Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 n.3 Texcoco Apr./May. 2019 Epub Mar 30, 2020
https://doi.org/10.29312/remexca.v10i3.1523
Articles
Rhizobacteria promoting growth and resistance to pathogens in pepper that favor their mycorrhization
1Universidad Autónoma Agraria Antonio Narro-Unidad Regional Laguna. Periférico y carretera a Santa Fe, Torreón, Coahuila, México. CP. 27000. (lleose@yahoo.com.mx; lucio.leos@colpos.mx).
2Colegio de Posgraduados en Ciencias Agrícolas-IRENAT. Carretera México-Texcoco km 35.5, Montecillo, Texcoco, Estado de México. CP. 56230. (juliandm@colpos.mx).
3Área de Suelos-Instituto Tecnológico de Torreón. Carretera Torreón-San Pedro km 7.5, Torreón, Coahuila. CP. 27170.
The success of agricultural production depends on the climate, soil, water and micro-organisms arbuscular mycorrhizal fungi (AMF) and plant growth promoting rhizobacteria (Rpcv). This study consisted in determining the germination of spores of AMF in vitro associated to Rpcv and in greenhouse, evaluation of the growth and damages caused by Phytophthora capsici (P. capsici) in pepper seedlings inoculated with AMF and Rpcv. At 32 days of in vitro germination, it was found that Pseudomona tolasii P-61 and Bacillus pumilus R-44, promoted the germination of Rhizophagus intraradices, Jalapa I and Cedro spores up to 60% while the spore germination without Rpcv was of 11.66%. In the greenhouse phase, the percentage of mycorrhizal colonization was greater in the treatment of AMF+Bpcv with respect to the treatment only with AMF. The height, the thickening of the stem, the dry weight of the plant and the root volume, were greater with the inoculation of AMF+Bpcv compared with the inoculation of AMF and the control without inoculation. In the resistance of plants to the attack of P. capsici, it was found that at 84 ddt the inoculation treatment AMF+Bpcv, presented 4.84% of dead plants, AMF 11.21% and the control 4.24%. (194 words)
Keywords: AMF spores; greenhouse; in vitro; pepper genotypes; rhizobacteria
El éxito de la producción agrícola depende del clima, suelo, agua y microorganismos hongos micorrizicos arbusculares (HMA) y rizobacterias promotoras del crecimiento vegetal (Rpcv). Este estudio consistió en determinar la germinación de esporas de HMA in vitro asociados a Rpcv y en invernadero, evaluación del crecimiento y los daños causados por Phytophthora capsici (P. capsici) en plántulas de chile inoculadas con HMA y Rpcv. A los 32 días de germinación in vitro, se encontró que Pseudomona tolasii P-61 y Bacillus pumilus R-44, promovieron la germinación de esporas de Rhizophagus intraradices, Jalapa I y Cedro hasta 60% mientras que la germinación de esporas sin Rpcv fue de 11.66%. En la fase de invernadero, el porcentaje de colonización micorrízica fue mayor en el tratamiento de HMA+Bpcv con respecto al tratamiento solo con HMA. La altura, el engrosamiento del tallo, el peso seco de planta y el volumen radical, fueron mayores con la inoculación de HMA+Bpcv en comparación con la inoculación de HMA y el testigo sin inoculación. En la resistencia de plantas al ataque de P. capsici, se encontró que a los 84 ddt el tratamiento de inoculación HMA+Bpcv, presentó 4.84% de plantas muertas, HMA 11.21% y el testigo 4.24%.
Palabras clave: esporas de HMA; genotipos de chile; invernadero; in vitro; rizobacterias
Introduction
The genus Capsicum sp. contains native plants of America, of great importance due to the diversity of uses (Cardona et al., 2008). It comprises 25 species and five of them are the most cultivated (Hunziker, 1979). China ranks first in global production of fresh peppers with 54%, followed by Mexico with 6.5% and then Indonesia, Turkey, Spain and the United States of America (FAOSTAT, 2014). In Mexico, the pepper is an important crop, the annual production is 2.3 million tons, in an area of 136 thousand hectares, representing 2.78% of the national total cultivated area (SIAP, 2015).
To increase yields in this crop, it is suggested to improve aspects such as its adaptation to various climatic regions, development of genotypes with higher production, early and tolerant to root diseases and better fruit quality (Ramírez, 1980). Recent technologies aimed at improving the production of pepper in Mexico involve the microorganisms of the rhizosphere, since some improve the growth of the plants. Such is the case of AMF that contribute to the adaptation of the plant to environmental conditions (Dakessian et al., 1986; Franco et al., 2007). In Mexico, there are about 44 species of AMF, equivalent to 22% of the world total. (Chamizo et al., 1998; Varela and Trejo, 2001; Ferrera-Cerrato and Alarcón, 2004).
The AMF, associated with Rpcv, favor the development of horticultural crops such as pepper (Borie et al., 2008; Castillo et al., 2008; Hallett et al., 2008). Among the benefits of AMF is the aggregation of soil via glomalin through fungal mycelium (Castillo et al., 2008), reduction of water stress in plants, control of root phytopathogens and participation in the ecological balance. Biological controllers for some diseases of crop plants have been reported since 1927 (Desai et al., 2002; Bautista-Calles et al., 2010).
There are no convincing results for the biological control of P. capsici, in field conditions (García, 2010; Bautista-Calles et al., 2010). The use of AMF and Rpcv, can be viable alternatives to promote the growth of pepper and control phytopathogens radicals. The objectives of the study were to determine if Rpcv favor the germination of AMF spores in vitro and to verify if the percentage of mycorrhizal colonization is increased by the inoculation effect of Rpcv, with effects on growth and resistance to P. capsici the plants of 23 pepper genotypes.
Materials and methods
Obtaining inocula of AMF
Six inocula of the Soil Microbiology Area-Postgraduate College, identified as Rhizophagus intrarradices, Tabasco-Naranja, Merida-Papaya, Jalapa I, Zac-19 and Cedro, the latter in identification phase, obtained in several ecosystems in the country. As a trap plant, annual ryegrass (Lolium multiflorum) cultivated in sterilized sand was used in an autoclave at 18 lb pulg-2 for three hours twice on a one-day interval.
The propagation lasted for 16 months, performing weekly irrigations with running water and nutrimental irrigation every four months with Long Ahston solution (Hewitt and Smith, 1974). The spores of 100 g of sample were extracted from each of the inocula by the wet decantation method (Gerdemann and Nicolson, 1963). The spores retained in the sieves of 43 and 73 μm of aperture were collected. The spores were kept refrigerated until disinfestation. Then they were transferred to a Petri dish (100 x 15 mm) of glass. They were observed on a stereoscope (Stereo Star AO), and extracted with Pasteur pipette. They were placed on 0.07 mm thick circular filter paper inside a Petri dish.
Disinfestation of AMF spores
The AMF spores were immersed in a 0.3% sodium hypochlorite solution for two min, then two rinses with sterile distilled water. Then, in a 2% chloramine T solution (Loreli et al., 2002), for six minutes and two rinses in sterile distilled water. Finally, they were immersed in a solution of antibiotics (5 μL of penicillin and 5 μL of gentamicin mL-1) for three min and three rinses in sterile distilled water. The spores were refrigerated until they were sown in a Petri dish.
Sowing of AMF spores
It was performed in the laboratory under in vitro conditions, placing the disinfected spores in Petri dishes with 33% agar-water, leaving one cm of separation, depositing 10 spores of each inoculum of AMF per Petri dish.
Germination of AMF spores
Observations were made from day six after the spores were placed on 33% water-agar. Results of the last observation made at 32 DDS are presented. The number of germinated spores was counted, and the germination percentage was calculated for each treatment.
Obtaining strains of Rpcv
The pure strains of Rpcv used were obtained from the Soil Microbiology Area Collection, Postgraduate School. The Pseudomonas tolasii strain (P-61), isolated from soil grown with potato (Solanum tuberosum) cv Rosita, in the Toluca Valley, Mexico, promotes growth, solubilizes phosphates, produces auxins and controls Rhizoctonia. The strain P. tolasii (A-46), isolated in the same region in soil cultivated with potato (Solanum tuberosum) cv Alpha, same characteristics as P-61, also promotes mycorrhization and increases germination. The Bacilus pumilus strain (R-44), isolated from soil cultivated with potato (Solanum tuberosum) cv Rosita in the Toluca Valley, Mexico, with the same characteristics as P-61 and the Paenibacillus sp. (BSP1.1), isolated from agricultural land in the Villa de Allende region, State of Mexico, similar to P-61, A-46 and R-44, in the solubilization of phosphates.
Strains were cultured in nutritious broth with incubation at 26 °C for 18 h under agitation, the bacterial suspension was centrifuged for 0.25 h at 7000 rpm, the pellet of bacteria was resuspended in 20 mL of sterile distilled water and decimal dilutions were made up to 10-5, then 3 μL of the 10-5 dilution was taken with a micropipette and deposited in each of the AMF spores. Petri dishes with AMF spores inoculated with Rpcv were incubated at 28 °C.
Treatments
These were established under a completely random Factorial experimental design with two factors. The factor ‘A’ corresponds to the inoculation in each of the four Rpcv, in addition to a treatment with the mixture of the four Rpcv and another treatment without inoculation. Factor ‘B’ corresponds to each of the AMF inoculants. With five repetitions, generating 160 experimental units.
Vegetal material
Twenty-three pepper genotypes were evaluated. Its description and origin: Pasilla type peppers: (1) variety UAA/Ags, (2) variety Perales 1/Zac, (3) variety Perales 2/Zac; peppers type Ancho: (4) variety UAA/Ags, (5) variety AP Neek/SLP, (6) variety Ancho 3 venas/Zac, (7) variety SR 2009/Dgo; Guajillo peppers: (8) Don Luis variety/SLP, (9) Perales/Zac variety, (10) Mirasol 3 venas variety/Zac, (11) Don Ramón variety/SLP; Puya peppers: (12) variety 91/SLP, (13) variety Saladillo 1/Zac, (14) variety Saladillo 2/Zac, (15) variety Caudillo/Dgo; Serrano peppers: (16) Hybrid Colossus/Tamps, (17) Hybrid HS44/Tamps, (18) Hybrid Centaur/Tamps; jalapeño peppers: (19) variety Don Benito 1/Tamps, (20) variety Don Benito 2/Tamps, (21) variety Don Pancho/Tamps, (22) variety Apache/Chih, (23) variety Isabel/Chih, from agricultural regions in Mexico.
Substrate preparation and sowing
In peat cellars with 200 cavities, peat and perlite (50:50, base V/V) were added. Two seeds per cavity were deposited at 0.5 cm depth.
Inoculation with AMF and Rpcv
The inoculation with AMF was carried out at the time of planting. 10 g of a mixture of the AMFs were mixed in each well. The AMF mixture contained 1024 spores and colonized root segments. The inoculation was carried out with a mixture of the four Rpcv. One mL of bacterial inoculum was placed in each cavity which contained 10-8 cells mL-1.
Transplant and harvest of pepper plants
At 11 dds, the emergence of the seeds began. The transplant was performed with 57 dds. The substrate was agricultural soil (sieved in mesh of 0.7 mm) and perlite (40:60, base V/V). Irrigation was made every five days. At 84 ddt, the aerial part and the root were separated, evaluating percentage of dry matter and root volume.
Inoculation with Phytophthora capsici
At 11 ddt, three grams of soil containing zoospores of P. capsici as a source of secondary inoculum was inoculated. The infested soil was obtained from lots cultivated with pepper in the agricultural region of Puebla, Mexico.
Variables evaluated
Plant height (Ap), stem diameter (Dt), dry weight (Ps), root volume (Vr) and mycorrhization percentage (Pm) were evaluated at 84 ddt. To determine the mycorrhizal colonization, the roots were stained according to the methodology of Phillips and Hayman (1970), Hyphas (H), vesicles (V), spores (E) and arbuscules (A) were counted.
Experimental design
The treatments in the greenhouse phase were established under a completely random Factorial experimental design with two factors. The ‘A’ factor corresponds to the 23 pepper genotypes. The factor ‘B’ corresponds to a witness, AMF and AMF+Rpcv. With six repetitions, obtaining 414 experimental units.
Statistical analysis
The data obtained were analyzed using the statistical package SAS 9.0 Spanish version (SAS, 2002), and Infostat-main components.
Results and discussion
Phase I. In vitro germination of AMF spores
The in vitro germination of the spores of the six inocula of AMF increased with the inoculation of at least one of the four strains of Rpcv at 32 dds (Table 1).
Table 1 In vitro germination of AMF spores inoculated with Rpcv, expressed as a percentage at 32 days after sowing (dds).
| Rhizophagus intraradices | Tabasco- naranja | Mérida- papaya | Jalapa I | Zac- 19 | Cedro | Mean | |
| Control | 10 | 10 | 10 | 20 | 0 | 20 | 11.6 b |
| Bacterium P-61 | 60 | 10 | 30 | 60 | 40 | 60 | 43.3 a |
| Bacterium A-46 | 10 | 20 | 30 | 50 | 10 | 30 | 23.3 ab |
| Bacterium R-44 | 20 | 50 | 40 | 20 | 30 | 50 | 35 ab |
| Bacterium BSP1.1 | 30 | 30 | 30 | 0 | 30 | 40 | 26.6 ab |
| All the Rpcv | 20 | 40 | 20 | 30 | 20 | 30 | 26.6 ab |
| Mean | 25 ab | 26.6 ab | 25 ab | 30 ab | 21.6 b | 38.3 a |
Means in the same column and with the same letter are statistically equal (Tukey, α= 0.05).
A difference was found between the Rpcv strains to promote in vitro germination of the AMF spores, with the Pseudomona tolasii strain P-61 (Table 1) standing out, which improved the germination of the Rhizophagus intraradices spores, Merida-Papaya, Jalapa I and Cedar (600, 300, 300 and 300% respectively).
In the germination of the Cedro spores, the effect of the Rpcv Bacillus pumilus R-44 with 250% increase with respect to the control was also highlighted. Germination is considered one of the most important processes in the life cycle of the AMF, depending on the success of the process of symbiosis with the plant (Rai, 2001; Fernández et al., 2005) almost all species are capable to germinate in the absence of hosts (Giovannetti, 2000). The disinfestation of spores is a very important requirement to achieve success in the formation of mycorrhiza under in vitro conditions (Breuninger and Requena, 2004).
Plant height
In the evaluation at 84 ddt, considering the average of the 23 pepper genotypes, the AMF+Rpcv treatment presented 7.19 cm, surpassing the AMF and control treatments (5.97 and 5.40 cm, respectively). In reference to the means by type of pepper (Pasilla, Ancho, Guajillo, Puya, Serrano and Jalapeño) it was observed that the AMF+Rpcv treatment exceeded the AMF and control treatments in all cases. Only in the types of Puya, Serrano and Jalapeño peppers the AMF treatment exceeded the control.
In 19 of the 23 pepper genotypes, the AMF+Rpcv treatment exceeded the AMF treatment. Only in genotype 7 of the Ancho type, 12 of the Puya type, 17 of the Serrano types and 23 of the Jalapeño type, was it found that the AMF treatment exceeded the AMF+Rpcv treatment (Table 2).
Table 2 Plant height and stem diameter (84 ddt) in 23 pepper genotypes inoculated with AMF and Rpcv.
| Pepper |
Plant height (cm) Control AMF AMF+Rpcv |
Mean |
Stem diameter (mm) Control AMF AMF+Rpcv |
Mean | |||||
| Pasilla | 1 | 6.2 b | 5.87 b | 9.05 a | 7.04 b | 13.65 b | 12.97 b | 15.67 a | 14.1 c |
| 2 | 6.87 b | 5.2 c | 9.55 a | 7.2 a | 13.77 c | 14.65 b | 15.92 a | 14.78 a | |
| 3 | 6.2 b | 5.35 c | 7.17 a | 6.24 c | 14.7 a | 14.85 a | 14.22 a | 14.59 b | |
| Mean | 6.42 b | 5.47 c | 8.59 a | 14.04 c | 14.15 b | 15.27 a | |||
| Ancho | 4 | 6.45 b | 5.65 c | 7.4 a | 6.5 a | 12.72 c | 15.7 a | 13.9 b | 14.1 c |
| 5 | 5.27 b | 5.3 b | 6.5 a | 5.69 c | 13.02 c | 14.45 b | 14.55 a | 14 c | |
| 6 | 5.2 c | 6.7 b | 6.97 a | 6.29 b | 14.32 b | 15.2 a | 14.45 b | 14.65 b | |
| 7 | 6.15 c | 7.07 a | 6.72 b | 6.5 a | 13.25 c | 16.9 a | 14.75 b | 14.96 a | |
| Mean | 5.76 b | 6.18 b | 6.89 a | 13.32 c | 15.56 a | 14.41 b | |||
| Guajillo | 8 | 6.65 c | 7.77 b | 8.69 a | 7.68 a | 14.55 b | 13.55 c | 15.77 a | 14.62 a |
| 9 | 7.15 b | 5.5 c | 8.27 a | 6.97 b | 12.77 b | 12.57 b | 14.52 a | 13.29 d | |
| 10 | 5.85 b | 5.85 b | 7.3 a | 6.33 d | 14.02 b | 12.27 c | 14.52 a | 13.6 c | |
| 11 | 5.35 c | 6.9 b | 8.45 a | 6.9 c | 13.2 c | 14.45 a | 14.42 b | 14.02 b | |
| Mean | 6.25 b | 6.5 b | 8.16 a | 13.62 b | 13.21 b | 14.8 a | |||
| Puya | 12 | 3 c | 6.1 a | 4.62 b | 4.57 d | 13.9 a | 13.37 a | 14.17 a | 13.81 c |
| 13 | 5.27 c | 5.82 b | 7.57 a | 6.22 c | 13.4 c | 15.27 b | 15.6 a | 14.75 a | |
| 14 | 6.1 c | 7.15 b | 7.8 a | 7.01 a | 15 a | 13.32 c | 14.55 b | 14.29 b | |
| 15 | 6.47 b | 5.17 c | 8.55 a | 6.73 b | 15.07 a | 12 c | 13.8 b | 13.62 c | |
| Mean | 5.21 c | 6.06 b | 7.13 a | 14.34 b | 13.49 c | 14.53 a | |||
| Serrano | 16 | 4.72 b | 5.17 b | 6.67 a | 5.52 b | 13.1 b | 12 c | 16.55 a | 13.88 b |
| 17 | 4.3 c | 5.22 a | 5.07 b | 4.96 c | 13.8 c | 14.57 b | 14.7 a | 14.35 a | |
| 18 | 4.55 c | 5.22 b | 6.85 a | 5.64 a | 13.07 b | 14.57 a | 12.45 b | 13.36 b | |
| Mean | 4.52 c | 5.4 b | 6.19 a | 13.32 b | 13.71 b | 15.46 a | |||
| Jalapeño | 19 | 3.9 c | 5.3 b | 5.9 a | 5.03 e | 13.2 b | 16.25 a | 13.65 b | 14.36 d |
| 20 | 4.65 c | 7.2 a | 7.2 a | 6.35 a | 15.55 c | 16.42 b | 17.35 a | 16.44 a | |
| 21 | 4.65 c | 5.25 b | 6.25 a | 5.38 d | 15.55 a | 15.37 a | 16.05 a | 16.65 b | |
| 22 | 4.6 c | 5.35 b | 6.9 a | 5.61 c | 12.05 b | 14.45 b | 15.37 a | 12.95 e | |
| 23 | 4.8 c | 6.72 a | 6.02 b | 5.85 b | 13.9 c | 16.82 a | 15.47 b | 15.4 c | |
| Mean | 4.52 c | 5.96 b | 6.45 a | 14.05 c | 15.26 b | 15.51 a | |||
| General mean | 5.4 b | 5.97 b | 7.19 a | 13.8 b | 14.3 b | 14.88 a | |||
AMF= arbuscular mycorrhizal fungi; AMF+Rpcv= arbuscular mycorrhizal fungi plus plant growth promoting rhizobacteria. Averages in the same row and in the same variable with the same letter are statistically equal; likewise, averages in the same column and for the same type of pepper are statistically equal (Tukey, α= 0.05).
In 14 of the 23 pepper genotypes it was found that the control without inoculation was surpassed by both inoculated treatments (AMF and AMF+Rpcv). These results coincide or differ from Luna et al. (2013) when inoculating pepper plants with Bacillus sp. MA 12, found an increase of 21.31%, with respect to the control at 60 ddt and an average height of the plant of 13.43 cm and 11.07 cm, for the control.
Stem diameter
The AMF+Rpcv treatment exceeded the AMF treatment and the control, in the evaluation at 84 ddt, according to average values for all pepper genotypes (Table 2). Regarding the averages of the types of pepper only in the Ancho, Puya and Jalapeño types, the AMF+Rpcv treatment was superior to the AMF and the control treatment. In 8 of the 23 genotypes, the AMF+Rpcv treatment surpassed the AMF and control treatment. The AMF treatment exceeded the AMF+Rpcv treatment in genotypes 4, 6, 7, 11, 18, 19, 21 and 23. In nine pepper genotypes, the control was surpassed by the AMF and AMF+Rpcv treatments. The AMF+Rpcv treatment with a mean value of 14.88 mm, exceeded the AMF treatment and the control (15.26 and 14.05 mm), the effect of the microorganisms being evident.
Dry weight
In the evaluation at 84 ddt, the AMF+Rpcv treatment (83.98) was superior to the AMF treatment (54.51) and the control (46.78), according to the general average of all the genotypes. Of the 23 pepper genotypes in 19 of them, the AMF+Rpcv treatment surpassed the AMF treatment and in 17 it outperformed both the AMF treatment and the control. The AMF treatment for its part managed to surpass the witness in genotypes 5, 6 and 7 of the Ancho types, 11 of the Guajillo, 12, 13 and 14 of the Puya, 17 of the Serrano, 19, 20, 22 and 23 of the Jalapeño. The control was superior to the AMF treatment in genotypes 1 and 2 of the Pasilla type, 7 of the Ancho, 15 of the Puya, 16 of the Serrano and 21 of the Jalapeño. In all cases, the mean per type of pepper from the AMF+Rpcv treatment exceeded that of the AMF treatment and that of the control (Figure 1).
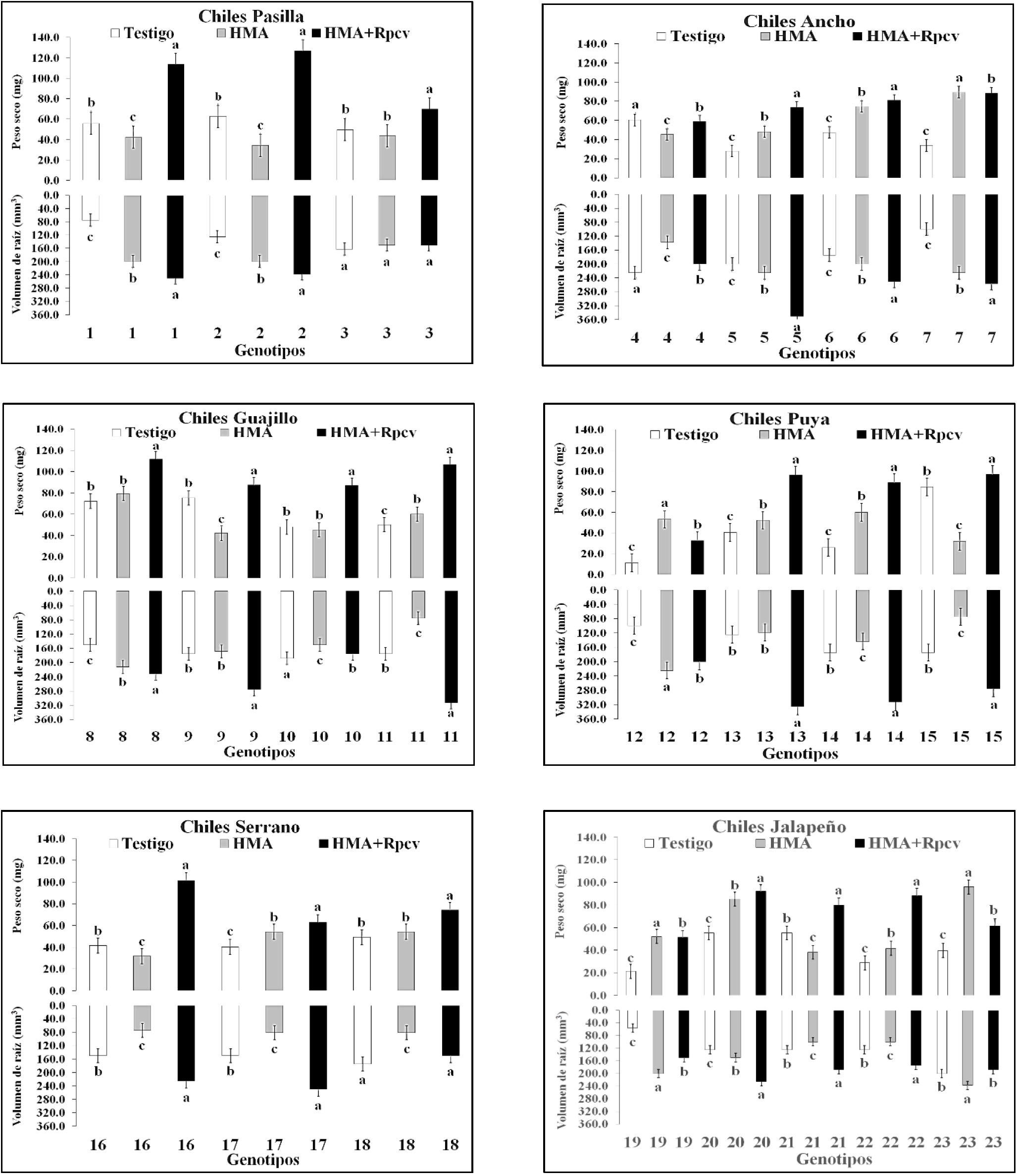
Figure 1 Dry plant weight and root volume (84 ddt) in 23 pepper genotypes inoculated with AMF and Rpcv.
The effect of Rpcv (Pseudomona sp. and Bacillus sp.) Is reflected in the growth of plants as mentioned (Gholami et al., 2009); likewise, Bashan et al. (2004), establish that there is an increase in biomass.
Root volume
In all cases, considering the average of the types of pepper, the AMF+Rpcv treatment exceeded the AMF treatment and the control. The AMF+Rpcv treatment showed superiority in 16 of the 23 pepper genotypes evaluated (Figure 1). The AMF treatment surpassed the control in 10 of the 23 pepper genotypes (1 and 2 of the Pasilla type, 5, 6 and 7 of the Ancho, 8 of the Guajillo and 20 of the Jalapeño). According to the means by type of pepper, the AMF+Rpcv treatment surpassed the AMF treatment and the control in all cases (Figure 1).
Strength indices
The principal components analysis (PCA) indicated that with component 1, it explained 84% of the total variance (Castañon-Najera et al., 2008). The ACP with the quantitative values of the 23 genotypes of pepper indicated that the eigenvectors of plant height, stem diameter, dry weight and root volume contributed 42, 39, 41 and 41% to Component 1 (Table 3).
Table 3 Vigor indexes (Iv) in 23 pepper genotypes inoculated with AMF and Rpcv.
| Pepper | Genotype | Control | AMF | AMF+Rpcv |
| Pasilla | 1 | -0.92 | -0.62 | 0.58 |
| 2 | 0.18 | -0.76 | 1.37 | |
| 3 | -0.49 | -1.74 | 0.44 | |
| Ancho | 4 | -0.39 | 0.29 | -0.2 |
| 5 | -0.29 | -0.56 | 0.11 | |
| 6 | -0.87 | -1.98 | 0.93 | |
| 7 | -0.17 | -0.52 | 0.11 | |
| Guajillo | 8 | -0.56 | -0.74 | 0.47 |
| 9 | -0.81 | -0.42 | 1.11 | |
| 10 | -0.2 | -0.42 | -0.64 | |
| 11 | -1.19 | -1.51 | 1.16 | |
| Puya | 12 | -2.37 | -0.22 | -0.82 |
| 13 | -2.13 | -0.58 | 0.44 | |
| 14 | -1.11 | -0.39 | 0.25 | |
| 15 | -0.89 | -1.53 | -0.86 | |
| Serrano | 16 | -1.28 | -2.54 | 0.23 |
| 17 | -1.63 | -0.74 | 0.54 | |
| 18 | -1.31 | -2.62 | -0.8 | |
| Jalapeño | 19 | -1.69 | -1.52 | -0.12 |
| 20 | -1.76 | 0.62 | 0.73 | |
| 21 | -2.68 | -0.83 | -0.22 | |
| 22 | -1.82 | -0.99 | -0.13 | |
| 23 | -2.18 | 0.21 | -1.02 |
AMF= arbuscular mycorrhizal fungi; Rpcv= Plant growth promoting rhizobacteria.
The characteristic values of this component were positive in their entirety for the AMF+Rpcv Treatment, but not for the AMF treatment and the control, which were negative as established Kaiser (1960); Castañon-Najera et al. (2008). the above, the additive effects of the AMF+Rpcv coinoculation are checked, compared with the inoculation of the AMF alone, as reported by Kaiser (1960); Bashan (1998).
Percentage of mycorrhizal colonization
The percentage of mycorrhizal colonization increased 79.07%. In 21 of the 23 pepper genotypes, the AMF+Rpcv treatment exceeded the AMF treatment. Only in two cases did the AMF treatment outperform the AMF Rpcv treatment in genotype 16 of the Serrano type and genotype 20 of the Jalapeño type (Table 4). In all cases, considering the average of the types of pepper, the AMF+Rpcv treatment exceeded the AMF treatment in the percentage of mycorrhization.
Table 4 Mycorrhizal colonization in 23 pepper genotypes inoculated with AMF and Rpcv under greenhouse conditions.
| Pepper | Genotype | AMF | AMF+AMF+Rpcv | Increase (%) |
| Pasilla | 1 | 61.9 | 63.64 | 2.8 |
| 2 | 36.84 | 107.14 | 190.82 | |
| 3 | 32.31 | 73.56 | 127.7 | |
| Mean | 43.68 | 81.45 | 86.44 | |
| Ancho | 4 | 31.94 | 98.33 | 207.83 |
| 5 | 40 | 71.08 | 77.71 | |
| 6 | 46.67 | 56.06 | 20.13 | |
| 7 | 24.64 | 70.59 | 186.51 | |
| Mean | 35.81 | 74.02 | 106.68 | |
| Guajillo | 8 | 21.84 | 47.13 | 215.79 |
| 9 | 28.79 | 31.75 | 10.28 | |
| 10 | 27.27 | 57.58 | 111.11 | |
| 11 | 25 | 75.38 | 201.54 | |
| Mean | 25.72 | 52.96 | 105.86 | |
| Puya | 12 | 29.51 | 43.28 | 46.68 |
| 13 | 21.21 | 70.77 | 233.63 | |
| 14 | 52.63 | 51.35 | -2.43 | |
| 15 | 25.42 | 77.05 | 203.06 | |
| Mean | 32.19 | 60.61 | 88.28 | |
| Serrano | 16 | 22.81 | 58.21 | 155.22 |
| 17 | 42.62 | 68.18 | 59.97 | |
| 18 | 59.57 | 57.35 | 96.27 | |
| Mean | 41.67 | 61.25 | 46.99 | |
| Jalapeño | 19 | 24.29 | 42.03 | 73.06 |
| 20 | 36.14 | 83.75 | 131.71 | |
| 21 | 18.46 | 53.23 | 188.31 | |
| 22 | 66.15 | 41.94 | -36.59 | |
| 23 | 36.92 | 56.36 | 52.61 | |
| Mean | 36.39 | 55.46 | 52.39 | |
| General mean | 35.35 | 63.29 | 79.07 |
AMF= arbuscular mycorrhizal fungi; Rpcv= Plant growth promoting rhizobacteria.
In two cases (Serrano peppers and Jalapeño peppers) the AMF+Rpcv treatment exceeded the AMF treatment in a percentage of mycorrhization less than 100 percent with 46.99 and 52.39%. Likewise, for the Ancho and Guajillo peppers, the AMF+Rpcv treatment exceeded the AMF treatment by more than 100% with 106.68 and 105.86% (Table 4).
The witness did not present mycorrhizal colonization. The previous results agree with the authors (Kaiser, 1960; Bashan, 1998; Rai, 2001), who report increases in the mycorrhization of plants due to the effect of co-inoculation with Rpcv.
Dead plants by P. capsici attack
The trend in number of dead plants was similar in all sampling dates (31, 59, 84 ddt). At 84 ddt, the control showed the highest number of plants killed by P. capsici, equivalent to 47%, followed by the AMF treatment with 37% and 16% for the AMF+Rpcv treatment. The Pasilla, Ancho and Serrano peppers of the AMF+Rpcv treatment presented the least amount of dead plants compared to the control. The joint inoculation of the microorganisms (AMF and Rcpv) managed to counteract the damage of P. capsici, greatly reducing the attack of the phytopathogen in the plant.
The Pasillas pepper showed higher resistance 19.39%, followed by the Guajillos 21.9%, the Ancho 26.47%, the Puyas 32.04%, the Jalapeños 36%, and the Serranos 46.67%, the latter being the most susceptible to the damage of phytopathogens (Table 5).
Table 5 Percentage of dead plants per attack of P. capsici, in the types of pepper and study treatments.
| Pepper | Treatments | 31 ddt (%) | 59 ddt (%) | 84 ddt (%) |
| Pasilla | Control | 1 | 1 | 2 |
| AMF | 1.74 | 2.61 | 4.35 | |
| AMF+Rpcv | 0 | 0 | 0.87 | |
| Ancho | Control | 3 | 3 | 5 |
| AMF | 3.48 | 3.48 | 6.09 | |
| AMF+Rpcv | 1.74 | 1.74 | 2.61 | |
| Guajillo | Control | 4 | 4 | 5 |
| AMF | 0.87 | 0.87 | 3.48 | |
| AMF+Rpcv | 0 | 1.74 | 1.74 | |
| Puya | Control | 5 | 7 | 11 |
| AMF | 4.35 | 4.35 | 3.48 | |
| AMF+Rpcv | 0.87 | 0.87 | 2.61 | |
| Serrano | Control | 3 | 4 | 7 |
| AMF | 3.48 | 5.22 | 9.57 | |
| AMF+Rpcv | 0.87 | 0.87 | 2.61 | |
| Jalapeño | Control | 10 | 12 | 17 |
| AMF | 3.48 | 3.48 | 5.22 | |
| AMF+Rpcv | 2.61 | 3.48 | 3.48 |
AMF= arbuscular mycorrhizal fungi; AMF+Rpcv= arbuscular mycorrhizal fungi plus plant growth promoting rhizobacteria; P. capsici= Phytophthora capsici; ddt= days after transplant.
The protection of plants against phytopathogenic diseases (Loreli et al., 2002; SIAP, 2015) can occur through AMF and Rpcv, natural enemies of Phytophthora, Fusarium, Rhizoctonia and others (Rillig, 2007).
The obtained results demonstrate the response of the Rpcv (Pseudomona tolasii P-61 and Bacillus pumilus R-44) in the in vitro germination of AMF spores (Rhizophagus intraradices, Merida-Papaya, Jalapa I and Cedro). In addition, the combined effect of AMF and higher Rpcv with respect to AMF and control in plant height, stem diameter, dry weight, root volume and percentage of mycorrhizal colonization. These microorganisms are known as plant growth promoters (Kloepper and Schroth, 1978), but with this study the foundations are laid for the development of a coinoculation technology (AMF and Rpcv) to improve the production of Mexican pepper genotypes.
Conclusions
The AMFs associated with the Rpcv, achieved significant increases in plant height, stem diameter, dry weight of the plant and root volume and decreased damage caused P. capsici.
The variables height of plant, diameter of stem, dry weight and volume of root kept a positive correlation, where in the measure that one showed effect the rest did it also.
The Pasilla peppers presented greater resistance to the attack of P. capsici and Jalapeño and Serrano were the ones with the least resistance.
Acknowledgments
To the National Council of Science and Technology (CONACYT), Postgraduate College-Campus Montecillo and the Agraria Antonio Narro Autonomous University-Laguna Regional Unit for the funding granted.
REFERENCES
Bashan, Y. 1998. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 16:729-770. [ Links ]
Bashan, Y.; Holguin, G. and De-Bashan, L. E. 2004. Azospirillum-plant relationships: agricultural, physiological, molecular and environmental advances. Canadian J. Microbiol. 50:521-577. [ Links ]
Bautista-Calles, J.; García-Espinosa, R.; Zavaleta-Mejía, E.; Pérez-Moreno, J.; Montes-Belmont, R.; Ferrera-Cerrato, R. y Huerta-Lara, M. 2010. Disminución de la marchitez del chile (Phytophthora capsici Leo) con complejidad ascendente de antagonistas en el sustrato de germinación del chile (Capsicum annuum L.). Inverciencia. 35(8):613-618. [ Links ]
Cardona, G.; Peña-Venegas, C. P. and Arcos, A. 2008. Occurrence of arbuscular micorrhizae fungi in red pepper (Capsicum sp.) in the Amazonian region of Colombia. Agron. Colomb. 26(3):459-470. [ Links ]
Castañón-Nájera, G.; Latournerie-Moreno, L.; Mendoza-Elos, M.; Vargas-López, A. y Cárdenas-Morales, H. 2008. Colección y caracterización de chile (Capsicum spp.) en Tabasco, México. Rev. Inter. Bot. Exp. 77:189-202. [ Links ]
Castillo, C.; Aztroza, I.; Borie, F. y Rubio, R. 2008. Efecto de cultivos hospederos y no hospederos sobre propágulos micorrízicos arbusculares. R. C. Suelo Nutrición Vegetal. 8:37-54. [ Links ]
Castillo, C.; Rubio, R.; Borie, F. and Sieverding, E. 2010. Diversity of arbuscular mycorrhizal fungi in horticultural production systems of Southern Chile. J. Soil Sci. Plant Nutr. 10:407-413. [ Links ]
Chamizo, A.; Ferrera-Cerrato, R. y Varela, L. 1998. Identificación de especies de un consorcio del género Glomus. Rev. Mex. Micol. 14:37-40. [ Links ]
Dakessian, M. S.; Brown, M. S. and Bathlen, G. J. 1986. Relationship of mycorrhizal growth enhancement and plant grwth with soil water and texture. Plant soil. 94:439-443. [ Links ]
Desai, S.; Reddy, M.S. and Kloepper, J. W. 2002. Comprehensive Testing of Biocontrol Agents. In: Gnanamanickam, S. S. (Ed.). Biological control of crop diseases. Dekker. Nueva York, NY, EE. UU. 387-420. [ Links ]
FAOSTAT. 2014. Food and Agriculture Organization of the United Nations Statistics. http://faostat3.fao.org/browse/Q/QC/S/2013. [ Links ]
Ferrera-Cerrato, R. y Alarcón, A. 2004. Biotecnología de los hongos micorrízicos arbusculares. In: Memoria Simposio de Biofertilización. Díaz, F. A.; Mayek, P. M.; Mendoza, A. y Maldonado, M. N. (Eds.). Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP)-Campo Experimental Río Bravo. Centro de Biotecnología Genómica- Instituto Politécnico Nacional (IPN). Río Bravo, Tamaulipas, México. 1-9 pp. [ Links ]
Franco-Ramírez, A.; Ferrera-Cerrato, R.; Varela-Fregoso, L.; Pérez-Moreno, J. and Alarcón, A. 2007. Arbuscular mycorrhizal fungi in chronically petroleum contaminated soils in Mexico and the effects of petroleum hydrocarbon son spore germination. J. Basic Microbiol. 47:378-383. [ Links ]
García, E. R. 2010. Agroecología y enfermedades de la raíz en los cultivos agrícolas. 1ª (Ed.). Colegio de Posgraduados. Montecillo, Texcoco, Estado de México. 130 p. [ Links ]
Gerdemann, J. W. and Nicolson, T. H. 1963. Spore of mycorrhizae endogen species extracted from soil by wet sieving and decanting. Transactions British Mycol. Soc. 46:235-244. [ Links ]
Gholami, A.; Shahasavani, S. and Nizarat, S. 2009. The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. World Academy Sci. Eng. Technol. 49:10-24. [ Links ]
Giovannetti, M. 2000. Spore germination and pre-symbiotic growth. In: Kapulnik, Y. and Douds, D. D. (Eds.). Arbuscular mycorrhizas: physiology and function. Klumer, Dordrecht. 47-67 pp. [ Links ]
Hunziker, A. T. 1979. South American Solanaceae: a synoptic survey. In: Hawkes, J. G.; Lester, R. N. and Skelding, A. D. (Eds.). The biology and taxonomy of the solanaceae. Linnean Society Symposium. 7:49-85. [ Links ]
Kaiser, H. F. 1960. The application of electronic computers to factor analysis. Educational and Phsychological Measurement. 20:141-151. [ Links ]
Kloepper, J. W. and Schroth, M. N. 1978. Plant growth-promoting rhizobacteria on radishes. In: Proc. of the 4th. International Conference on Plant Pathogenic Bacteria. Clarey, G. (Ed.). Station de pathologie vegetale etphyto-bacteriologie. Angers, France. 879-882. [ Links ]
Lorelí, M.; Ortega, E.; Rodés, R. y Fernández, F. 2002. Métodos efectivos para la desinfección total de esporas de hongos micorrizógenos arbusculares (HMA): aislamiento y caracterización de Bacterias endospóricas en Glomus clarum. Cultivos Tropicales. 23(1):21-24. [ Links ]
Rai, M. K. 2001. Current advances in mycorrhization in micropropagation. In vitro Cell. Dev. Biol. Plant. 37:158-167. [ Links ]
Ramírez, V. J. y Cova, S.R. 1980. Supervivencia de Phytophthora capsici Leo., agente causal de la marchitez del chile. Agrociencia. 39:9-12. [ Links ]
Rillig, M. 2007. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 84:355-363. [ Links ]
SAS. 2002. Institute Inc., Cary, NC, USA. Version 9.0. [ Links ]
SIAP. 2015. Servicio de Información Agroalimentaria y Pesquera. http://www.siap.gob.mx /agricultura-produccion-anual/2014. [ Links ]
Varela, L. y Trejo, D. 2001. Los hongos micorrizógenos arbusculares como componentes de la biodiversidad del suelo en México. Acta Zoológica Mexicana (nueva serie). 1:39-51. [ Links ]
Received: March 01, 2019; Accepted: May 01, 2019











 text in
text in 


