Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 spe 22 Texcoco Mar./Abr. 2019
https://doi.org/10.29312/remexca.v0i22.1863
Articles
Effect of entomopathogenic nematodes on nymphs of Aeneolamia albofasciata and its persistence in sugarcane soils of Veracruz
1Posgrado en Fitosanidad, Entomología y Acarología-Colegio de Postgraduados-Campus Montecillo, Texcoco, Estado de México. CP. 56230.
2Programa de Innovación Agroalimentaria Sustentable-Campus Córdoba-Colegio de Postgraduados. Carretera Federal Córdoba-Veracruz, Congregación Manuel León, Amatlán de los Reyes, Veracruz. CP. 94953.
3Facultad de Ciencias Biológicas y Agropecuarias-Campus Peñuela-Universidad Veracruzana. Carretera Peñuela-Amatlán km 177, Córdoba, Veracruz. CP. 94500.
4Programa de Innovación en el Manejo de Recursos Naturales-Campus San Luis Potosí-Colegio de Postgraduados. Iturbide 73, Salinas de Hidalgo, San Luis Potosí. CP. 78600.
The objective of this study was to evaluate the pathogenicity of nematodes, Heterorhabditis sp. (CPVG13) and Steinernema spp. (CPVC12, CPVC13) on the spittlebug, Aeneolamia albofasciata and determine its persistence in the soil of three sugar mills Potrero, Constancia and Motzorongo, of the state of Veracruz. In the first experiment the pathogenicity of the entomopathogenic nematodes was evaluated in nymphs of spittlebug, A. albofasciata of 3rd and 4th instar, 120 (infective juvenile) JI/nymph were applied on boxes with six cavities, previously covered with filter paper, each cavity contained a grass stolon (Cynodon sp.) and a nymph per cavity. In the second experiment, the persistence of entomopathogenic nematodes (NEPs) in soil was evaluated; the experiment lasted 70 days, with independent weekly evaluations, of 32 experimental units per week. The pathogenicity experiment revealed that both nematodes were able to cross the saliva that protects the nymphs, kill and develop in the corpses producing new progeny of JI. The highest mortality (62.5%) was observed with Heterorhabditis (CPVG13), also producing the highest concentration of JI per nymph (3500JI/nymph). Significant differences were found between the persistence profiles between Heterorhabditis (CPVG13) and Steinernema (CPVC12). A significant effect of the soil on the survival of both nematodes was found. The isolation that persisted most in soil was the Steinernema compared with that of Heterorhabditis (CPVG13). Deepening these studies, will allow to determine the impact of the interaction between spittlebug-NEPs and the soil.
Keywords: entomopathogenic nematodes; spittlebug; sugarcane
El objetivo de este estudio fue evaluar la patogenicidad de los nematodos, Heterorhabditis sp. (CPVG13) y Steinernema spp. (CPVC12, CPVC13) sobre el salivazo, Aeneolamia albofasciata y determinar su persistencia en el suelo de tres ingenios azucareros Potrero, Constancia y Motzorongo, del estado de Veracruz. En el primer experimento la patogenicidad de los nematodos entomopatógenos se evaluó en ninfas de mosca pinta, A. albofasciata de 3° y 4° instar, se aplicaron 120 (juveniles infectivos) JI/ninfa sobre cajas con seis cavidades, cubiertas previamente con papel filtro, cada cavidad contenía un estolón de pasto (Cynodon sp.) y una ninfa por cavidad. En el segundo experimento, se evaluó la persistencia de los nematodos entomopatógenos (NEPs) en suelo, el experimento tuvo una duración de 70 días, con evaluaciones semanales independientes, de 32 unidades experimentales por semana. El experimento de patogenicidad revelo que ambos nematodos fueron capaces de atravesar la saliva que protege a las ninfas, matar y desarrollarse en los cadáveres produciendo nueva progenie de JI. La mortalidad más alta (62.5%) se observó con Heterorhabditis (CPVG13), produciendo además la mayor concentración de JI por ninfa (3500JI/ninfa). Se encontraron diferencias significativas entre los perfiles de persistencia entre Heterorhabditis (CPVG13) y Steinernema (CPVC12). Se encontró un efecto significativo del suelo en la supervivencia de ambos nematodos. El aislamiento que persistió más en suelo fue el Steinernema comparado con el de Heterorhabditis (CPVG13). Profundizar en estos estudios, permitirá determinar el impacto de la interacción entre salivazo-NEPs y el suelo.
Palabras clave: caña de azúcar; nematodos entomopatógenos; salivazo
Introduction
Spittlebug, Aeneolamia albofasciata (Lallemand) (Hemiptera Cercopidae), is one of the most damaging pests of sugarcane. It occurs in large areas of the Gulf of Mexico and coastal area of the Pacific Ocean (Flores-Caceres, 1994; Peck, 2001; Gómez, 2007; López-Collado et al., 2013, Alatorre-Rosas and Hernández-Rosas, 2015). Alatorre-Rosas et al. (2013); Alatorre-Rosas and Hernandez-Rosas (2015) mention that A. albosfaciata has an altitudinal distribution ranging from 10 to 1 700 meters above sea level. The effects generated by feeding nymphs and adults cause water stress, delaying the growth of the plant, reducing biomass production (Dinardo-Miranda et al., 2000; Valerio et al., 2001; Alatorre-Rosas and Hernández-Rosas, 2015). A. albofasciata causes losses of up to 9 t ha-1 of sugarcane (De la Cruz et al., 2005; Alatorre-Rosas and Hernández-Rosas, 2015).
The nymphs appear a few days after the onset of the rains and last throughout the rainy season, with population peaks of nymphs and adults appearing staggered and overlapping. The newly hatched nymphs begin the production of saliva, which covers them completely, gives them shelter, serves as a defense against their natural enemies and at the same time protects against adverse weather conditions (Marshall, 1966; Whittaker, 1970; Bodegas, 1973; Alatorre-Rosas and Hernández-Rosas, 2015).
For the control of adults, it is recommended the use of insecticides in severe infestations and of the entomopathogenic fungus Metarhizium anisopliae (Metschnikoff) Sorokin (Ascomycota: Hypocreales) (Torriello et al., 2008; Hernández-Rosas et al., 2013; Alatorre-Rosas and Hernández-Rosas, 2015). The use of M. anisopliae as a biological insecticide has been generalized in different cane areas, mainly for the regulation of adults (Flores-Caceres, 1994; Berlanga et al., 1997; Badilla, 2002; Carballo and Falguni, 2004; Castillo-Zeno 2006; Bustillo and Castro, 2011; Alatorre-Rosas and Hernández-Rosas, 2015). To date, formulated in inert wettable powder with spores of M. anisopliae and native isolates of entomopathogenic nematodes for the regulation of populations of the nymph or spittlebug, because saliva is an effective barrier against natural enemies and other control strategies (Alatorre-Rosas and Hernández-Rosas, 2015; Grifaldo-Alcántara et al., 2017).
The entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae have generated interest as a strategy of biological control of nymphs of different spittlebug species, in this sense the species Steinernema spp. and Heterorhabditis spp. against spittlebug, Mahanarva fimbriolata have achieved mortalities between 96 and 100% under laboratory conditions (Leite et al., 2002). Field studies showed that Heterorhabditis spp. in doses of 5 x 1010 JI/h, it caused mortalities of 42.3% and with H. bacteriophora in doses of 1.5 x 1011 JI/ha, mortality on nymphs of A. varia was 76% (Moreno et al., 2012).
Abiotic factors can have a positive or negative effect on the performance, effectiveness and level of success of entomopathogenic nematodes in the control of insect pests. That is why the application of entomopathogenic nematodes can be unpredictable in natural conditions. In this sense (Kaya, 1990; Kaya and Koppenhöfer, 1996; Smith, 1996) mention the importance of taking into account the intrinsic factors: behavior and physiological, as well as temperature, humidity, soil texture and radiation (extrinsic), in addition to biotic factors such as competition, which affect its persistence.
For this reason, it is important to carry out this study with some of the indicators that allow to evaluate the type of soils, the existing biological diversity and determine the effect on the entomopathogenic nematode’s permanence. The objective of this investigation was to evaluate the pathogenicity of native isolates of Steinernema spp. (CPVC12; CPVC13) and Heterorhabditis sp. (CPVG13) and its persistence in soil from three sugar mills in the state of Veracruz.
Material and methods
Susceptibility of nymphs of A. albofasciata towards entomopathogenic nematodes nymphs of A. albofasciata
The third and fourth instar nymphs of A. albofasciata were collected from field with cane cultivated in Atoyaquillo of sugarmill El Potrero, coordinates 18° 59’ 38.74 North latitude and 96° 46’ 50 West longitude. The nymphs were collected with the support of stainless-steel spatulas, were separated by gender and placed individually in plastic plates with cavities, which contained moist moistened paper toweling and stolons of star grass (Cynodon sp.). A preliminary test with grass stolons showed that rootlets of different lengths are formed in the area of the nodes, which remain active for several days (10-12). It was considered that if the nymphs could adhere to the rootlets and stay at least 4-5 days producing the excretion of saliva, it would constitute a favorable period for the trials proposed in this investigation.
The nymphs collected were transferred to the laboratory and kept under observation for 24 h, those that survived the management were selected. Subsequently, each nymph was confined individually in boxes Costar® (Corning Inc. NY USA) with 6 cavities, covered with moistened filter paper and a grass stolon (as food). During the experiment, the nymphs were kept at room temperature. The nymphs that survived and continued to produce saliva were used in the experiment, due to their adaptation to space and food exchange.
Entomopathogenic nematodes
The nematode isolates used for this research were obtained from larvae of G. mellonella that were used as bait insects in soil samples from three sugar mills in the state of Veracruz, Mexico (Central Progreso, Constancia and La Gloria). The experiments were carried out with two isolates of Steinernema sp. (CPVC12, CPVC13) and one of Heterorhabditis sp. (CPVG13) (Table 1). The morphological and molecular taxonomic determination of the isolates is currently in process and will be reported in a later article.
Table 1 Nematodes used for the pathogenicity test.
| Treatment | UE (a plate) | Reply (plate) | Nymphs totals (Reply) | Repetitions in time | Total nymph/UEs | Total plates |
| 1. Steinernema (CPV12) | 1 | 4 | 24 | 2 | 48 | 8 |
| 2. Steinernema sp. (CPVC13) | 1 | 4 | 24 | 2 | 48 | 8 |
| 3. Heterorhabditis (CPVG13) | 1 | 4 | 24 | 2 | 48 | 8 |
| 4. Control (without nematodes) | 1 | 4 | 24 | 2 | 48 | 8 |
| Totals | 16 | 96 | 192 | 32 |
The nematodes were propagated on last instar larvae of G. mellonella (L.) and harvested as infective juvenile (JI) by the White trap method (Kaya and Stock, 1997), sterilized with 0.01% benzalkonium chloride and two changes of sterile distilled water. The suspension of infective juvenile (JI) was centrifuged at 500 rpm for 5 min, in order to eliminate dead nematodes by decanting the supernatant. The nematodes were kept at room temperature in distilled water, oxygenating them with a fish tank pump continuously to reduce mortality. The concentration of JI in suspension was estimated using aliquots of 20 μL, using the average of 7 counts, adjusting the concentration required in the experiment.
Experimental procedure
The process of inoculation of the different isolates was the same (Table 1). Costar® plates with 6 cavities were used. Each cavity was covered with two layers of filter paper previously sterilized and moistened. In each cavity, a stolon (3.5 cm) of star grass (Cynodon sp.) where the formation of rootlets was induced, on these a nymph of A. albofasciata was placed. The inoculation was carried out applying a single concentration of infective juvenile, 120 JI cm-2 according to Alves et al. (2005). The application was made on the filter paper, allowing the displacement of the JI and search for the host insect.
The treatments were incubated at 25 ±2 °C and relative humidity (74% min and 84% max) and photoperiod of 9 and 15 h dark-light. In the control, the nymphs were treated only with sterile water. The nymphs were reviewed every 48 h and mortality was determined by plaque taking into account the symptoms (red or beige color, flaccidity) of the nymphs. Each dead nymph was removed from the plate and placed in a humid chamber, which consisted of a Petri dish with moistened filter paper, the emergence of JI was determined by infected nymph. The JI were collected during a period of 4 to 5 days and the total JI was determined in 10 ml of the suspension collected by nymph. This value was taken as indicative of the reproductive capacity of the nematodes. Each treatment had four repetitions (four plates/24 nymphs) and the whole experiment was repeated on three different occasions. The experiment lasted 7 days after the application of infective juvenile. The entire laboratory phase was carried out under aseptic conditions.
Effect of soil with the persistence of entomopathogenic nematodes
Soil samples from 8 sites were selected, two belonging to the Constancia sugarmill, two to Central Motzorongo and four to El Potrero. The soil samples were taken in an area 30 x 30 cm long by wide and approximately 10 cm deep using a shovel. From each site thirteen samples (a total of 5 kg) were obtained and were stored at 4 °C for no more than two weeks. Composite samples were prepared, combining the 13 sub samples of each site by ingenuity.
To avoid the effect of native nematodes in the persistence experiment, soil sampling was carried out using the bait insect method with G. mellonella (Zimmerman, 1986). Each soil sample was moistened with sterile distilled water at field capacity (60%). Samples of 500 g of each locality were taken by placing them in plastic containers of 500 mL of capacity (experimental unit), seven larvae of G. mellonella of the last instar were individually confined in metal mesh cages and introduced in the containers with soil and distributed randomly. Each container was covered with a lid with perforations to allow constant aeration, the vessels were inverted (Miduturi and Moens, 1997) and incubated in a breeding chamber at 25 ±2 °C for 7 days, to confirm the presence of native nematodes. This procedure was repeated twice.
Cultivable microbial populations
Within the first week of collection, the natural populations of bacteria, fungi and actinomycetes of each soil sample were estimated. Samples of 10 g of soil from each location, sieved in No. 10 mesh, were placed in a 250 mL Erlenmeyer flask containing 90 mL of sterile distilled water. The samples were shaken for 18 minutes in an orbital shaker (Thermolyne Cimarec® 3), to subsequently prepare serial dilutions (Dhingra and Sinclair, 1985). A final dilution factor of 10-3 and 10-5 was considered adequate for fungal isolation, 10-4 and 10-6 for bacteria and actinomycetes 10-3 and 10-5.
Planting was done by plaque extension in Petri dishes with nutritive agar (NA) for bacteria, potato dextrose agar (PDA) for fungi and Czapeck Dox agar with pH 8.0 for actinomycetes with three replications by dilution. Plates were incubated at 27 °C/5d, 7d and 10d for bacteria, fungi and actinomycetes, respectively. After the incubation period, the number of colony forming units (UFC) was estimated by selecting the dilution in each medium that favored the proper enumeration (10-3 for bacteria and fungi and 10-6 for actinomycetes). The UFC/gram of dry soil (air) was calculated by multiplying the arithmetic average of the number of UFC per plate by the dilution factor. The result was divided by the estimated dry soil weight. The physical and chemical characteristics of the soil were carried out by the Soil Fertility Laboratory of the Edaphology Program, Postgraduate School.
Experimental procedure
For this experiment, soil from El Potrero, Central Motzorongo and Constancia sugarmills were used, these were selected because the presence of nematodes was not recorded. Two isolates, CPVC12 (Steinernema spp.) and CPVG13 (Heterorhabditis sp.) were used. The procedure was the same for each type of soil and nematode evaluated. For each soil and nematode combination, 22 containers with 500 g of soil each were prepared, in the center of 11 of the containers, 5 000 infective juveniles were suspended, suspended in 2.5 mL of sterile distilled water (ADE), in the 11 remaining containers only 2.5 mL of ADE was deposited, these being the control treatment.
The survival of the nematodes was quantified by the proportion of larvae of G. mellonella infected when in contact with soil inoculated by nematodes. For this, in two containers, with and without nematodes, they were introduced in seven cages made of metal mesh in each container. Cages 1 cm in diameter by 4 cm in length contained a larva of G. mellonella. Seven days later, this procedure was repeated using two different containers (with and without nematodes) and so on at 14, 21, 28, 35, 42, 49, 56, 63 and 70d after inoculation of the nematodes. The experiment was incubated at 25 °C in total darkness. The larvae of G. mellonella were left in contact with the soil for 6 d and then they were removed from the soil and the number of dead larvae was quantified.
Each larva was placed in a cavity of a plate of six cavities containing moist filter paper, and incubated to the same experimental conditions. The presence of infective juvenile (JI) was determined by observations between 10 a 15d. The experiment was carried out under a completely randomized design, where each soil/nematode combination had two replicates, and the experiment was repeated twice (four repetitions in total).
The data were analyzed using logistic regression (assuming that the data had a binomial distribution), where each number of dead larvae was a proportion of the total larvae evaluated. When it was necessary, the presence of greater dispersion of the data under the assumption of the binomial distribution was allowed, comparing the ratio of the mean deviation of the treatments and the residual mean deviation against a F distribution, instead of comparing the deviation of the treatments with a distribution of χ2. Before comparing between treatments, the results obtained between repetitions in time were compared to be combined. With the combined repetition data, survival between nematode isolates, between the 4 soil types, between the evaluation times and finally the interaction between these factors was first compared. The analysis was carried out in the GenStat v. 8 (Payne et al., 2005).
Results
Susceptibility of nymphs of A. albofasciata against Heterorhabditis sp. and Steinernema spp.
In general, differences were observed in mortality caused by Steinernema spp. (CPVC12; CPVC13) and Heterorhabditis sp. (CPVG13). The percentage of mortality (62.5%) was higher for Heterorhabditis sp. at 25 °C, compared to Steinernema (CPVC12) (56.25%) and Steinernema sp. (CPVC13) (33.33%). Although mortality was observed in the treatments, it was not possible to perform a statistical analysis, since the control treatment showed high mortality from other causes. However, this experiment showed that the isolates of both Steinernema and Heterorhabditis had the ability to cross the saliva produced by the nymphs, penetrate the insect cavity and cause infection (Figure 1b, 1c).

Figure 1 Spittlebug nymphs: a) healthy nymph; b) nymph infected by Heterorhabditis. (CPVG13); c) infected by Steinernema (CPV12). Dissections made to parasitized nymphs; d) adult nematodes of (CPVG13) Heterorhbaditis; e) adult nematodes of (CPV12) Steinernema; f) meristemed nematodes; and g) nymphs with presence of necrosis, possible presence of bacteria.
The dissections of the corpses of infected nymphs allowed to verify the reproductive capacity of the entomopathogenic nematodes (Figure 1d, 1e). The nymphs exposed to Heterorhabditis (CPVG13) and Steinernema sp. (CPVC12) showed differences in abundance of infective juvenile (Table 2). In the case of Heterorhabditis, the highest proportion corresponded to 3 500 JI per nymph (Table 2).
Table 2 Estimation of the number of infected nymphs and JI found in nymphs of A. albofaciata.
| Species NEP/repetition | Infected larvae | Population of JI/nymph | |
| Minimum | Maximum | ||
| Steinernema R1 | 13 | 4 | 1 500 |
| Steinernema R2 | 14 | 7 | 1 500 |
| Heterorhabditis 1 | 12 | 1 000 | 3 500 |
| Heterorhabditis 2 | 18 | 10 | 3 500 |
| S. (Constancy) 1 | 6 | 9 | 1 000 |
| S. (Constancy) 2 | 10 | 4 | 1 000 |
| Control | 0 | - | - |
Cultivable microbial populations
The largest population of bacteria and fungi was present in Constancia soil (3X109 UFC g-1 of soil and 1.5 x 104 UFC g-1 of soil for bacteria and fungi, respectively) (Figure 2a) while in Central Motzorongo the proportion of bacteria (3 x 108 UFC) and fungi (4 x 104 UFC g-1 of soil) was considered low (Figure 2b). The largest actinomycete population was found in site 2 of El Potrero plantation (7 x 105 UFC g-1 of soil) and the lowest population was obtained in Constancia (8.6 x 104 UFC g-1 of soil) and Motzorongo (1.7 x 195 UFC g-1 of soil) (Figure 2c). The physical and chemical characteristics of the soil (Table 3).

Figure 2 Colony forming units. a) bacteria; b) fungi; and c) actinomycetes, obtained from cane soil cultivated in the Constancia, Motzorongo plant and El Potrero sugarmills.
Table 3 Physical-chemical characteristics of the soil of three sugar mills.
| Identificación | pH | CE | MO (%) | N* | P | K | Texture Bouyoucos |
| PotreroS1 | 4.8 | 0.32 | 1.7 | 0.09 | 41 | 0.4 | Clay |
| PotreroS2 | 5.1 | 0.28 | 5.4 | 0.27 | 64 | 0.7 | Clay |
| PotreroS3 | 6.4 | 0.26 | 3.3 | 0.16 | 12 | 0.3 | Clay loam |
| PotreroS4 | 5.3 | 0.14 | 5.3 | 0.26 | 15 | 0.3 | Clay |
| ConstanciaS1 | 5.9 | 0.21 | 3.7 | 0.19 | 39 | 0.3 | Clay loam |
| ConstanciaS2 | 6.5 | 0.16 | 5.3 | 0.26 | 31 | 0.4 | Clay |
| MotzorongoS1 | 5.2 | 0.21 | 2.2 | 0.11 | 23 | 0.6 | Clay loam |
| MotzorongoS2 | 6 | 0.23 | 1.4 | 0.07 | 18 | 1 | Sandy clay loam |
CE= electrical conductivity; MO= organic material; N= nitrogen; P= phosphorus; K= potassium.
Effect of soil with the persistence of entomopathogenic nematodes
There were no significant differences between the data of the four repetitions (
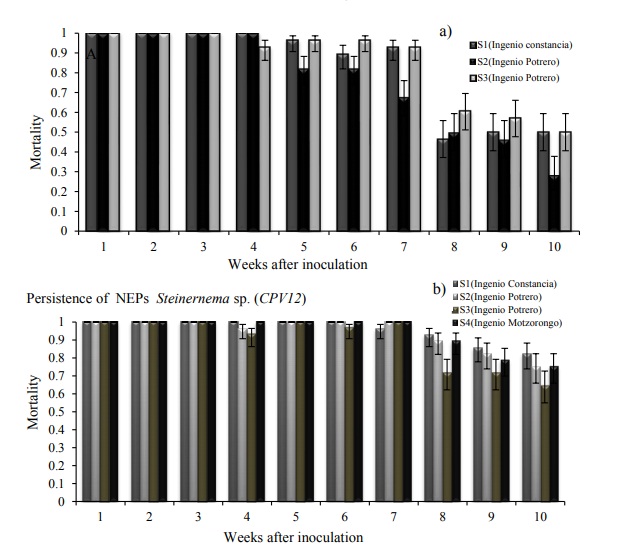
Figure 3 Proportions of mortality a) Heterorhabditis sp.; and b) Steinernema sp. in larvae of G. mellonella. The error bars represent confidence limits of 95%, transformed from the logistic scale. The persistence of JI constitutes the average value of dead larvae of G. mellonella by date of evaluation at 25 °C.
Discussion
These results show that isolates of entomopathogenic nematodes CPVC13; CPVC12 (Steinernema) and CPVG13 (Heterorhabditis) can infect nymphs of Aeneolamia albofasciata, being able to cross the physical barriers of the insect, mainly the foamy mass of saliva that serves them as protection. The infection capacity of the nematode isolates used can be confirmed by the reproduction of these in their host, especially by the CPVG13 isolation of Heterorhabditis (Table 2), as well as by previous reports of infection in other species of hemiptera under conditions of laboratory, greenhouse and field (Ferrer et al., 2004; Leite et al., 2005; Rosero- Guerrero, 2011; Morero et al., 2012). There was a greater infection caused by Heterorhabditis compared to Steinernema, which could be due to the fact that Heterorhaditides have the ability to penetrate through the cuticle of the host with the help of a small tooth they possess (Bedding and Molyneux, 1982; Aguilera, 2001). The high mortality of A. albofasciata nymphs in the control was probably caused by pathogens brought from the field, as well as by the manipulation at the time of collection.
One of the factors of natural mortality observed in this investigation was the presence of mermitid nematodes of the genus Hexamermis sp., a phenomenon that has already been previously reported in A. varia (Poinar and Linares, 1985) and Manhanarva fimbriolata (Bennett, 1984). For this reason, it is considered important that, in future experiments, the biological material comes from colonies of insects kept under controlled conditions, thus avoiding high mortality during the bioassay and obtaining statistically reliable results.
In the experiment of persistence of entomopathogenic nematodes, significant differences were observed between treatments and nematode strains, which means that there was a decrease in the initial population of nematodes over time. In both Steinernema and Heterorhabditis the persistence of JI measured as the ability to kill bait insects over time, showed slight variations from the fourth week, in the case of Steinernema the mortality remained close to 100% until the seventh week; however, in the eighth week, the mortality ratio showed variations of 0.65 to 0.9 depending on the soil where it was incubated (Figure 3B). In the case of Heterorhabditis, the persistence of JI showed more evident variations in the following weeks. Just like Steinernema, from the eighth week there was a reduction in the persistence of JI, but in this case the fall was more drastic (0.25) (Figure 3B).
Heterorhabditis showed problems of persistence in the different types of soil (Figure 3A), in clayey soil, clay loam and sandy loam, with acidic to neutral pH (Table 3) Heterorhabditis showed a drastic drop in the mortality curve, which means that soil represents an important factor for the persistence of this entomopathogenic nematode (Kung et al., 1990; Portillo-Aguilar et al., 1999).
According to (Kaya, 1990; Kaya and Koppenhöfer, 1996; Epsky et al., 1998) the interaction between soil microorganisms and entomopathogenic nematodes can affect the survival of the JI, affect the establishment and development of the symbiont and affect the insect once invaded by the nematode. However, in this study differences were found in the concentration of bacteria, fungi and actinomycetes present in the soil, which may be related to the persistence of entomopathogenic nematodes. The nematode vs microbiota interaction could affect the nematode strain differently, causing greater mortality in Heterorhabditis (CPVG13). However, more in-depth studies are needed to corroborate the effect of soil-microbiota on the persistence and parasitic activity of entomopathogenic nematodes.
Conclusions
Isolates of entomopathogenic nematodes of the genus Steinernema and Heterorhabditis had the ability to infect nymphs (spittlebug) of Aeneolamia albofasciata, for their ability to cross the frothy saliva barrier that covers the nymph. In addition to showing biological effectiveness for its virulence to develop and reproduce in the host. In particular, by the genus Heterorabititis (CPVG13).
The persistence of the isolates of Steinernema sp., as of Heterorhabditis sp., regarding JI in both cases was maintained, in the case of Steinernema at the seventh week the mortality was almost 100%, only that at the eighth week there was variations between 0.65 to 0.9 depending on the type of soil where it was maintained, however Heterorhabditis the persistence of JI was similar only that its sudden drop was highlighted by the mortality that was more pronounced and this was observed in both clayey soil, clay loam, and loam. sandy as in acidic to neutral pH.
Acknowledgments
To the National Council of Science and Technology (CONACYT) for the scholarship to Oscar Parada Dominguez. This study was funded by the Foundation Produce Veracruz AC, a national project: Design of a contemporary management program Integrated spittlebug in sugarcane (2012-2013). It was supported by Projects of Scientific Research and Technological Development 2013; through, of the trust No. 167304, Postgraduates College.
REFERENCES
Aguilera, M. M. 2001. Nematóides do bem. Cultivar. Grandes culturas. Pelotas. 25:52-54. [ Links ]
Alatorre-Rosas, R.; Carrillo, B. G.; Grifaldo, A. P. F.; Valdez, C. J.; Guzmán, F. A. W.; Romero, N. J.; Segura, L. O.; Hernández-Rosas, F.; López, C. J. y Villanueva, J. J. A. 2013. Identificación de especies de mosca pinta. Proyecto nacional. Diseño de un programa contemporáneo de manejo integrado de mosca pinta en caña de azúcar. FMP-002. 2 p. [ Links ]
Alatorre-Rosas, R. y Hernández-Rosas, F. 2015. Mosca pinta, Aeneolamia spp. y Prosapia spp. (Hemiptera: Cercopidae). In: Arredondo-Bernal, H. and Rodríguez del Bosque L. A. Casos de control biológico en México. (Ed.) Colegio de Postgraduados. 141-164 pp. [ Links ]
Alves, L. F. A.; Rhode, C. y Alves, V. S. 2005. Patogenicidad de Steinernema glaserie, S. carpocapsae (Nematoda: Rhabditida) contra o cascudinho, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Neotropical Entomol. 34(1):139-141. [ Links ]
Badilla, F. F. 2002. Un programa exitoso de control biológico de insectos plaga de la caña de azúcar en Costa Rica. Manejo Integrado de Plagas y Agroecología (Costa Rica). 64:77-87. [ Links ]
Bedding, R. A. and Molyneux, A. 1982. Penetration of insect cuticle by infective juveniles of Heterorhabditis spp. (Heterorhabditidae: Nematoda). Nematológica. 28(3):354-359. [ Links ]
Bennett, F. D. 1984. Discusión sobre las posibilidades de control biológico de la candelilla. In: Seminario problemas de la candelilla y el taladrador de la caña de azúcar y pastos (Barquisimeto, 1984). Unión de productores de azúcar . 39-48 pp. [ Links ]
Berlanga-Padilla, A. M. y Hernández, V. V. M. 1997. Control microbial de mosca pinta Aeneolamia spp. con Metarhizium anisopliae. Dirección General de Sanidad Vegetal-Comisión Nacional de Sanidad Agropecuaria. Ficha técnica. CB-08. 4 p. [ Links ]
Bodegas, V. P. R. 1973. Aspectos biológicos sobre la mosca pinta de los pastos, con énfasis en el periodo de incubación de los huevecillos de Aeneolamia occidentalis (Fennah). Tesis de Maestría en Ciencias. Monterrey, NL. ITESM. 36-111 pp. [ Links ]
Bustillo, P. A. E. y Castro, V. U. 2011. El salivazo de la caña de azúcar Aeneolamia varia. Red Mundial http://www.cenicaña.org/pdf/serie-divulgativa/sd11/sd.pdf. [ Links ]
Carballo, M. y Falgumi, G. 2004. Control biológico de plagas agrícolas. Serie técnica: manual técnico núm. 53. Nicaragua. 232 p. [ Links ]
Castillo, Z. S. 2006. Uso de Metarhizium anisopliae para el control biológico del salivazo (Aeneolamia sp. y Prosapia sp.) en pastizales de Brachiaria decumbens en el Peten, Guatemala. Tesis de Maestría en Ciencias. CATIE. 7 p. [ Links ]
De la Cruz, L. J. J.; Vera, G. J.; López, C. J.; Pinto, V. M. and Garza, G. R. 2005. Una técnica simple para el desarrollo de ninfas de Aeneolamia postica (Homoptera: Cercopidae). Folia Entomológica Mexicana. 44(1):91-93. [ Links ]
Dhingra, O. D. and Sinclair, J. B. 1985. Basic plant pathology methods, CRC Press Inc., Boca Raton. 448 pp. [ Links ]
Dinardo, M. L. L.; Ferreira, J. M. G. e Carvalho, P. A. M. 2000. Influência das cigarrinhas das raízes, Mahanarva fimbriolata, sobre a qualidade tecnológica da cana-de-açúcar. STAB. Açúcar, Álcool e Subprodutos. 19(2):34-35. [ Links ]
Epsky, N. D.; Walter, D. E. and Capinera, J. L. 1998. Potential role of nematophagus microarthropods as biotic mortality factors of enthomogenous nematodes (Rhabditida: Steinernematidae, Hterorhabditidae). J. Econ. Entomol. 81(3):821-825. [ Links ]
Ferrer, F.; Arias, M.; Trelles, A.; Palencia, G.; Navarro, J. y Colmenares, R. 2004. Posibilidades del uso de nematodos entomopatógenos para el control de Aeneolamia varia en caña de azúcar. Manejo Integrado de Plagas y Agroecología. 72:39-43. [ Links ]
Flores, C. S. 1994. Las plagas de la caña de azúcar en México. Servicios gráficos OREL. Primera Edición. Veracruz, México. 350 p. [ Links ]
Gómez, L. A. 2007. Manejo del salivazo Aeneolamia varia en cultivos de caña de azúcar en el valle del río Cauca. Cenicaña, Cali, Colombia. 10-17 pp. [ Links ]
Grifaldo, A. P. F.; Alatorre, R. R.; Segura, L. O. and Hernández, R. F. 2017. Steinernema ralatorei n. sp. Isolated from sugarcane areas at Veracruz, Mexico. Southwestern Entomologist. 42(1):171-190. [ Links ]
Hernández, R. F.; Cruz, T. M.; Pacheco, C. R.; González, V. G. T. and Ortíz, M. J. 2013. Esporas de M. anisopliae y efecto en mosca pinta. Proyecto nacional. Diseño de un programa contemporáneo de manejo integrado de mosca pinta en caña de azúcar. FMP-008. 2 p. [ Links ]
Kaya, H. K. 1990. Soil ecology. In: Gaugler, R. and Kaya, H. K. (Eds.). Entomopathogenic Nematodes in Biological Control. CRC Press, Boca Raton, FL. 93-116 pp. [ Links ]
Kaya, H. K. and Koppenhöfer, A. M. 1996. Effects of microbial and other antagonistic organism and competition on entomopathogenic nematodes. Bio. Sci. Technol. 6(3):333-345. [ Links ]
Kaya, H. K. and Stock, S. P. 1997. Techniques in insect nematology. In: Lacey, L. A. (Ed.). Manual of techniques in insect pathology. Academic Press, New York. 281-324 pp. [ Links ]
Kung, S. P.; Gaugler, R.; Kaya, H. K. and Vail, P. 1990. Soil type and entomopathogenic nematode persistence. J. Invertebrate Pathol. 55(3):401-406. [ Links ]
Leite, L. G.; Machado, L. A.; Aguilera M. M.; Rodrigues R. C. D. and Negrisoli, A. S. Jr. 2002. Patogenicidad de Steinernema, Heterorhabditis (Nematoda: Rhabditida) contra ninfas da cigarrinha-das-raízes da cana-de-açúcar, Mahanarva fimbriolata (Hemiptera: Cercopidae). Rev. Agric. 78(1):139-148. [ Links ]
Leite, L. G.; Machado, L. A.; Goulart, R. M.; Tavares, F. M. and Filho, B. A. 2005. Screening of entomopathogenic nematodes (Nemata: Rhabditida) and the efficiency of Heterorhabditis sp. against the sugarcane root spittlebug Mahanarva fimbriolata (Fabr.) (Hemiptera: Cercopidae) Neotropical Entomol. 34(5):785-790. [ Links ]
López, C. J.; Pérez, A. W. A.; Villanueva, J. J. A. and Hernández, F. F. 2013. Distribución de especies de mosca pinta y su riesgo potencial. Proyecto nacional. Diseño de un programa contemporáneo de manejo integrado de mosca pinta en caña de azúcar. FMP-003. 2 p. [ Links ]
Marshall, T. A. 1966. Spittle-production and tube-building by cercopid larvae (Homoptera) IV. Mucopolysaccharide associated with spittle-production. Department of Zoology. University of Hong Kong China. J. Insect Physiol. 12(6):635-644. [ Links ]
Miduturi, J. S. and Moens, M. 1997. Distribution of entomopathogenic nematodes in grassland. Russian J. Nematol. 5(1):67-70. [ Links ]
Moreno, A. C.; Bustillo, P. E. A.; Núñez, L. C. J.; Valderrama, C. U. and Ramírez, S. D. G. 2012. Virulencia de nematodos entomopatógenos para el control del salivazo Aeneolamia varia (Hemiptera: Cercopidae) en caña de azúcar. Rev. Colomb. Entomol. 38(2):260-265. [ Links ]
Payne, R. W.; Murray, D. A.; Harding, S. A.; Baird, D. B. and Soutar, D. M. 2005. GenStat for Windows. 8th (Ed.). Introduction VSN International, Hemel Hempstead. [ Links ]
Peck, C. D. 2001. Diversidad y distribución geográfica del salivazo (Homoptera: Cercopidae) asociado con gramíneas en Colombia y Ecuador. Rev. Colomb. Entomol. 27(3-4):129-136. [ Links ]
Poinar, G. O. and Linares, B. 1985. Hexamermis dactylocerus sp. n. (Mermithidae: Nematoda) a parasite of Aeneolamia varia (Cercopidae: Homoptera) in Venezuela. Revue de Nematologie. 8(2):109-111. [ Links ]
Portillo, A. C.; Villani, M. G.; Tauber, M. J.; Tauber, C. A. and Nyrop, J. P. 1999. Entomopathogenic nematode (Rhabditida: Heterorhabditidae and Steinernematidae) response to soil texture and bulk density. Environ. Entomol. 28(6):1021-1035. [ Links ]
Rosero, G. M. 2011. Evaluación de la virulencia de nematodos entomopatógenos para el control del salivazo de la caña de azúcar, Aeneolamia varia (F) (Hemiptera: Cercopidae). Tesis Magister en Ciencias Agrarias, énfasis protección de cultivos. Facultad de Ciencias Agropecuarias. Sede Palmira- Escuela de Posgrados. Universidad Nacional de Colombia. Cenicaña. 80 p. [ Links ]
Smith, P. 1996. Post-application persistence of entomopathogenic nematodes. Bio. Sci. Technol. 6:379-387. [ Links ]
Valerio, J. R.; Cardona, C.; Peck, D. C. and Sotelo, G. 2001. Spittlebugs: bioecology, host plant resistance and advances in IPM. In: proceedings of the 19th International Grass Congress, 11-21 February 2001, Sao Pedro, Sao Paulo, Brazil. CENARGEN, EMBRAPA, Brasilia. 217-221 p. [ Links ]
Whittaker, B. J. 1970. Cercopid spittle as a microhabitat. Department of biological sciences. University of Lancaste. Copenhage. OIKOS. 21(1):59-64. [ Links ]
Zimmermann, G. 1986. The Galleria baits method for detection of entomopathogenic fungi in soil. J. Appl. Entomol. 102(1-15):213-215. [ Links ]
Received: February 2019; Accepted: April 2019











 texto em
texto em 


