Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 spe 22 Texcoco Mar./Apr. 2019
https://doi.org/10.29312/remexca.v0i22.1864
Articles
Chemical composition of volatiles from sugarcane leaves and pastures by gas chromatography
1Colegio de Postgraduados-Campus Veracruz. Carretera Federal Veracruz-Xalapa km 88.5, Rancho Tepetates, Manlio Fabio Altamirano, Veracruz, México. CP. 91690.
2Programa de Innovación Agroalimentaria Sustentable-Campus Córdoba-Colegio de Postgraduados. Carretera Federal Córdoba-Veracruz, Congregación Manuel León, Amatlán de los Reyes, Veracruz, México. CP. 94953.
3Programa de Innovación en el Manejo de Recursos Naturales-Campus San Luis Potosí-Colegio de Postgraduados. Iturbide 73, Salinas de Hidalgo, San Luis Potosí, México. CP. 78600.
This work analyzed the volatiles by obtaining extracts of green leaves of sugar cane in the mature state of eight varieties of sugarcane and of two pasture species by hydrodistillation with a Clevenger trap. The varieties of sugar cane used in this study were: CP 72-2086, MEX 69-290, RD 75-11, ITV 92-1424, MEX 79-431, L 77-50, COLPOS CT MEX 05-223, COLPOS CT MEX 05-204. The grasses evaluated were: itchgrass (Rottboellia cochinchinensis) and African star grass (Cynodon nlemfuensis). In the evaluated material three main compounds were found, these are: (Z) -3-hexen-1-ol, 1-octen-3-ol and phytol. These compounds were more representative in the varieties CP 72-2086 and MEX 69-290. The compound with greater frequency was (Z) -3-hexen-1-ol, which was present in seven varieties as well as in the two pastures evaluated. The abundance of (Z) -3-hexen-1-ol in the samples of the varieties of sugarcane varies from 20.35% to 57.36%, the greater amount of (Z) -3-hexen-1-ol was detected in African star grass with an abundance of 59.69%.
Keywords: Cynodon nlemfuensis; Saccharum officinarum; Rottboellia cochinchinensi
Este trabajo analizó las volátiles mediante la obtención de extractos de hojas verdes de caña de azúcar en estado maduro de ocho variedades de caña de azúcar y de dos especies de pastos mediante hidrodestilación con una trampa Clevenger. Las variedades de caña de azúcar utilizadas en este estudio fueron: CP 72-2086, MEX 69-290, RD 75-11, ITV 92-1424, MEX 79-431, L 77-50, COLPOS CT MEX 05-223, COLPOS CT MEX 05-204. Los pastos evaluados fueron: zacate peludo (Rottboellia cochinchinensis) y pasto estrella africana (Cynodon nlemfuensis). En el material evaluado se encontraron tres compuestos principales, estos son: (Z)-3-hexen-1-ol, 1-octen-3-ol y fitol. Estos compuestos fueron más representativos en las variedades CP 72-2086 y MEX 69-290. El compuesto con mayor frecuencia fue el (Z)-3-hexen-1-ol, que estuvo presente en siete variedades al igual que en los dos pastos evaluados. La abundancia de (Z)-3-hexen-1-ol en las muestras de las variedades de caña de azucar varia desde 20.35% hasta 57.36%, la mayor cantidad de (Z)-3-hexen-1-ol se detecto en el pasto estrella africana con una abundancia de 59.69%.
Palabras clave: Cynodon nlemfuensis; Rottboellia cochinchinensi; Saccharum officinarum
Introduction
The compounds produced by the plants are of great importance in the control of insect pests, because the insect plant relationship is a fundamental part in the search for an efficient control of pests such as the use of pheromones, kairomonas or simply, attractants, such as example we can mention the attraction of the aphids to the pheromone (E) -7,11- dimethyl-3-methylene-1,6,10-dodecatriene, or more commonly (E) -β-farnesene, this compound works for the attraction of different species of insects (Verheggen et al., 2010).
Another example is the specific attraction of the cochineal (Dactylopius ceylonicus) to the cactus (Opuntia vulgaris), which was used to provide control of this insect in India since 1863, so also the moths are attracted by several compounds, this depends of the species being studied, being the most common compounds linalool, geraniol, β-ocimeno, and β-caryophyllene (De Bruyne and Baker, 2008).
Therefore, the plant-insect relationship is an important aspect for decision-making (Scala, 2013), which can work in favor of production in the field, provided that the mode of action of the volatile compounds that relate said activity can be identified. interaction (Rodríguez, 2004). The volatile compounds produced in sugarcane leaves play a very important role in the agroecosystem, these compounds can influence the choice of the host crop of the plague insects (Martínez, 2013).
The identification of these compounds could be useful to have an efficient control in the management of pests such as the sugarcane borer (SAGARPA, 2006) and the spittlebugs (Hernández-Rosas et al., 2010; Alatorre-Rosas and Hernández-Rosas, 2015), these compounds being attractive or insect repellent and in some cases, due to their high concentrations, they can have different uses, from pharmaceuticals to use in cosmetic products (Singh et al., 2015). The objective was, the identification of volatile compounds of the green leaves of varieties of sugarcane and pastures.
Materials and methods
Vegetal material
The varieties of sugar cane used in this study were: CP 72-2086, MEX 69-290, RD 75-11, ITV92-1424, MEX 79-431, L 77-50, COLPOS CT MEX 05-223, COLPOS CT MEX 05-204 (Figure 1). In the same way the leaves of two grasses were used: itchgrass (Rottboellia cochinchinensis) and African star grass (Cynodon nlemfuensis). The evaluation was carried out by obtaining extracts of green leaves of sugarcane in the mature state of the sugarcane varieties and of the pastures mentioned, by hydrodistillation using a Clevenger trap (Rodríguez-Álvarez et al., 2012; Lima et al., 2017).
Gas chromatography
Evaluation by gas chromatography was performed using an HP® 6890 chromatograph coupled to an HP® 5973 mass selective detector. The chromatographic system used an HP-5MS® 30 x 0.250 mm column and 0.25 μm stationary phase thickness for separation of cane leaves compounds the oven temperature started at 40 °C and higher than 5 °C min-1 until reaching 70 °C and subsequently increased 2 °C min-1 to 80 °C and finally increased 7 °C min-1 until reaching 168 °C. For the separation of pasture leaf compounds the furnace temperature started at 40 °C and increased 5 °C min-1 to reach 173 °C.
Helium (He) was used as the carrier gas with a purity grade of 99.9% in flow ramp mode for cane leaves, it started with a flow of 1.9 L min-1 and was maintained for 5 min to subsequently decrease at a rate of 1 mL min-1 to 0.2 mL min-1 for 3 min, to increase at a rate of 1 mL min-1 to 1.5 mL min-1 for 16 min. For the leaves of pastures the flow started at 1.9 mL min-1 for 8 min and then decreased at a rate of 1 mL min-1 until reaching a flow of 1.3 mL min-1, which was maintained for 18 min.
For both materials the temperature of the injection port in splittless mode was 220 °C, and 280 °C as the interface temperature. For the mass detector, the temperature of the ion source was 230 °C and 250 °C for the quadrupole. The ionization energy was 70 eV. The dilution of the samples was 1:1000 (V:V) in dichloromethane, from this 1 μL was taken as injection volume. The separations and identifications were made in triplicate. The identification of the compounds was made by comparing the ion spectra of the sample and the NIST V. 2008 library. To give greater support to the identity of the compounds, an injection of n-alkanes (C7-C30 and C8-C40) was carried out, to calculate the modified Kovat's indexes using the following formula (Lubeck and Sutton, 1983).
The formula is repeated for each of the compounds found and was developed in the following way: to obtain the retention index (RI), the retention time of the carbons obtained with the n-alkane sample was taken as reference. The compounds that are between the retention time of the first carbon and the second took as reference the first carbon, then the compounds with retention time between the second carbon range and the third took the second carbon as reference and so on. To do this, multiply the carbon number (C) corresponding to the compound by 100+100 and this by the corrected retention time for the sample (T’r) x minus the corrected retention time of the minor alkane (T’r) c-1, divided by and the corrected retention time of the major alkane (T’r) c+1 minus the corrected retention time of the minor alkane (T’r) c-1.
Because the retention time (TR) of the last compounds exceeded the retention time of the last carbon obtained in the results of the evaluated sample, a linear regression was performed to determine the retention time of the next carbon, this was necessary for the samples of sugar cane as in the pasture samples and for this the following equation was used.
Results and discussion
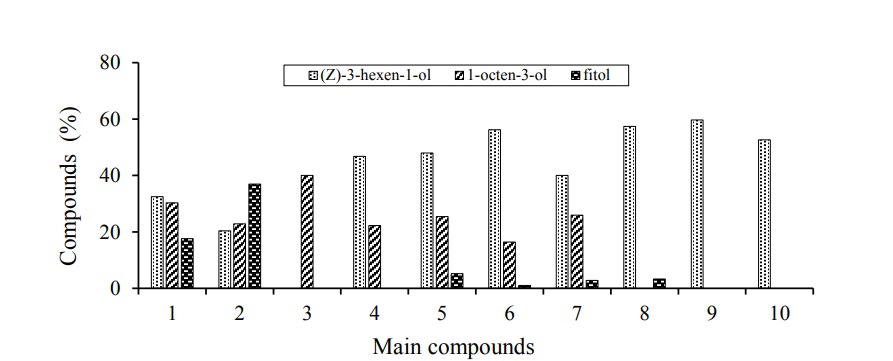
There were differences in the number and type of compounds of the aromatic extract of cane leaves of the varieties evaluated. The aromatic fraction of the leaves of the varieties of cane and grasses analyzed are composed of alcohols, esters, sesquiterpenes, alkenes, aldehydes and ketones. The major compound in the case of all cane varieties, except RD 75-11, was (z) -3-hexen-1-ol, followed by 1-octen-3-ol. The following tables show the compounds detected in three of the 8 varieties and the two grasses evaluated, each of them is accompanied by their retention time (TR), the percentage of composition within each sample (%) of composition and the result obtained from the Kovats indexes (IK). The most abundant compounds in the CP 72-2086 variety were (z) -3-hexen-1-ol, 1-octen-3-ol and phytol with 32.5, 30.28 and 17.56% presence, respectively (Table 1).
Table 1 Compounds of the variety CP 72-2086.
| Compound number | Compound | TR | (%) of composition | IK |
| 1 | (Z)-3-hexen-1-ol | 4.54 | 32.5 | 384 |
| 2 | 1-Chloro-2-methylpropene | 5.19 | 1 | 441 |
| 3 | 2-pentanol, propanoate | 9.48 | 8.25 | 666 |
| 4 | 1-octen-3-ol | 9.95 | 30.29 | 743 |
| 5 | 3-octanol | 10.8 | 1.06 | 882 |
| 7 | Benzeneacetaldehyde | 12.18 | 1.1 | 391 |
| 8 | Trasn-2-undecen-1-ol | 12.85 | 0.59 | 797 |
| 9 | Terpineol | 15.93 | 0.65 | 913 |
| 11 | (Z)-7-hexadeceneo | 21.68 | 0.77 | 1016 |
| 12 | 3-Buten-2-one,4-(2,2,6-trimethyl) | 21.97 | 0.33 | 1058 |
| 14 | Phytol | 23.01 | 17.56 | 282 |
As the variety CP 72-2086, the variety MEX 69-290 presented as major components to (z) -3-Hexen-1-ol and 1-octen-3-ol, only in this variety is the phytol the most abundant compound with almost 37% (Table 2), unlike the variety CP 72-2086 where the six-carbon alcohol is the most abundant compound.
Table 2 Compounds of the variety MEX 69-290.
| Compound number | Compound | TR | (%) of composition | IK |
| 1 | (Z)-3-hexen-1-ol | 4.52 | 20.35 | 391 |
| 2 | 3,4,4-trimethyl-1-tentin-3-ol | 9.52 | 8.03 | 672 |
| 3 | 1-octen-3-ol | 9.98 | 22.9 | 748 |
| 4 | 3-octanol | 10.82 | 0.81 | 885 |
| 5 | Benzyl alcohol | 11.92 | 2.2 | 277 |
| 6 | Benzeneacetaldehyde | 12.19 | 1.04 | 395 |
| 7 | (E)-2-decen-1-ol | 12.86 | 2.01 | 735 |
| 8 | Propionate | 13.69 | 0.97 | 1047 |
| 9 | Terpineol | 15.94 | 0.43 | 917 |
| 10 | (E)-5-octadecene | 21.68 | 0.61 | 1136 |
| 11 | 3-Buten-2-one,4-(2,2,6-trimethyl) | 21.96 | 0.6 | 1050 |
| 12 | Phytol | 22.98 | 36.97 | 267 |
In the case of the variety RD 75-11, the behavior was different from the rest of the varieties, being the only one that does not present as a component to (z)-3-Hexen-1-ol. This variety presented twice as much 1-octen-3-ol as the rest of the varieties (Table 3) and as the second major compound to an alkene with seven carbons (1-methyl-Cyclohexene), and both make up more than 50% of the composition of the aromatic extract of the leaves of the RD 75-11 variety. Octenol is also found in corn and cowpea, although in lower concentrations (Gouinguene, 2005).
Table 3 Compounds of the variety RD 75-11.
| Compound number | Compound | TR | (%) of composition | IK |
| 1 | Cyclohexene, 1-methyl | 5.26 | 0.5 | 734 |
| 2 | 1-butanol,3-methyl-, propanoate | 7.68 | 20.85 | 370 |
| 3 | 1-octen-3-ol | 8.06 | 40.06 | 433 |
| 4 | 3-octanol | 9.2 | 2.3 | 620 |
| 5 | 2, pentene, (E) | 10.3 | 2.38 | 533 |
| 6 | 2-cyclohexen-1-one | 10.44 | 1.84 | 640 |
| 7 | Benzyl alcohol | 10.9 | 1.13 | 799 |
| 8 | Benzeneacetaldehyde | 11.14 | 3.13 | 71 |
| 9 | Cyclooctyl alcohol | 11.68 | 7.93 | 237 |
| 10 | Propionate | 12.43 | 0.56 | 573 |
| 11 | 2-methylene-bornane | 15.75 | 0.29 | 911 |
| 13 | 2,4-decadienal, (E, E) | 17.2 | 0.81 | 418 |
| 14 | Eugenol | 18.05 | 0.48 | 841 |
| 15 | 2-Buten-1-one,1-(2,2,6-trimethyl) | 18.59 | 0.45 | 14 |
| 16 | 3-Buten-1-one,4-(2,2,6-trimethyl) | 19.17 | 0.9 | 418 |
| 17 | 3-Buten-2-one,4-(2,2,6-trimethyl) | 20.49 | 0.83 | 1337 |
This compound has been widely evaluated as a kairomona (Sant’Ana et al., 2002) in hematophagous insects because it is found in different sources, such as the breath of bovines and humans (Torres et al., 2014; Torto, 2002-2015), specifically the mosquitoe Lutzomyia longipalpis is able to detect 1-octen-3-ol in air currents (Sant’Ana et al., 2002; Lazzari, 2011). Due to the attraction efficiency with mosquitoes it is considered an efficient control of the diseases transmitted by this type of vectors (Laporta and Sallum, 2011). In a shrub species, such as Lantana camara it was found that 1-octen-3-ol is one of the main compounds that have attraction of the tse-tse fly, evaluating the behavior of the insect, it was determined that it is one of the chemical stimulants for the recipient cells (Syed and Guerin, 2004).
The varieties ITV 92-1424, MEX 79-431, L 77-50, COLPOS CT MEX 05-223, COLPOS CT MEX 05-204 were those that showed the highest abundance of (z)-3-hexen-1-ol with 46.8, 47.96, 56.2, 39.99 and 57.36%, respectively. On the other hand, these same varieties present 1-octen-3-ol as the second major compound, with the exception of the COLPOS CT MEX 05-204 variety, which presents benzyl alcohol, benzenaldehyde and phytol as the most abundant compounds after (z)-3-hexen-1-ol.
Regarding the pastures analyzed that serve as alternate hosts to the cane; it should be noted that they also presented as a major compound the (z)-3-Hexen-1-ol with composition percentage close to 60% in both pastures (Table 4 and 5). Although the absence of 1-octen-3-ol was observed, it was found present in almost all varieties of cane attacked by the spittlebugs.
Table 4 African star grass compounds.
| Compound number | Compound | TR | (%) of composition | IK |
| 1 | (Z)-3-hexen-1-ol | 4.57 | 52.73 | 400 |
| 2 | 2-butoxyethanol | 5.62 | 0.56 | 66 |
| 3 | Pentane,3-ethyl | 6.51 | 0.79 | 356 |
| 4 | Benzaldehyde | 6.91 | 0.84 | 482 |
| 5 | Cyclohexane, 1,2-dimethyl,-cis | 7.19 | 0.95 | 641 |
| 6 | Cis-1,2-dihydrocatechol | 7.66 | 1.44 | 628 |
| 7 | 3-buten-2-ol, 2,3-dimethyl | 8.32 | 0.77 | 87 |
| 8 | 2-cyclohexen-1-one | 8.4 | 2.47 | 105 |
| 9 | 3-cyclohexen-1-ol, 3-methyl | 8.56 | 0.97 | 160 |
| 10 | Benzyl alcohol | 8.95 | 0.92 | 257 |
| 11 | Benzeneacetaldehyde | 9.27 | 7.32 | 380 |
| 12 | 4,6-Heptadiene-2-one, 3,6-dimethyl | 12.83 | 0.66 | 717 |
| 13 | 10-undecin-1-ol | 13.21 | 1.09 | 809 |
| 14 | Terpineol | 13.95 | 1.59 | 1004 |
| 15 | Methyl salicylate | 14.05 | 1.19 | 851 |
| 16 | Vanillin | 19.72 | 2.08 | 893 |
Table 5 Hairy grass compounds.
| Compound number | Compound | TR | (%) of composition | IK |
| 1 | (Z)-3-hexen-1-ol | 4.55 | 59.69 | 393 |
| 2 | 1-butanol, 3-methyl-acetate | 4.99 | 4.96 | 6.35 |
| 3 | 2-butanol,2,3-dimethyl | 5.62 | 3.46 | 66 |
| 4 | 3-hexanol, 4-methyl | 5.67 | 6.37 | 91 |
| 5 | 3H-pyrazol-3-one,1,2-dihydro-5-me | 6.06 | 4.88 | 134 |
| 6 | Propionate | 11.14 | 4.45 | 4 |
The varieties of cane most attacked by spittlebugs (CP 72-2086 and MEX 69-290) are those that produce the least amount of (z)-3-Hexen-1-ol and 1-Octen-3-ol. The (z)-3-Hexen-1-ol is directly related to green leaf aromas, volatiles are released in the grasses from the damaged leaves immediately after the beginning of damage by feeding or even immediately after the plant is mechanically damaged (Röse and Tumlinson, 2004; D’Auria et al., 2007), are formed by enzymatic degradation and reduction of fatty acid (Cortes et al., 2005) and have been evaluated as attractants of autumn swarm females (Hyphantria cunea) using the electroantenogram technique (Tang et al., 2012). It was shown that this compound of plant origin emits chemical signals that attract beneficial insects to crops (Gurr and Reynolds, 2009).
In Nicotiana tabacum it has the function of repellent (Torto, 2002-2015), the reports in corn refer to it as an attractant of the elotero worm (Huang, 2009) and of some wasps, as in the cotton crops and the so-called cow chicharo (Gouinguene, 2005). In the case of the variety MEX 69-290, a high content of phytol was detected in the aromatic extract (36.97%), this compound can come from the degradation of chlorophyll in the leaves of cane that were boiling in the flask of the hydro-sink (Suzuki and Shioi, 1999). This compound was detected by means of gas chromatography on leaves of the karaya tree (Sterculia urens) with a percentage similar to that detected in sugarcane leaves (37.78%). The phytol has a wide application in the food, bakery and dairy industry.
The gum that is extracted from the karaya is in great demand inside and outside of India. It can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1 (Sermakkani and Thangapandian, 2012; Konovalova et al., 2013; Mohan, 2014; Nanadagopalan et al., 2015). Phytol can also include increased energy and fight against infections, as well as an anti-mycobacterial activity against mycobacterial tuberculosis. It is used as antidiabetic, antibacterial, anticancer, antioxidant, antispasmodic, analgesic and diuretic (Nanadagopalan et al., 2015).
Conclusions
In each of the evaluations different compounds were found, the coincidences between varieties were given with three main compounds that are (z)-3-hexen-1-ol; 1-octen-3-ol and phytol. The most frequently detected compound was (z)-3-hexen-1-ol, which coincided in seven varieties (it was not found in the variety RD 75-11) and was also detected in the two grasses evaluated. The abundance of (z)-3-hexen-1-ol in the samples of the sugar cane varieties varies from 20.35% in the variety MEX 69-290 to 57.36% in the variety COLPOS CT MEX 05-204, taking in the pasture species evaluated, we can determine that the highest amount of (z)-3-hexen-1-ol was detected in African star grass with an abundance of 59.69% in the sample. The 1-octen-3-ol was expressed in seven varieties (except in the variety COLPOS CT MEX 05-204) with an abundance ranging from 16.37% in the variety L 77-50 to 40.06% in the variety RD 75-11. For its part, phytol was expressed with less regularity, since it was present in six varieties, the abundance of this compound starts at 1.02% in the L 77-50 variety, the highest abundance of phytol was detected in the variety mex 69-290 with 36.97%. The appearance of these three main compounds were expressed in five varieties, these are CP 72-2086, MEX 69-290, MEX 79-431, L 77-50 and COLPOS CT MEX O5-223 where the first three varieties susceptible to being damaged of pests like spittlebugs (Aeneolamia and Prosapia).
Acknowledgment
To the National Council of Science and Technology (CONACYT) for the scholarship awarded to Nelson José Ramírez-Medorio. This study was funded by the Foundation Produce Veracruz AC, a national project: ‘Design of a contemporary program for integrated management of spittlebugs in sugarcane’ (2012-2013).
REFERENCES
Alatorre, R. R. y Hernández, R. F. 2015. Mosca pinta, Aeneolamia spp. y Prosapia spp. (Hemiptera: Cercopidae). In: Arredondo-Bernal, H. and Rodríguez del Bosque, L. A. Casos de control biológico en México. (Ed.). bba-Colegio de Postgraduados 2. 141-164 pp. [ Links ]
Cortés, S.; Gil, M. A. and Fernández, E. 2005. Volatile composition of traditional and industrial Orujo spirits. Food Control. 16(4):383-388. [ Links ]
D’Auria, J. C.; Pichersky, E.; Schaub, A.; Hansel, A. and Gershenzon, J. 2007. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 49(2):194-207. [ Links ]
De Bruyne, M. and Baker, T. C. 2008. Odor detection in insects: volatile codes. J. Chem. Ecol. Springer Sci. 34(7):882-897. [ Links ]
Gouinguene, S. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volátiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 31(5):1023-1038. [ Links ]
Gurr, G. M. and Reynolds, O. L. 2009. Synergizing biological control: scope for sterile insect technique, induced plant defences and cultural techniques to enhance natural enemy impact. Biol. Control. 52(3):198-207. [ Links ]
Hernández, R. F.; Figueroa, S. B. y Figueroa, R. K. A. 2010. Biologia de la mosca pinta. Comunidades rulales agrarias, ejidos y conocimiento local. Colegio de Postgraduados LPI 13. Ficha técnica núm. 1. 2 p. [ Links ]
Huang, C. H. 2009. Volatiles induced by the larvae of the Asian corn borer (Ostrinia furnacalis) in maize plants affect behavior of conspecific larvae and female adults. Insect Sci. 16(4):311-320. [ Links ]
Konovalova, O.; Gergel, E. and Herhel, V. 2013. GC-MS Analysis of bioactive components of Shepherdia argentea (Pursh.) Nutt. from Ukrainian Flora. The Pharma Innovation J. 2(6):7-12. [ Links ]
Laporta, Z. G. and Sallum, M. M. A. 2011. Effect of CO2 and 1-octen-3-ol attractants for estimating species richness and the abundance of diurnal mosquitoes in the southeastern Atlantic forest, Brazil. Mem Inst Oswaldo Cruz. 106(3):279-284. [ Links ]
Lazzari, R. C. 2011. Ecología sensorial de insectos vectores. In: Simposio ecología sensorial de insectos vectores 2011. Institut de Recherche sur la Biologie de l’Insecte, Faculté des Sciences, Université François Rabelais, Tours, Francia. XV Congreso Colombiano de Parasitología y Medicina Tropical. 41-49 pp. [ Links ]
Lima, A. V. A.; Barbosa, M. A. S.; Cunha, L. C. S.; de Morais, S. A. L.; de Aquino, F. J. T.; Chang, R. and do Nascimento, E. A. 2017. Volatile compounds obtained by the hydrodistillation of sugarcane vinasse, a residue from ethanol production. Rev. Virtual Quim. 9(2):764-773. [ Links ]
Lubeck, A. J. and Sutton, D. L. 1983. Kovats retention indices of selected hydrocarbons through C10 on bonded phase fused silica capillaries. J. High Resolution Chromatography & Chromatography Communications. 328-332 pp. [ Links ]
Martínez, A. 2013. Introducción a la ecología química y su uso en el manejo de insectos plaga en sistemas forestales. Serie técnica: manejo integrado de plagas forestales. INTA EEA Bariloche. Río Negro. Argentina. Cuadernillo núm. 17. 14 p. [ Links ]
Mohan, D. 2014. GC-MS Analysis of leaf and stem bark of Cleidion Nitidum (Muell. -Arg.) Thw. Ex Kurz. (Euphorbiaceae) Asian J. Pharm. Clin. Res. 7(2):41-47. [ Links ]
Nanadagopalan, V.; Johnson, G. M. and Doss, A. 2015. GC-MS analysis of biomolecules on the leaves extract of Sterculia urens Roxb. J. Pharmacognosy Phytochem. 3(6):193-196. [ Links ]
Rodríguez, A. M.; Alcaraz, M. L. y Real, C. S. M. 2012. Procedimientos para la extracción de aceites esenciales en plantas aromáticas. Centro de Investigaciones Biológicas del Noroeste, SC. Instituto Politécnico Nacional (IPN). La Paz, BCS. México. 38 p. [ Links ]
Rodríguez, H. C. 2004. Plantas atrayentes de insectos plaga. Ciencias ambientales y agricultura. Benemérita Universidad Autónoma de Puebla. Puebla, México. Publicación especial. 203-234 pp. [ Links ]
Röse, U. and Tumlinson, J. 2004. Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta. 218(5):824-832. [ Links ]
SAGARPA. 2006. Manejo integrado para el control de gusano barrenador en caña de azúcar en el estado de Morelos. SAGARPA-INIFAP. Centro de Investigacion Regional del Centro Campo Experimental Zacatepec, Zacatepec, Morelos. Folleto para productor núm. 44. 13 p. [ Links ]
Sant’Ana, L. A.; Eiras, A. E. and Cavalcante, R. R. 2002. Electroantennographic responses of the Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva) (Diptera: Psychodidae) to 1-octen-3-ol, Neotrop. Entomol. 31(1):13-17. [ Links ]
Scala, A. 2013. Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Inter. J. Mol. Sci. 14(9):17781-17811. [ Links ]
Sermakkani, M. and Thangapandian, V. 2012. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharmaceutical and Clinical Res. 5(2):90-94. [ Links ]
Singh, A.; Uma, R. L.; Hayat, M. M.; Prabh, S. S.; Gagan, S. and Ravi, K. D. 2015. Phytochemical profile of sugarcane and its potential health aspects. Pharmacognosy Reviews. 9(17):45-54. [ Links ]
Suzuki, Y. and Shioi, Y. 1999. Detection of chlorophyll breakdown products in the senescent leaves of higher plants. Plant Cell Physiol. 40(9):909-915. [ Links ]
Syed, Z. and Guerin, P. M. 2004. Tsetse flies are attracted to the invasive plant Lantana camara. Institute of Zoology. J. Insect Physiol. 50(1):43-50. [ Links ]
Tang, R.; Su, M. W. and Zhang, Z. N. 2012. Electroantennogram responses of an invasive species fall webworm (Hyphantria cunea) to host volatile compounds. Chinese Science Bulletin. 57(35):4560-4568. [ Links ]
Torres, M. J.; Barrouin, M. S. M.; Goncalves, C. A.; da Rocha, S. F. B.; Machado, V. E.; Govone, J. S. and Pinto, M. C. 2014. A laboratory evaluation of alcohols as attractants for the sandfly Lutzomyia longipalpis (Diptera: Psychodidae). Parasites & Vectors. 7(60):1-5. [ Links ]
Torto, B. 2004. Chemical signals as attractants, repellents and aggregation stimulants. In: Encyclopedia of life support systems (EOLSS), developed under the auspices of the UNESCO. Eolss Publishers, Oxford, UK. (http://www.eolss.net). [ Links ]
Verheggen, F. J.; Haubruge, E. and Mescher, M. C. 2010. Alarm pheromones- Chemical signaling in response to danger. In: Gerald Litwack (Ed.). Vitamins and Hormones. 83(9):215-240. [ Links ]
Received: December 2018; Accepted: March 2019











 text in
text in