Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 no.7 Texcoco sep./nov. 2019 Epub 04-Dic-2020
https://doi.org/10.29312/remexca.v10i7.1756
Articles
Tomato-associated phytophagous mites in northern Sinaloa, Mexico
1Posgrado en Ciencias Biológico Agropecuarias-Universidad Autónoma de Nayarit. Carretera Tepic-Compostela km 9, Xalisco, Nayarit, México. CP. 63780. Tel. 687 1819975. (mals-23@hotmail.com).
2Unidad Académica de Agricultura-Universidad Autónoma de Nayarit. Carretera Tepic-Compostela km 9, Xalisco, Nayarit, México. CP. 63780. Tel. 311 1184365. (ricardo-flores-uan@hotmail.com).
3Colegio de Ciencias Agropecuarias-Universidad Autónoma de Sinaloa. Calle 16 y Avenida Japaraqui, Juan José Ríos, Ahome, Sinaloa, México. CP. 81110. Tel. 668 1025140. (nisiordia@gmail.com).
Due to the great importance that Sinaloa has in the production of red tomato in Mexico, the need arose to carry out the present investigation whose objective was to determine the species of phytophagous mites associated with tomato cultivation in the north of Sinaloa and to know their population fluctuation. The work was carried out in the north of the state, in four sampling sites and two production systems (shade mesh and open field). Sampling was carried out on a biweekly basis during the period from December 2015 to May 2016, randomly 10 plants per plot were selected and 30 leaflets per plant, 10 per stratum (upper, middle and lower) were considered as the sampling unit. 1 548 mites in shade mesh and 1 314 in open field were collected, of the total mites collected, 71.5% corresponded to Tetranychus urticae, 13.2% to Polyphagotarsonemus latus and 15.3% to Phytonemus pallidus. The T. urticae species was present during all stages of crop development, its population fluctuation fluctuated according to temperature conditions, with lower presence records during January-February and higher incidence during April-May. The presence of the cyclamen mite, Phytonemus pallidus in the shade mesh production system during the reproductive stage of the crop represents the first record of the presence of this species on the crop in northern Sinaloa.
Keywords: Phytonemus pallidus; Polyphagotarsonemus latus and Tetranychus urticae; shadow mesh
Debido a la gran importancia que tiene Sinaloa en la producción de jitomate rojo en México, surgió la necesidad de realizar la presente investigación cuyo objetivo fue determinar las especies de ácaros fitófagos asociados al cultivo de jitomate en el norte de Sinaloa y conocer su fluctuación poblacional. El trabajo se realizó en el norte del estado, en cuatro sitios de muestreo y dos sistemas de producción (malla sombra y campo abierto). Los muestreos se realizaron en una periodicidad quincenal durante el periodo de diciembre de 2015 a mayo de 2016, aleatoriamente se seleccionaron 10 plantas por parcela y se consideraron 30 foliolos por planta, 10 por estrato (superior, medio e inferior) como unidad de muestreo. Se recolectaron 1 548 ácaros en malla sombra y 1 314 en campo abierto, del total de ácaros recolectados, 71.5% correspondió a Tetranychus urticae, 13.2% a Polyphagotarsonemus latus y 15.3% a Phytonemus pallidus. La especie T. urticae estuvo presente durante todas las etapas de desarrollo del cultivo, su fluctuación poblacional osciló conforme a las condiciones de temperatura, con menores registros de presencia durante enero-febrero y mayor incidencia durante abril-mayo. La presencia del ácaro ciclamen, P. pallidus en sistema de producción malla sombra durante la etapa reproductiva del cultivo representa el primer registro de presencia de esta especie sobre el cultivo en el norte de Sinaloa.
Palabras clave: Phytonemus pallidus; Polyphagotarsonemus latus y Tetranychus urticae; malla sombra
Introduction
The Mexican red tomato Solanum lycopersicum L. (Solanaceae) is the vegetable that generates more currencies in Mexico, with a 21% share of the value of world exports (SAGARPA, 2014). Statistics provided by the Agrifood and Fisheries Information Service (SIAP, 2016), show that in Mexico 51 000 hectares are destined for tomato production, with a production of 2.8 million tons, of which 15 307 were established in Sinaloa , with an annual production of 867 164 tons, which is equivalent to 20% of the volume produced throughout the country: this places Sinaloa as the main producer of export tomatoes.
The 100% of the area of Sinaloa is sown for irrigation and is affected by insects, mites and diseases that reduce crop yields (Sánchez-Peña et al., 2006). In Mexico, farmers face serious phytosanitary problems that cause economic losses, including diseases caused by viruses, fungi and bacteria (Jaramillo et al., 2007), as well as insects and mites (Nuño-Moreno, 2007).
There is a great diversity of pests that affect tomato production such as whitefly (Bemisia argentifolii Bellows & Perring), leaf miner (Liriomyza trifolii Burgess), green peach aphid (Myzus persicae Sulzer), paratrioza (Bactericera cockerelli Sulc), the thrips of flowers (Frankliniella occidentalis Pergande), pinworm (Keiferia lycopersicella Walsingham), the red spider (Tetranychus urticae Koch), and the roasted tomato mite (Aculops lycopersici Massee), among others. T. urticae and A. lycopersici cause severe damage to many crops on the leaves and fruits, so that the injured areas discolor and the edges of the leaves show deformations as a result of the extraction of sap from the tissues that influences the growth, defoliation, discoloration, decrease in production and finally the death of the plant (Fasulo, 2000).
Damage by mites in all types of crops increased progressively, from secondary pests to primary pests in agriculture (Jeppson et al., 1975). Globally, these arthropods are represented 109 families (Zhang, 2011) and 54 617 species, of which 2 625 are present in Mexico, distributed in five orders, representing 4.8% of the world’s wealth (Pérez et al. , 2013), located in the families Eriophyidae, Tetranychidae, Tenuipalpidae and Tarsonemidae, which include species of agricultural importance (Evans, 1992), in the particular case of Tetranychidae, this family is made up of 1 200 species grouped into 70 genera, within of which, the species belonging to Tetranychus are those that produce the greatest losses to agriculture (Zhang, 2003).
In Sinaloa 135 species of mites are registered, a very low number for the enormous quantity that must exist in the different terrestrial and aquatic habitats of the state; but, a systematic study of fauna mite in the entity is lacking, since there are only sporadic and casual data of certain species of medical and veterinary importance (Hoffmann et al., 2000). Therefore, the objective of this study was to determine the mite species associated with tomato cultivation in northern Sinaloa and to know their population fluctuation.
Materials and methods
The study was carried out in four sampling sites: Agricola Los Reyes (25° 78’ 34” north latitude, -108° 88’ 42” west longitude), Agricola San José (25° 81’ 54” north latitude, 108° 75’ 58” west longitude), Agricola Moreno (25° 71’ 23” north latitude, -108° 67’ 39” west longitude) and Agricola Meza (25° 74’ 10” north latitude, -108° 82’ 83” longitude west), located in the Municipalities of Ahome and Guasave, in the north of Sinaloa. For the first two sites, the production system was in the open field, while in the other sites it was in shade mesh.
With a biweekly frequency, the sampling was carried out during the period from December 20, 2015 to May 25, 2016. In each plot diagonal linear transects were made, on which the samples of the mites were taken randomly (Bautista et al., 2004). With the purpose of specifying the location of the species, 10 plants per plot were randomly selected, 10 leaflets of each stratum of the plant (upper, middle and lower) (Rabinovich, 1980).
The leaflets were deposited in 30 x 25 cm polyethylene bags, labeled and placed in a cooler for conservation, they were transferred to the Entomology Laboratory of the Faculty of Agriculture of the Valle del Fuerte, of the Autonomous University of Sinaloa. The collected leaves were checked in a stereoscopic microscope brand LBM Luxeo 4Z. The mites of each sample were deposited in a vial with 70% alcohol with a brush; for assembly, the mites were clarified in lactic acid according to the methodology of Krantz and Walter (2009).
The dried lamellae were deposited in entomological cases and were identified according to the taxonomic keys of Tuttle et al. (1974), Jeppson et al. (1975) and Krantz (1978). The corraboration of species was carried out by Dr. Gabriel Otero Colina, of the Montecillo Campus Graduate College. The adult mite images were taken with a Carl Zeiss Tessovar microscope and subsequently treated with the Gimp software version 2.8.14, in order to make the drawings. The effect of factors such as precipitation and temperatures on the population density of mites was analyzed using Pearson’s correlation coefficient.
Results and discussion
1 548 adult mites were collected under shade mesh conditions and 1 314 mites in open field conditions. Of the total mites collected, 71.5% correspond to the species T. urticae Koch, 15.3% to P. pallidus Banks and 13.2% to P. latus Banks. In the open field, the distribution of specimens in T. urticae for the upper, middle and lower strata was 528, 434 and 96, respectively, while for P. latus it was 282, 253 and 48 collected, respectively, under mesh conditions In the shade the distribution of T. urticae was 584, 348 and 56 specimens, respectively, for P. latus the distribution of specimens per stratum was 3, 81 and 37, respectively, in turn, the P. pallidus distribution was 57, 279 and 103 copies, respectively (Table 1).
Table 1 Mites associated with tomato cultivation in shade mesh and open field production systems in northern Sinaloa, Mexico.
Sampling sites |
Family |
Genus |
Species |
Individuals |
Stratum |
Agricola Meza |
Tetranychidae |
Tetranychus |
urticae |
368 |
upper |
125 |
middle |
||||
37 |
lower |
||||
Tarsonemidae |
Phytonemus |
pallidus |
57 |
upper |
|
279 |
middle |
||||
103 |
lower |
||||
Agricola Los Reyes |
Tetranychidae |
Tetranychus |
urticae |
282 |
upper |
253 |
middle |
||||
48 |
lower |
||||
Tarsonemidae |
Polyphagotarsonemus |
latus |
62 |
upper |
|
104 |
middle |
||||
90 |
lower |
||||
Agricola San Jose |
Tetranychidae |
Tetranychus |
urticae |
246 |
upper |
181 |
middle |
||||
45 |
lower |
||||
Agricola Moreno |
Tetranychidae |
Tetranychus |
urticae |
216 |
upper |
223 |
middle |
||||
19 |
lower |
||||
Tarsonemidae |
Polyphagotarsonemus |
latus |
3 |
upper |
|
81 |
middle |
||||
37 |
lower |
The results of the correlation analysis are shown in Table 2. It was observed that precipitation was only associated with the populations of T. urticae in two sites, Agricola Moreno and Agricola San Jose, while the population density of the observed species is found highly related to temperatures, in coincidence with Solano et al. (2008) in terms of population fluctuation of Brevipalpus spp., which is directly proportional to the increase in temperature, oscillating between 24 and 33 °C favors population development.
Table 2 Correlation coefficient (Pearson) between precipitation and temperatures with population density of T. urticae, P. latus and P. pallidus in northern Sinaloa, Mexico.
Sites |
Species |
Precipitation |
Maximum temperature |
Minimum temperature |
Average temperature |
A. Los Reyes |
T. urticae |
0.13737 |
0.7144 |
0.64055 |
0.70875 |
|
|
0.6871 |
0.0135 |
0.0337 |
0.0146 |
|
P. latus |
0.10315 |
0.87177 |
0.71804 |
0.84535 |
|
|
0.7628 |
0.0005 |
0.0128 |
0.001 |
A. Moreno |
T. urticae |
-0.67077 |
0.82448 |
0.77311 |
0.80933 |
|
|
0.0239 |
0.0018 |
0.0053 |
0.0025 |
|
P. latus |
0.46193 |
0.64427 |
0.63595 |
0.64694 |
|
|
0.1526 |
0.0324 |
0.0354 |
0.0314 |
A. San Jose |
T. urticae |
-0.63687 |
0.93362 |
0.90595 |
0.93099 |
|
|
0.0351 |
<0.0001 |
0.0001 |
<0.0001 |
A. Meza |
T. urticae |
-0.23932 |
0.76043 |
0.78211 |
0.78605 |
|
|
0.4785 |
0.0066 |
0.0044 |
0.0041 |
|
P. pallidus |
-0.18814 |
0.77319 |
0.64699 |
0.74097 |
|
|
0.5796 |
0.0053 |
0.0314 |
0.0091 |
Correlation coefficient (CC) Prob > lRl; *= the variables are correlated (α= 0.05).
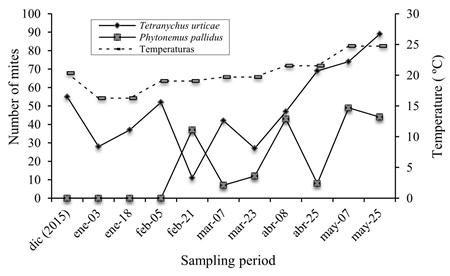
In Agricola Meza, under shade mesh conditions, 530 individuals of T. urticae and 439 of P. pallidus were collected. The presence of T. urticae was recorded in the middle and upper strata of the plants; the highest population densities occurred in April and May, because they coincided with the ideal conditions (Aucejo-Romero, 2005), which caused the leaves to be completely covered with cobweb, because the farmer did not carry out the phytosanitary measures given the low prices of tomato in the market.
The species P. pallidus (Figure 1 and 2), preferred the middle layer of the plant, preferring dark and humid habitats (DGSV-CNRF, 2014) and its fluctuation varied within the crop, with different population peaks with presence during the months from February to May, with favorable temperature conditions (Cloyd, 2010).
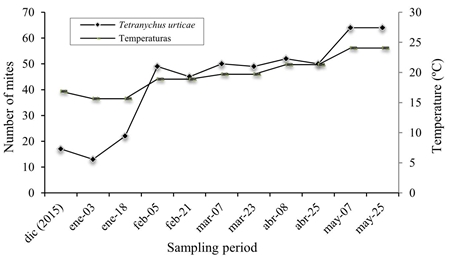
For the open field system at Agricola Los Reyes (Figure 3), a total of 839 mites were collected, of which 583 correspond to T. urticae and 256 to P. latus. The highest populations of T. urticae occurred in May, coinciding with the temperature increases; this species prefers the middle and upper strata of the plant.
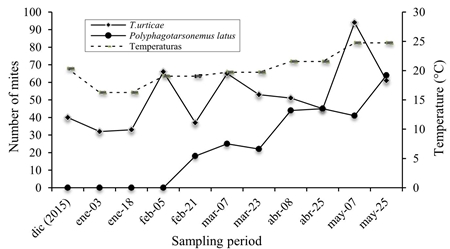
Figure 3 Population fluctuation of T. urticae and P. latus in open field tomatoes in Agricola Los Reyes.
The results obtained indicate that there is a relationship between temperature and mite population, which coincides with Da Silva (2002) and Prasliĉka and Huszar (2004), who argue that hot and dry summers favor the reproduction of mites. The P. latus species was collected in February and increased its population in April and May, where it showed preference for the middle and lower strata of the plants, since they are humid, shady places and these mites feed on the lower surface of the leaf (Peña and Bullock, 1994).
In Agricola San Jose (Figure 4), 475 individuals of T. urticae were collected in tomato, an open-field production system established, whose presence was located in the middle and upper strata of the plant, being the only species found in this site; likewise, its presence was recorded from the first sampling, which maintained its population from February to May and fluctuated according to the high temperatures that ranged from 20 ºC to 30 ºC, favorable for its development and biological cycle, as well as low relative humidity and presence of some predatory insects such as Stethorus sp. (Coleoptera: Coccinallidae), registered in the study.
In Agricola Moreno under conditions of shade mesh, a total of 579 specimens were obtained, of which 458 corresponded to T. urticae and 121 to P. latus (Figure 5). The species T. urticae was presented from the first sampling in December 2015 with low populations during the first months, with the highest population peaks in April and May 2016, this species was located feeding more preferably in the middle and upper parts of the plants. The P. latus species was found in the lower part of the plant in February and raised its populations in April and May, until the end of the crop where it presented the largest number of individuals in the middle part of the plant.

Figure 5 Population fluctuation of T. urticae and P. latus in tomato under shade conditions in Agricola Moreno.
It should be noted that the percentage of phytophagous mites associated with tomato cultivation in northern Sinaloa (Figure 6) is much higher compared to the number of predatory and commensalistic mites, some result that coincides with that obtained indicates that (Oligonychus punicae Tutle) and (Olygonychus perseae Hirst) (Tetranychidae), are the most representative and harmful species of phytophagous mites in the avocado crop (Persea americana Mill, 1768) in Michoacan also depend on the agronomic management of the crop, is decisive in the number of mites present in each batch and in each sampling.
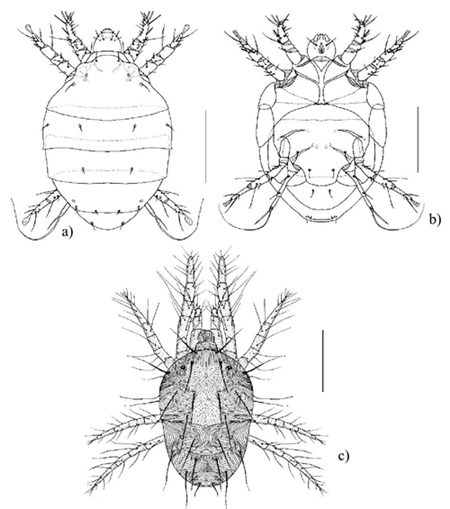
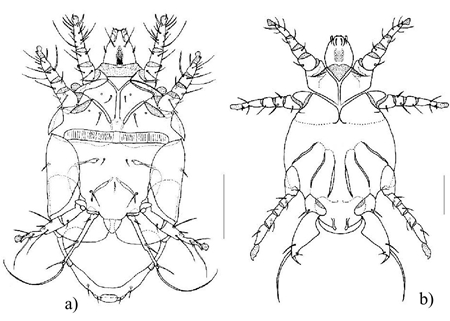
100 µm scale
Figure 6 a) Dorsal view of Polyphagotarsonemus latus male. b) ventral view of female P. latus; and c) dorsal view of male Tetranichus urticae.
Which coincides with the results obtained in this investigation, since the lots that showed greater deficiency in the agronomic management of the tomato presented greater abundance of mites and therefore, greater damage (Chow et al., 2009; Flores et al., 2011). It is important to note that at the end of the crop cycle the species that presented the lowest percentage of population incidence was P. pallidus (84%) and the agricultural ones Meza and Moreno presented intermediate values with (57 and 60%), respectively.
In Agricola Los Reyes and Agricola San José showed the incidence of mites in the open field, from 22 to 37%, in contrast under shade mesh conditions that show the incidence of mites in the two production systems, the presence of higher incidence is presented as the temperatures were favorable for the development of the mite.
Conclusions
T. urticae Koch, P. latus Banks and P. pallidus Banks were determined as the species of phytophagous mites associated with tomato cultivation in the different production systems in northern Sinaloa, which have great potential for impact of the damage on the tender leaves and the fruits. During the development of the crop T. urticae was present during all stages presented its lowest population in January, February and its highest incidence in April and May respectively, which is related to temperature.
The P. latus Banks species was found in tomato cultivation in the flowering and fruiting stages and P. pallidus Banks was present in shade mesh during the reproductive stage of the crop.
Literatura citada
Aucejo-Romero, S. 2005. Manejo Integrado de Tetranychus urticae Koch (Acari: Tetranychidae) en clementinos: agregación, dinámica e influencia del estado nutricional de la planta huésped. Tesis Doctoral, Universidad Politécnica de Valencia. [ Links ]
Bautista, Z.; Delfín, H. G. H. y Palacio-Prieto, J. L. 2004. Técnicas de muestreo para manejadores de recursos naturales. Instituto de Ecología-Instituto de Geografía UNAM-UAY. 507 p. [ Links ]
Cloyd, R. A. 2010. Broad mite and cyclamen mite: management in greenhouses and nurseries. Kansas State University. 4 p. [ Links ]
Chow, A.; Chau, A. and Heinz, K. M. 2009. Reducing fertilization for cut roses: effect on crop productivity and twospotted spider mite abundance, distribution, and management. J. Econ. Entomol. 102(5):1896-1907. [ Links ]
Da Silva, C. 2002. Biología e exigencias térmicas do ácaro-vermelho (Tetranychus ludeni Zacher) em folhas de algodoeiro. Pesquisa Agropecuária Brasileira. 37(5):573-580. [ Links ]
DGSV-CNRF. 2014. Ácaro del ciclamen, Phytonemus pallidus (Banks). Dirección General de Sanidad Vegetal- Centro Nacional de Referencia Fitosanitaria. Ficha técnica. México, DF. 13 p. [ Links ]
Evans, G. O. 1992. Principles of acarology. CAB International. 563 p. [ Links ]
Fasulo, T. R. 2000. Two spotted spider mites. Publication number EENY-150. University of Florida. http://entnemdept.ufl.edu. [ Links ]
Flores, C. R. J.; Mendoza, V. R.; Landeros, F. J.; Cerna, C. E.; Robles, B. A. e Isiordia, A. N. 2011. Caracteres morfológicos y bioquímicos de Rosa x hybrida contra Tetranychus urticae Koch en invernadero. Rev. Mex. Cienc. Agríc. 3:473-482. [ Links ]
Hoffmann, A. y López-Campos, G. 2000. Biodiversidad de los ácaros en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). México, DF. 230 p. [ Links ]
Jaramillo, J.; Rodríguez, V. P.; Guzmán, M.; Zapata, M. y Rengifo, T. 2007. Manual técnico de buenas prácticas agrícolas (BPA) bajo condiciones protegidas en la producción de jitomate. Medellín. Colombia. 331 p. [ Links ]
Jeppson, L. R.; Keifer, H. H. and Baker, H. W. 1975. Mites injurious to economic plants. University of California Press, Berkeley. Los Angeles, USA. 614 p. [ Links ]
Krantz, G. W. 1978. A manual of acarology. Oregon State University Book Stores, Inc. Corvallis, Oregon. 509 p. [ Links ]
Krantz, G. W. and Walter, D. E. 2009. A manual of acarology. Third Edition. Texas Tech University Press. Lubbock, Texas. 807 p. [ Links ]
Nuño-Moreno, R. 2007. Manual de producción de jitomate rojo bajo condiciones de invernadero para el Valle de Mexicali, Baja California. México. 26 p. [ Links ]
Peña, J. E. and Bullock, R. C. 1994. Effects of broad mite, Polyphagotarsonemus latus feeding on vegetative plant growth. Florida Entomologist. 77(1):180-184. [ Links ]
Pérez, T. M.; Guzmán-Cornejo, C.; Montiel-Parra, G.; Paredes-León, R. y Rivas, G. 2013. Biodiversidad de ácaros en México. Departamento de Zoología-Universidad Autónoma de México (UNAM). México, DF Rev. Mex. Biod. 84:399-407. [ Links ]
Praslicka, J. and Huszár, J. 2004. Influence of temperature and host plants on the development and fecundity of the spider mite Tetranychus urticae (Acarina: Tetranychidae). Plant Protection Sci. 40(4):141-144. [ Links ]
Rabinovich, J. E. 1980. Introducción a la ecología de poblaciones de animales. CECSA. México, 313 p. [ Links ]
SAGARPA. 2014. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Comunicado de prensa. http://www.sagarpa.gob.mx/saladeprensa/2014/paginas /2014B615.aspx. [ Links ]
Sánchez-Peña, P. K.; Oyama, J.; Núñez-Farfán, J.; Fornoni, S.; Hernández-Verdugo, J. y Garzón- Tiznado J. A. 2006. Sources of resistance of whitefly (Bemisia spp.) in wild populations of Solanum lycopersicum var. cerasiforme (Dunal) Spooner, G.; Anderson, J. and Jansen, R. K. in Northwestern Mexico. Genetic Resources and Crop Evolution. 53(4):711-719. [ Links ]
SIAP. 2016. Servicio de Información Agroalimentaria y Pesquera. http://www.siap.sagarpa.gob.mx/. [ Links ]
Solano, D. A.; Álvarez, J. G. y Rodríguez, J. A. 2008. Distribución espacial de Brevipalpus phoenicis, vector de la leprosis de los cítricos en el cultivo de naranja valencia (Citrus sinenesis) en Yopal, Casanare (Colombia). Agron. Colomb. 26(3):399-410. [ Links ]
Tuttle, D. M.; Baker, E. W. and Abbatiello, M. 1974. Spider mites from northwestern and north central México (Acarina: Tetranychidae). Smithsonian Contributions to Zoology. 171:1-18. [ Links ]
Zhang, Z. Q. 2003. Mites of greenhouses. Identification, Biology and Control. CABI Publishing. (Eds). 235 p. [ Links ]
Zhang, Z. Q. 2011. Phylum Arthropoda von Siebold, 1848. In: animal biodiversity: an outline of higher-level classification and survey of taxonomic richness, Z. Q. Zhang (Ed.). Zootaxa. 3148(Special issue):99-103. [ Links ]
Received: July 01, 2019; Accepted: September 01, 2019











 texto en
texto en