje de aves Passeriformes presentes en dos zonas de bosque tropical con diferente grado de perturbación. Rev. Cienc. 20(2):125-137. [ Links ]
Hutto, R. L.; Pleschet, S. M. and Hendricks, P. 1986. A fixed-radius point count method for non-breeding and breeding season use. The Auk. 103:593-602. [ Links ]
IEEG. 2002. Programa de manejo del Área Natural Protegida Peña Alta. Instituto de Ecología del estado de Guanajuato (IEEG). PDOEG. el 10-09-2002. Guanajuato, México. 27-43 pp. [ Links ]
JMP IN: Statistics for the Apple Macintosh. 2018. Statistics and graphics guide. Version 14.0. 1. Academic SAS Institute Inc., Cary, North Carolina. https://support.sas.com/downloads/package.htm?pid=2323. [ Links ]
Johnson, M. D.; Sherry, T. W.; Strong A. M. and Medori, A. 2005. Migrants in neotropical bird communities: an assessment of the breeding currency hypothesis. J. Animal Ecol. 74(2):333-341. [ Links ]
Karr, J. R. 1981. Surveying bird with mist nets. In: Ralph, C. J. and Scott, J. M. (Eds.): estimating numbers of terrestrial birds. Stud. Avian Biol. 6:62-67. [ Links ]
Lambert, F. R. 1992. The consequences of selective logging for Bornean lowland forest birds. Philos T Roy Soc B. 335:443-457. [ Links ]
Latta, S. C. and Wunderle, J. M. Jr. 1998. The assemblage of birds foraging in Native West Indian Pine (Pinus occidentalis) forests of the Dominican Republic during nonbreeding season. Biotropica. 30(4):645-656. [ Links ]
Lešo, P. and Kropil, R. 2007. A comparison of three diferent approaches for the classification of bird foraging guilds: an effect of leaf phenophase. Folia Zool. 56(1):51-70. [ Links ]
Levey, D. J. and Stiles, F. G. 1994. Birds: ecology, behavior, and taxonomic affinities. In: McDade, L. A.; Bawa, K. S.; Hespenheide, H. A. and G. S. Hartshorn, G. S. (Eds.) 1994. La selva. Ecology and natural history of a neotropical rain forest. University of Chicago Press. Chicago, USA. 217-228 pp. [ Links ]
Lovette, I. J. and Hochachka, W. M. 2006. Simultaneous effects of phylogenetic niche conservatism and competition on avian community structure. Ecology. 87(7):S14-S28. [ Links ]
MacArthur, R. H. and Levins, R. 1964. Competition, habitat selection and character displacement in a patchy environment. Proc N A S Zool. (51):1140-1207. [ Links ]
Magurran, A. 2004. Measuring biological diversity. Blackwell Science Ldt. Blackwell Publishing Company. Oxford, UK. 256 pp. [ Links ]
Mahon, T. E. 1992. The role of Marbled Murrelets in mixed-species feeding flocks in British Columbia. Wilson Bulletin. 104(4):738-743. [ Links ]
Medianero, E.; Valderrama, A. y Barrios, H. 2003. Diversidad de insectos minadores de hojas y formadores de agallas en el dosel y sotobosque del bosque tropical. Acta Zoológica Mexicana. 89:153-168. [ Links ]
Meller, D. A. 2013. Registro del Zorzal Azulado (Turdus flavipes) en el extremo noroeste de Río Grande do Sul, Brasil. El Hornero. 28(1):35-38. [ Links ]
Michalski, M.; Nadolski, J.; Marciniak, B.; Loga, B. and Bańbura, J. 2011. Faecal analysis as a method of nestling diet determination in insectivorous birds: a case study in Blue Tits Cyanistes caeruleus and Great Tits Parus major. Acta Ornithologica. 46(2):164-172. [ Links ]
Microsoft Excel. 2016. Excel. Microsoft Office para Windows. [ Links ]
Mills, K. L. 1998. Multispecies seabird feeding flocks in the Galápagos Islands. The Condor. 100(2):277-285. [ Links ]
Montaldo, N. H. 2005. Aves frugívoras de un relicto de selva subtropical ribereña en Argentina: manipulación de frutos y destino de las semillas. Hornero. 20(2):163-172. [ Links ]
Murdoch, W. W.; Evans, F. C. and Peterson, C. H. 1972. Diversity and pattern in plants and insects. Ecology. 53(5):819-829. [ Links ]
Navarro, S. A. y Gordillo, A. 2006. Catálogo de autoridades taxonómicas de las aves de México. Facultad de Ciencias-Universidad Nacional Autónoma de México (UNAM). Base de datos del Sistema Nacional de Información sobre Biodiversidad. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Proyecto CS010. México, DF. 38 p. [ Links ]
Nocedal, J. 1984. Estructura y utilización de las comunidades de pájaros en bosques templados del Valle de México. Acta Zoológica Mexicana. 1(6):1-45. [ Links ]
Norris, A. R. and Martin, K. 2012. Red-breasted nuthatches (Sitta canadensis) increase cavity excavation in response to a mountain pine beetle (Dendroctonus ponderosae) outbreak. Ecoscience. 19(4):308-315. [ Links ]
Oosting, H. J. 1956. The study of plant communities. Freeman. San Francisco, USA. 185 p. [ Links ]
Ostrand, W. D. 1999. Marbled murrelets as initiators of feeding flocks in Prince William Sound, Alaska. Waterbirds. 22(2):314-318. [ Links ]
Platt, H. M.; Shaw, K. M. and Lambshead, P. J. D. 1984. Nematode species abundance patterns and their use in the detection of environmental perturbations. Hydrobiologia. 118:59-66. [ Links ]
Pyke, G. H.; Pullman, H. R. and Charnov, E. L. 1977. Optimal foraging: a selective review of theory and tests. The Quarterly Review of Biology. 52(2):137-154. [ Links ]
Quilarque, E.; Marín, G.; Carvajal, Y. y Ferrer, H. 2010. Componentes de la dieta de Sporophila minuta, S. intermedia (Emberizidae), Myiozetetes similis y Elaenia flavogaster (Tyrannidae), en un ecotono bosque palustre-basimontano de Venezuela. Boletín del Centro de Investigaciones Biológicas. 44(2):161-172. [ Links ]
R: Copyright 2018. The R foundation for statistical computing Version 3.5.1. (2018-07-10). ISBN 3-900051-07-0. [ Links ]
Ralph, C. J.; Geupel, G. R.; Pyle, P.; Martín, T. E.; DeSante, D. F. y Milá, B. 1996. Manual de métodos de campo para el monitoreo de aves terrestres. Department of Agriculture and Forest Service. USA. 46 p. [ Links ]
Remsen, J. V. Jr. and Robinson, S. K. 1990. A classification scheme for foraging behavior of birds in terrestrial habitats. Studies Avian Biol. 13:44-160. [ Links ]
Rosas-Espinoza, V. C.; Maya-Elizarraras, E.; Reyna-Bustos, O. F. and Huerta-Martínez, F. M. 2008. Diet of acorn woodpeckers at La Primavera forest, Jalisco, Mexico. The Wilson J. Ornithol. 120(3):494-498. [ Links ]
Rosenberg, K. V. and Cooper, R. J. 1990. Quantification of diets approaches to avian diet analysis. Studies Avian Biol. 13:80-90. [ Links ]
Rzedowski, J. 2006. Vegetación de México. 1a. Edición digital. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). México, DF. 504 p. [ Links ]
Sainz-Borgo, C. 2015. Nota: consumo de obreras Eciton burchellii (Hymenoptera: Formicidae) por varias especies de aves en condiciones urbanas. Ecotrópicos. 28(1-2):38-42. [ Links ]
Schowalter, T. D. 1994. Invertebrate community structure and herbivory in a tropical rain forest canopy in Puerto Rico following Hurricane Hugo. Biotropica. 26(3):312-319. [ Links ]
Somasundaram, S. and Vijayan, L. 2008. Foraging behavior and guild structure of birds in the montane wet temperate forest of the Palni Hills, South India. Podoces. 3(1/2):79-91. [ Links ]
Stiles, E. W. 1978. Avian communities in temperate and tropical alder forests. Condor. 80:276-284. [ Links ]
Wen, D. M.; Yu, L. F.; Liu, Y. H.; Yan, X. F.; Lu, P. F. and Luo, Y. Q. 2016. Trabala vishnou gigantina yang (Lepidoptera: Lasiocampidae) larval fitness on six sympatric plant species in sea-buckthorn forest. J. Insect Behavior. 29(5):591-604. [ Links ]
Whitaker, J. O. Jr. 1988. Food habits analysis of insectivorous bats. In: ecological and behavioral methods for the study of bats (Ed.). TH Kunz. Smithsonian Institution Press, Washington, DC. 171-189 pp. [ Links ]
Zar, J. H. 1999. Biostatistical analysis. Prentice-Hall, Inc. New Jersey, USA. 32-45 pp. [ Links ]
Received: January 2019; Accepted: April 2019











 texto em
texto em