Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 no.2 Texcoco Fev./Mar. 2019
https://doi.org/10.29312/remexca.v10i2.1603
Articles
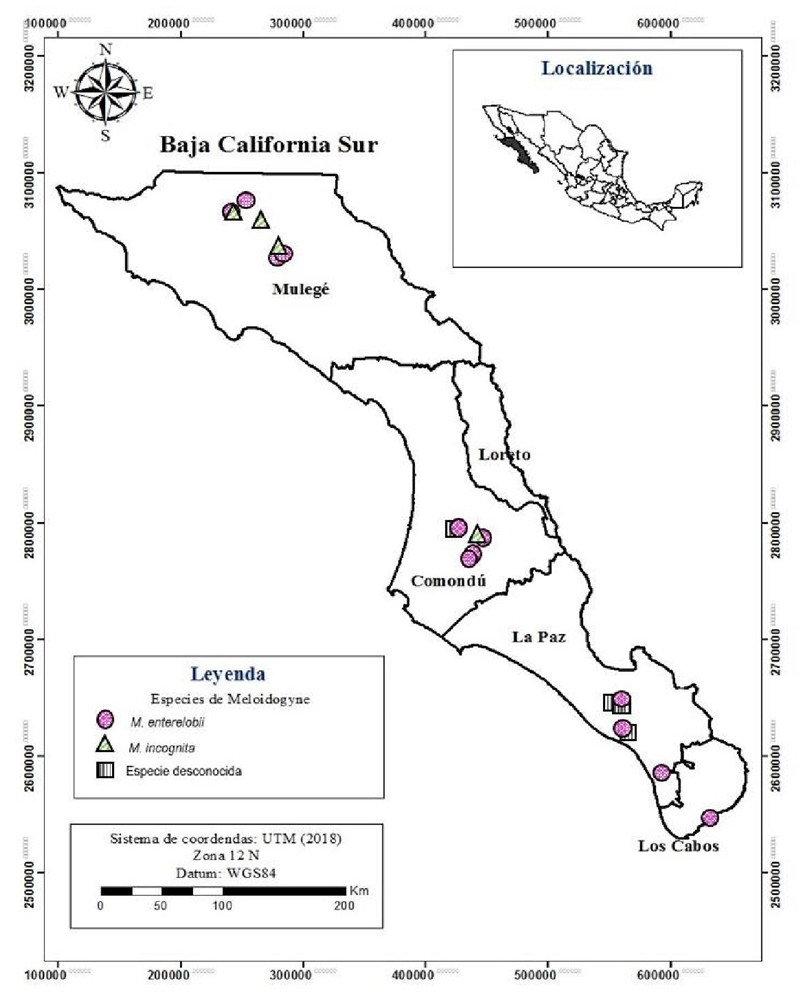
Identification and distribution of Meloidogyne species in Baja California Sur, Mexico
1Universidad Autónoma de Baja California Sur. Carretera al sur km 5.5, Col. El Mezquitito, La Paz, Baja California Sur. Tel. 01(612) 1238800, ext. 5155. CP. 23080. (manletmacias@outlook.com; mrojas@uabcs.mx; saulhr@gmail.com; jduarte@uabcs.mx).
2Centro de Investigación en Alimentación y Desarrollo, AC. Carretera Culiacán-Eldorado km 5.5, Culiacán, Sinaloa. Tel. 01 (667) 7605536. CP. 80110. (acarrillo@ciad.mx).
Agriculture in Baja California Sur presents a serious problem due to the damage caused by the root-knot nematode Meloidogyne spp. The incidence of damage caused by this type of pathogen is high, however, its distribution and the species present in the entity are not currently known. Taking into account that this information is key in the strategies for the management of said phytonematode, the objective of the present study was to identify by morphological characters and molecular methods, the Meloidogyne species present in the sampled crops, as well as their distribution in the state. During the 2017-2018 agricultural cycle, soil and plant roots were sampled with wilt and yellowing symptoms in the different crops of four municipalities of the state. From 50 collected roots, 50 females of Meloidogyne spp. of the sampled crops, which were identified by morphological characterization of perineal patterns. Subsequently, genomic DNA was extracted from these females and specific genes reported in the literature for different species were amplified. The results obtained show that the identified species correspond to Meloidogyne incognita and Meloidogyne enterelobii, where the incidence of the populations was 30% and 70% respectively. Of the two-species identified, M. enterelobii was the one that predominated in the agricultural areas of Baja California Sur. While M. incognita was only found in two municipalities. This information will be useful in the effective implementation of control measures against the different Meloidogyne species in the State.
Keywords: Meloidogyne spp.; root knot nematode; vegetables
La agricultura en Baja California Sur presenta un grave problema debido al daño provocado por el nematodo agallador Meloidogyne spp. La incidencia de daños provocados por este tipo de patógenos es alta, sin embargo, actualmente no se conoce su distribución ni las especies presentes en la entidad. Tomando en cuenta que esta información es clave en las estrategias para el manejo de dicho fitonemátodo, el objetivo del presente estudio fue identificar mediante caracteres morfológicos y métodos moleculares, las especies de Meloidogyne presentes en los cultivos muestreados, asi como su distribución en el estado. Durante el ciclo agrícola 2017-2018, se realizaron muestreos de suelo y raíces de plantas con síntoma de marchitez y amarillamiento en los diferentes cultivos de cuatro municipios de la entidad. A partir de raíces colectadas, se extrajeron 50 hembras de Meloidogyne spp. de los cultivos muestreados, las cuales, se identificaron mediante caracterización morfológica de patrones perineales. Posteriormente se extrajo ADN genómico de dichas hembras y se amplificaron genes específicos reportados en la bibliografía para diferentes especies. Los resultados obtenidos muestran que las especies identificadas corresponden a Meloidogyne incognita y Meloidogyne enterelobii, donde la incidencia de las poblaciones fue 30% y 70% respectivamente. De las dos especies identificadas M. enterelobii fue la que predominó en las áreas agrícolas de Baja California Sur. Mientras que M. incognita solo se encontró en dos municipios. Esta información será útil en la implementación eficaz de medidas de control contra las diferentes especies de Meloidogyne en el Estado.
Palabras clave: Meloidogyne spp.; hortalizas; nematodo agallador
Introduction
In Baja California Sur, agriculture represents one of the most important economic activities, which is carried out under organic or conventional production systems. Currently, there are 44 167 ha of cultivation, with more than 50 types of horticultural products and more than 12 species of fruit trees distributed throughout the entire entity, considered of high strategic priority, given its high competitiveness and its socioeconomic importance (SAGARPA, 2017). Among the main phytopathological problems, is the root-knot nematode (Meloidogyne spp.).
Its main symptom in the plants, is the generation of numerous galls in the roots, preventing the absorption and transport of water and nutrients to the foliage, which results in yellowing of leaves, growth retardation and later the death of the plant, generating production losses of 20-50% (Zeng et al., 2017). Currently, the application of chemical products and the use of resistant varieties are the most common strategies for the control of this pathogen (Beira et al., 2016).
However, they have not shown the expected efficacy. The main species reported worldwide are M. incognita, M. arenaria, M. hapla and M. javanica (Kaur and Attri, 2013). Although, new species related to this genus continually appear, where their severity is greater, since they possess the capacity to break the resistance of the crops used and react differently to the chemical groups used in the field, which hinders the success of the control methods.
The precise identification of pathogenic microorganisms and the use of distribution maps of the species are one of the useful tools in the design of programs for the implementation of effective management strategies (Nicol et al., 2011), which avoids the application indiscriminate agrochemicals, which promote the generation of resistant species, as well as environmental pollution and the elimination of beneficial fauna and flora of the soil (Kloepper et al., 1991).
Currently, in different states of Mexico, several studies have been carried out to identify Meloidogyne species. However, in Baja California Sur, there is no record of the species associated with the crops, nor their location in the different areas of production, which would be of great importance for the agricultural sector. Therefore, the objective of the present study was to identify, through molecular and morphological methods, the Meloidogyne species, associated with crops of economic importance. As well as its distribution in the main agricultural areas of the state.
Materials and methods
Study area
This study was carried out during the 2017-2018 agricultural cycle, in the phytopathology laboratory of the Academic Department of Agronomy, of the Autonomous University of Baja California Sur (UABCS), located at the geographic coordinates 24° 06’ 03’’ North latitude 110° 18’ 54’’ West longitude, in La Paz, Baja California Sur.
Sampling in the field
A total of 16 crops were sampled in the four municipalities of major agricultural importance in Baja California Sur: Mulege (26° 53’ 30’’ North latitude 111° 58’ 51” West longitude), Comondu (25° 30’ North latitude 112° 00’ West longitude), La Paz (24° 08’ 32’’ North latitude 110° 18’ 39” West longitude) and Los Cabos (22° 52’ 52’’ North latitude 109° 54’ 49” West longitude). In each field, 10 samples of plants were obtained at random, directed only at those that showed symptoms of yellowing, leaf wilt and reduction in growth. In addition, 10 soil subsamples of the rhizosphere, obtained at a depth of 30 cm with the help of a shovel, using the zig-zag method proposed by Shurtleff and Averre III (2000).
The subsamples were homogenized to form a single sample (2 kg), were placed in plastic bags and then taken to the laboratory for the corresponding analysis. The geographic coordinates of the different places were determined by means of a global positioning device (navigator, Etrex, Garmin®) and the respective field information was taken (cultivation, phenological stage and type of management).
Frequency of occurrence, incidence and gall index in the sampled fields
In each area sampled, the frequency of occurrence, incidence and gall index caused by the root-knot nematode was determined. The frequency of occurrence was obtained by the prevalence of Meloidogyne when calculating the number of fields with root-knot nematodes/total number of fields evaluated. Likewise, the incidence percentage of the disease was determined by obtaining the number of plants with galling/total number of plants evaluated. The galling index (IG) was evaluated according to the scale proposed by Taylor and Sasser (1978), where: 0= 0 galls, 1= 1-2 galls, 2= 3-10 galls, 3= 11-30 galls, 4= 31-100 galls and 5= more than 100 galls.
Extraction of individuals for the determination of populations in soil and root
The extraction of males and juveniles was carried out using the Baerman sieve-funnel technique as described by Southey (1986). 100 g of soil were obtained from each original sample previously homogenized and diluted in 200 ml of distilled water. Subsequently, the suspension was passed; through, sieves (brand Fiicsa) of 200 and 325 mesh. From the latter, the suspension was recovered and emptied into a funnel with the help of a pizeta. The determination of juveniles in roots, was done by the method of maceration and filtering (Hooper et al., 2005), where 25 g of roots were diluted in 200 ml of water, and liquefied for 30 s.
The suspension was decanted through 200 and 325 mesh sieves. With the help of a pizeta, the nematodes retained in the 325 sieve were transferred to a funnel. After 48 h of rest, 20 ml of the solution was extracted, from which a 1 ml aliquot was taken and deposited on a watch glass. Under a compound microscope (Labomed model Lx 400), 5 counts of each sample were made to obtain the average value of phytoparasitic nematodes.
Pathogenicity test
After the extraction method, the pathogenicity of each population was determined, tomato seeds cv ‘Río Grande’ were planted in plastic trays containing terralite® commercial substrate. When presenting the fourth true leaf, 30 seedlings were transplanted into 4 kg plastic pots, which contained soil from each sampling site. The control group contained sterilized soil. The plants were placed in a shaded mesh house (40% shade), under an average temperature of 18-30 °C and relative humidity 40-80%.
Two irrigations per week were carried out, and one fertilization per month with Triple 17® (17-17-17 nitrogen, phosphorus and potassium, respectively). Three months later, all the roots were examined to determine the presence of galls and confirm the pathogenicity of Meloidogyne spp.
Morphological identification of Meloidogyne spp.
The identification of Meloidogyne species collected in each locality was carried out by comparing perineal patterns (Eisenback and Hirschmann, 1981). 50 mature globose females were randomly extracted from roots with galls and subjected to dissection of the back of their body with the aid of a fine knife. Subsequently, mounts were prepared on slides and examined under a compound microscope at 45X (Carl Zeiss). The species were identified based on the distribution and shape of the longitudinal and transverse grooves of the perineal region and were compared with the characteristics of perineal patterns proposed by Taylor and Sasser (1983); Yang and Eisenback (1983).
Molecular identification of Meloidogyne spp.
The molecular identification of each of the populations of Meloidogyne, was performed through the method of genomic DNA extraction of 50 adult females, which were obtained with a dissection needle and were deposited in a 1.5 ml microcentrifuge tube, subsequently, an aliquot of 45 μl of lysis buffer (50 mM NaOH) was added, it was subjected to heat lysis at 95 °C for 10 min, an aliquot of 45 μl of Tris-HCl (pH 8) was added and centrifuged for 3 min at 10 000 rpm (Hu et al., 2011), the supernatant was recovered, to proceed with the PCR. The primers used for the analysis of the genomic DNA of the nematode species are listed in Table 1.
Table 1 Primers used for the molecular identification of Meloidogyne species.
| Species | Code of primers | Sequence of the primers 5" to 3" | Fragment (pb) | Specificity and source |
| Meloidogyne spp. | MF | GGGGATGTTTGAGGCAGATTTG | 500 | 28S rRNA Nunn (1992) |
| MR | AACCGCTTCGGACTTCCACCAG | |||
| Meloidogyne incognita | Mi-F | GTGAGGATTCAGCTCCCCAG | 900 | (SCAR), Meng et al. (2004) |
| Mi-R | ACGAGGAACATACTTCTCCGTCC | |||
| Meloidogyne javanica | Fjav | GGTGCGCGATTGAACTGAGC | 700 | (SCAR) Zijlstra et al.(2000) |
| Rjav | CAGGCCCTTCAGTGGAACTATAC | |||
| Meloidogyne enterelobii | Me-F | AACTTTTGTGAAAGTGCCGCTG | 256 | (SCAR), Long et al. (2006) |
| Me-R | TCAGTTCAGGCAGGATCAACC | |||
| Meloidogyne arenaria | Ma-F | TCGAGGGCATCTAATAAAGG | 950 | (SCAR), Dong et al. (2001) |
| Ma-R | GGGCTGAATATTCAAAGGAA | |||
| Meloidogyne hapla | Mha-F | GGCTGAGCATAGTAGATGATGTT | 1 500 | (SCAR), Dong et al. (2001) |
| Mha-R | ACCCATTAAAGAGGAGTTTTGC |
All PCR amplifications were developed in a total of 25 μl containing 5 μl of 5x dropq buffer, 3 μl of MgCl2 (25 mM), 0.2 μl of Taq polymerase, 0.5μl of dNTP, 1 μl of each primer, 2 μl of genomic DNA and supplemented with sterile nanopure water. The amplification of the DNA was carried out in a thermal cycler (BIO-RAD T100), under the following amplification conditions: 94 °C for 2 min, 35 cycles of 94 °C for 30 s, 64 °C for 30 s, 68 °C for 1 min, followed by a final extension at 72 °C for 5 min. The PCR products were separated by electrophoresis in Tris-EDTA (TAE) buffer on a 2% agarose gel, stained with gel network at 60 V for 60 min and then visualized under UV light.
Results and discussion
Frequency of occurrence and incidence
16 crops in the production stage were evaluated in four of the five municipalities of the state of Baja California Sur. 13 of these were vegetables, such as tomato saladette, bola, grape, cherry, bell pepper, California pepper, beet, chard and pumpkin. Two of aromatic type (basil) and one fruity (fig tree). 12 of the 16 crops were produced under a conventional system, while the remaining four belonged to an organic production system. Of these, 160 root samples were analyzed, as well as 160 soil samples, of which 157 roots (98%) and 160 (100%) soil samples showed infestation of the root-knot nematode.
The frequency of occurrence of Meloidogyne in Mulege, Comondu, La Paz and Los Cabos was 100%, that is, all the fields evaluated in each municipality, where the samplings were carried out, showed damage by the root-knot nematode. The incidence of the root-knot nematode was 100% in all crops evaluated except in fig trees where it presented 70% damage. This indicates that the ten evaluated plants of each crop, showed the presence of galls in their roots and only seven plants belonging to the cultivation of fig tree showed root galling.
Gilt index
The roots of the different crops showed differential galling (Table 2). The conventional type saladette tomato from the Mulege area was one of the crops that showed the highest galling index when showing a scale of 5, followed by the organic type saladette tomato with 4.8 and one of the grape-1 cultivars with 4.3. For the case of ball tomato, grape-2 and fig, the gall index was lower when showing a scale of 3.3, 1.5 and 1.1, respectively.
Table 2 Incidence, gall index and population of Meloidogyne spp.
| Municipality | Hosting | Production system | (%) of incidence | Galling index | Population of nematodes | |
| J2/25 g root | J2/100 g soil | |||||
| Mulege | Tomato ball | Conventional | 100 | 3.3 | 657 | 1 004 |
| Tomato grape-1 | Conventional | 100 | 4.3 | 595 | 448 | |
| Tomato saladette-1 | Organic | 100 | 4.8 | 770 | 216 | |
| Tomato saladette-2 | Conventional | 100 | 5 | 418 | 1 116 | |
| Tomato grape-2 | Conventional | 100 | 1.5 | 44 | 96 | |
| Fig tree | Conventional | 70 | 1.1 | 130 | 72 | |
| Comondu | Tomato saladette-3 | Conventional | 100 | 3.4 | 500 | 452 |
| Beetroot | Conventional | 100 | 2.1 | 35 | 126 | |
| Chard | Conventional | 100 | 3.7 | 965 | 566 | |
| pumpkin | Organic | 100 | 2 | 976 | 260 | |
| Cherry tomato | Organic | 100 | 2.1 | 37 | 101 | |
| La Paz | Chile bell | Conventional | 100 | 4.1 | 280 | 916 |
| Tomato saladette-4 | Conventional | 100 | 4.7 | 281 | 2 284 | |
| California pepper | Conventional | 100 | 2.7 | 871 | 1 192 | |
| Basil-1 | Conventional | 100 | 3.3 | 753 | 1 028 | |
| Los Cabos | Basil-2 | Organic | 100 | 4.2 | 1 671 | 1 050 |
In Comondu, the highest galling index was 3.7 in chard, followed by tomato saladette (3.4), while in beet, cherry tomato and squash the damage was lower showing a galling on the scale of 2.1, 2.1 and 2 respectively. In the case of the La Paz area, a higher gall rate was observed in tomato saladette (4.7), following in descending order the cultivation of bell pepper (4.1), basil (3.3) and to a lesser extent the cultivation of California pepper (2.7). Likewise, in Los Cabos, the basil crop had a galling index of 4.2.
Plant response to nematode infection
The tomato plants grown in soil infested with the different populations of Meloidogyne, showed root galling at 60 days after the transplant. During this time, the galls presented variable sizes and grew individually. However, at the end of the evaluation (90 dds), the galls were grouped to produce larger galls, close to a scale of 4 or 5, within a galling index. This damage response demonstrates and confirms the infective capacity of the nematode populations obtained at each sampling site.
Population of nematodes in soil
The populations of the root-knot nematode per 100 g of soil and 25 g of root varied among the sampling sites (Table 2). The populations in soil varied from 72 to 2 284 individuals of the second stage (J2), while in roots it was 35 to 1 671 nematodes. The crops that presented the lowest population of the root-knot nematode in the soil were fig, grape-2 tomato and cherry tomato with 72, 96 and 101 J2 respectively in 100 g of soil. Followed by these, crops with populations greater than 100 nematodes, such as beet (126), tomato saladette-1 (216) and pumpkin (260) were presented.
While in tomato grape-1, tomato saladette-3 and chard, the population exceeded 400 nematodes per sample when presenting 448, 452 and 556 nematodes respectively in 100 g of soil. However, the largest populations were observed in bell peppers (916), tomate ball (1,004), basil (1 028 and 1 050), tomate saladette-2 (1 116) and California pepper (1 192). Being the tomato saladette-4, where the maximum population density was presented with 2 284 nematodes in 100 g of soil.
Population of nematodes in root
The maximum population of root nematodes was observed in basil-2, located in Los Cabos, which showed 1 671 nematodes, followed by pumpkin crops (976), chard (965), California pepper (871), tomato-1 (770) and basil-1 (753), present in the different municipalities sampled. In the case of tomato saladette-2, tomato saladette-3, tomato grape-1 and tomato ball, the population exceeded 400 nematodes present in the samples of roots to obtain 418, 500, 595 and 657 nematodes respectively. While to a lesser extent, the nematode population was present in tomato saladette-4 (281), bell pepper (280), fig tree (130), grape-2 tomato (44), cherry (37) and beet (35).
Morphological identification
When analyzing the morphology of the perineal pattern of adult females microscopically, the different fields sampled revealed the presence of two different types of perineal patterns. The type of pattern 1, was characterized by presenting generally a shape elongated with a dorsal arch high and square, with soft grooves to wavy with some bifurcations in the lateral lines. This morphology coincided with that described in the literature for M. incognita (Figure 1) (Taylor and Sasser, 1983).
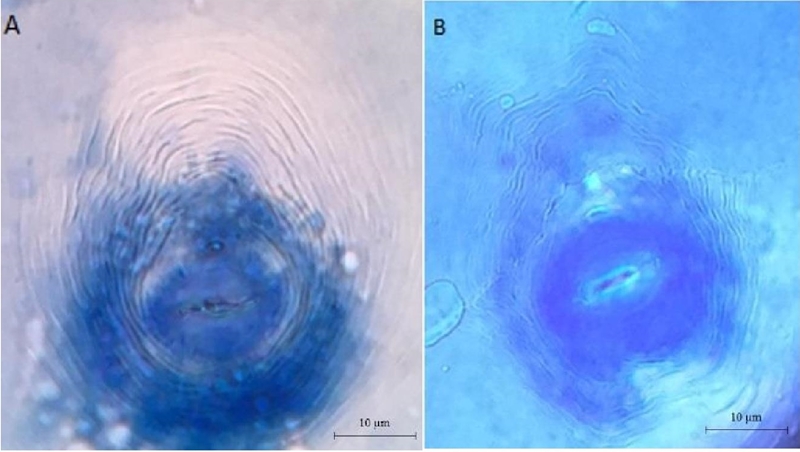
Figure 1 Microphotography of perineal patterns obtained from Meloidogyne females in agricultural crops of Baja California Sur: Meloidogyne enterelobii: A) high and round dorsal arch. Meloidogyne incognita; and B) high and square dorsal arch. 40X.
The type 2 pattern presented an ovoid to round morphology, with a high dorsal arch rounded, lateral ridges with smooth to wavy streaks. These characteristics are typical of Meloidogyne enterelobii (Yank and Eisenback, 1983).
Molecular identification
The amplification of the ribosomal DNA, with the universal primers MF/MR produced a specific fragment of 500 bp for the genus Meloidogyne in the samples belonging to all the localities. The specific primers were used to identify and differentiate the Meloidogyne species in the samples of collected roots. A single specific band of 256, 500 and 900 bp in size was produced; through PCR amplification only for the specific primers of M. enterelobii Me-F/Me-R from 11 populations, MF/MR universal primers from Meloidogyne spp. of four populations and specific primers of M. incognita Mi-F/Mi-R of four populations respectively (Figure 2).
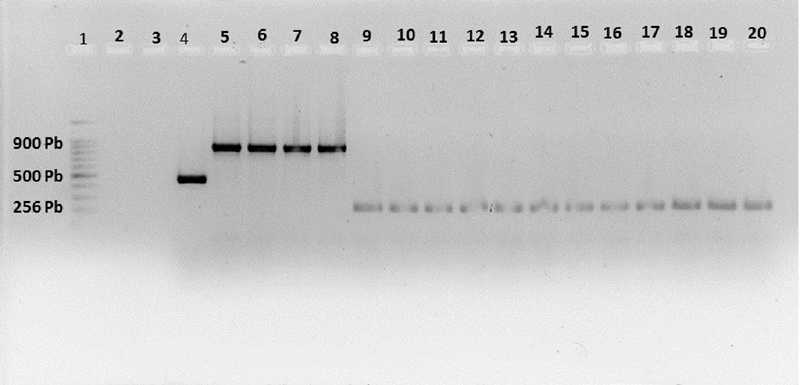
Figure 2 PCR product obtained from specific and universal primers, from DNA from populations of nematodes present in roots with galls. Lane 1= 100 bp DNA marker. Lane 2= negative control. Lane 3= white. Lane 4= positive control Meloidogyne spp. Lane 5-8= Mi-F/Mi-R. Lane 9-20= Me-F/Me-R.
The fragment obtained with the primers of M. enterelobii, coincides with that reported by Martínez et al. (2015) in their investigations when obtaining a 256 bp PCR product during their analysis of the identification of Meloidogyne species in tomato. While in the case of M. incognita, the amplicon generated was 900 bp, which corresponds to that reported by Meng et al. (2004). The negative control and the blank, as expected, did not amplify a PCR product with the primers used.
It is important to mention that the amplification was negative for the rest of the specific primers, corresponding to the species of M. hapla, M. arenaria and M. javanica. Four of the samples amplified for the Meloidogyne genus; however, no amplicon was expressed for any of the evaluated species, which could indicate a species different from these and new in the genus. Figure 3 shows the result obtained from the analysis in the Comondu, Mulege, La Paz and Los Cabos samples, where only a specific band of 500 bp was presented, derived from PCR amplification only for the Meloidogyne spp. MF/MR. In the rest of the samples they did not show any amplification to the previously evaluated species.
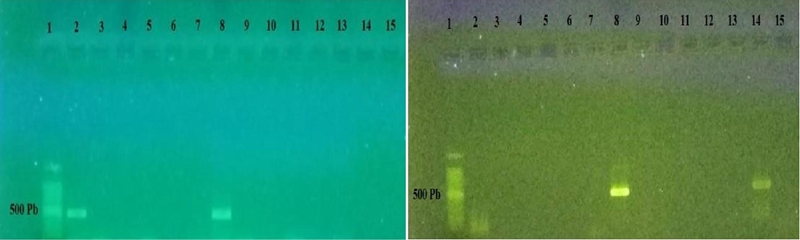
Figure 3 PCR product obtained from specific and universal primers, from DNA from populations of nematodes present in roots with galls. Photo left and right; amplification of a single band of 500 bp only for MF/MR in lanes 2, 8, 8 and 14. In the rest of the lanes it did not amplify for any other species.
Distribution of species
Meloidogyne incognita and Meloidogyne enterelobii were found co-infecting two (12.5%) evaluated tomato fields, in the area of Mulege and Comondu. As well as two (12.5%) of the fields were infested with M. enterelobii and Meloidogyne spp. While Meloidogyne enterelobii was found in 12 (75%) of the 16 evaluated and M. incognita in four (25%) only. M. enterelobii, was determined as the most common nematode species found in Baja California Sur, when it was detected in all the districts sampled in the present investigation, such as Mulege, Comondu, La Paz and Los Cabos, with the establishment of tomato, chili, basil, beet, chard, squash and fig. Meloidogyne incognita was less common and was detected only in two districts (Mulege and Comondu), infecting only tomato in four fields (Figure 4).
Galling nematode species are morphologically very similar to each other and identification at the species level is difficult. In addition, on several occasions more than one species is found in the same root of the plants (Devran and Sogut, 2009). Therefore, the rapid and precise identification of these microorganisms is necessary for the optimal management and improvement of crops (Powers et al., 2005). This is only possible; through regular and exhaustive studies of the detection of populations of Meloidogyne species in the agricultural areas of each region.
With this objective, the present study reveals the main Meloidogyne species that are currently present in the production areas of various agricultural crops in Baja California Sur. 100% of the crops under study showed damage by the root-knot nematode. The highest rate of galling was presented by the saladette tomato, showing large and irregular gills in its roots. This, probably due to the fact that it is one of the most common and constant vegetables in the fields, besides that it is one of the main host species of this pathogen (Oliveira et al., 2017).
It has been established that, although there are tomato crops with the Mi resistance gene, to inhibit the damage of the Meloiodgyne incognita species, it still presents considerable gall damage that impacts the yield and production costs of the different vegetables (Jacquet et al., 2005). Several studies have determined that this type of response is due to the process of selection pressure that gives rise to the appearance of virulent biotypes of Meloidogyne incognita, with the ability to reproduce in commercial crops established in the field (Castagnone-Sereno, 2002).
Likewise, the presence in the soil of several Meloidogyne species has been confirmed (Quénéhervé et al., 2011), where some are exceeding the resistance effect by the Mi gene and have the faculty to break this resistance and facilitate the increase of the incidence of damage to plants (Ornat et al., 2001; Pérez-Almeida et al., 2015).
Such is the case of this study, where the main species found were M. incognita, which is one of the most common species with a wide range of hosts, infecting different types of crops and of wide geographical distribution, when settling in different environments (Taylor et al., 1982). And M. enterelobii, a species considered one of the most destructive and severe, due to its ability to break the resistance that the Mi gene confers on plants and its rapid development and reproduction (Rodríguez et al., 2007).
Currently, the latter has established itself as an emerging pathogen, which has been rapidly distributed around the world causing losses in the production of vegetables such as tomatoes, up to 70% (Freitas et al., 2017). In the present study, it was determined that M. enterelobii, is distributed throughout the state, this species being the one with the highest incidence in the agricultural crops of the region, unlike M. incognita.
This incidence could be related to the characteristics of the soil type of the region and the climatic conditions of each sampled area. Particularly, Baja California Sur, is characterized for being an arid state and for having sandy soils, poor in organic matter. This type of soil within the production areas, generates the rapid loss of water in the soil, so the irrigation established in agricultural systems is constant, which causes greater humidity, facilitating these two factors the rapid movement of nematodes towards their host plants, to penetrate and reproduce rapidly within the roots, which leads to the increase of their populations in the field (Prot and Van Gundy, 1981).
The high prevalence and incidence of these nematodes suggests their importance as a potential risk in the production of crops of economic interest, mainly vegetables such as tomatoes or chili, which are commonly established as monocultures and where producers indicate that they have been established. continuous form for more than 27 years in the fields evaluated.
Conclusions
The characteristics in the different isolates of Meloidogyne coincided with those described for M. incognita and M. enterelobii. Where the distribution of the latter was greater when presented in the four municipalities of the state sampled. The high reproduction rate of M. enterelobii and its ability to break the Mi resistance gene can significantly affect the agricultural production of the state. This is the first report of the Meloidogyne species present in the state of Baja California Sur. This type of information can form a basis to formulate and design strategies for control methods against this pathogen, with the rational use of chemical products and crop rotation.
Acknowledgments
To the Program for the Professional Development of Teachers (PRODEP), for the resource granted in the 2017 call, as part of the incorporation of new full-time professors (NPTC), with a release letter for the resource 511-6/17-8557.
REFERENCES
Beira, H. M.; Heinz, W. D. and Hallmann, J., 2016. Population dynamics and damage potential of Meloidogyne hapla to rose rootstock species. J. Phytopathol. 164:711-721. Doi: 10.1111/jph.12492. [ Links ]
Castagnone, S. P. 2002. Genetic variability of nematodes: a threat to the durability of plant resistance genes? Euphytica. 124:193-199. [ Links ]
Devran, Z. and Söğüt, M. A. 2009. Distribution and identification of root-knot nematodes from Turkey. J. Nematol. 41:128-133. [ Links ]
Dong, K.; Dean, R. A.; Fortnum, B. A. and Lewis, S. A. 2001. Development of PCR primers to identify species of root-knot nematodes: Meloidogyne arenaria, M. hapla, M. incognita, and M. javanica. Nematropica 31:271-280. [ Links ]
Eisenback, J. D. and Hirschmann, H. 1981. Identification of Meloidogyne species on the basis of head and stylet morphology. J. Nematol. 12:23-32. [ Links ]
Freitas, M. V.; Silva, J. G. P.; Gomes, B. C.; Castro, J. M. C.; Correa, V. R. and Carneiro, M. D. G. R. 2017. Host status of selected cultivated fruit crops to Meloidogyne enterolobii. Eur. J. Plant Pathol. 148:307-319. Doi 10.1007/s10658-016-1090-8. [ Links ]
Hooper, D. J.; Hallmann, J. and Subbotin, S. A. 2005. Methods for extraction, processing and detection of plant and soil nematodes. In: Luc, M.; Sikora, R. A.; Bridge, J. (Eds.). Plant parasitic nematodes in subtropical and tropical agriculture. 2a (Ed.). UK, CABI. 53-86 pp. [ Links ]
Hu, M.; Zhuo, K. and Liao J. 2011. Multiplex PCR for the simultaneous identification and detection of Meloidogyne incognita, M. enterolobii, and M. javanica using DNA extracted directly from individual galls. Phytopathology. 101:1270-1277. [ Links ]
Jacquet, M.; Bongiovanni, M.; Martinez, M.; Verschave, P.; Wajnberg, E. and Castagnone-Sereno, P. 2005. Variation in resistance to the root-knot nematode Meloidogyne incognita in tomato genotypes bearing the Mi gene. Plant Pathol. 54:93-99. Doi: 10.1111/j.1365-3059.2005.01143.x. [ Links ]
Kaur, H. and Attri, R. 2013. Morphological and Morphometrical Characterization of Meloidogyne incognita from different host plants in four districts of Punjab, India. J. Nematol. 45(2):122-127. [ Links ]
Kloepper, J. W.; Mahaffee, W.; McInroy, J. A. and Backman, P. A. 1991. Comparative analysis of methods for recovering plant growth promoting rhizobacteria from roots. Can. J. Microbiol. 37:953-957. [ Links ]
Long, H.; Liu, H. and Xu, H. 2006. Developmet of a PCR diagnostic for the root-knot nematode Meloidogyne enterolobii. Acta Phytopathol. Sinica. 36:109-115. [ Links ]
Martínez, G. J. A.; Díaz, V. T.; Allende, M. R.; García, E. R. S. y Carrillo, F. J. A. 2015. Primer reporte de Meloidogyne enterolobii parasitando tomate en Culiacán, Sinaloa, México. Rev. Mex. Cienc. Agríc. Pub. Esp. Núm. 11:2165-2168. [ Links ]
Meng, Q. P.; Long, H. and Xu, J. H. 2004. PCR assays for rapid and sensitive identification of three major root-knot nematodes, Meloidogyne incognita, M. javanica and M. arenaria. Acta Phytopathol. Sin. 34:204- 210. [ Links ]
Nicol, J. M.; Turner, S. J. D.; Coyne, D. L.; Nijs, L. D.; Hockland, S. and Maafi, Z. T. 2011. Current nematode threats to world agriculture. in J. Jones, G. Gheysen, and C. Fenoll, eds. Genomics and molecular genetics of plant-nematode Interactions, Springer, Dordrecht. 21-43 pp. https://DOI.org/10.1007/978-94-007-0434-3-2. [ Links ]
Nunn, G. B. 1992. Nematode molecular evolution. Ph.D. dissertation. University of Nottingham, UK. https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.334855. [ Links ]
Oliveira, S. J.; Vinícius, S. M.; Levorato, F. L.; Silva, F. B. and Rúbia, R. M. 2017. Biocontrol agents in the management of Meloidogyne incognita in tomato agentes de biocontrole no manejo de Meloidogyne incognita em tomateiro. Ciencia Rural, Santa María, Crop Protection. ISSNe 1678-4596 pp. http://dx.DOI.org/10.1590/0103-8478cr20161053. [ Links ]
Ornat, C.; Verdejo, L. S. and Sorribas, F. 2001. A population of Meloidogyne javanica from Spain virulent to the Mi resistance gene in tomato. Plant Dis. 85:271-276. [ Links ]
Perez, A. I.; Vegas, G. A.; Pérez, D.; Muñoz, J. y Malyshev, S. 2015. Presencia del marcador Mi-23 de resistencia a Meloidogyne incognita como apoyo a la caracterización del germoplasma de tomate en Venezuela. Bioagro. 27(1):11-16. [ Links ]
Powers, T. O.; Mullin, P. G.; Harris, T. S.; Sutton, L. A. and Higgins, R. S. 2005. Incorporating molecular identification of Meloidogyne spp. Into a large-scale regional nematode survey. J. Nematol. 37:226-235. [ Links ]
Prot, J. C. and Van Gundy, S. D. 1981. Effect of soil texture and the clay component on migration of Meloidogyne incognita second stage juveniles. J. Nematol. 13:213-217. [ Links ]
Quénéhervé, P.; Godefroid, M.; Mège, P. and Marie, L. S. 2011. Diversity of Meloidogyne spp. parasiting plants in Martinique Island, French West Indies. Nematropica. 41:191-199. [ Links ]
Rodríguez, M.; Gomez, L. y Peteira, B. 2007. Meloidogyne mayaguensis Rammah y Hirschmann, plaga emergente para la agricultura tropical y subtropical. Rev. Protec. Veg. 22:183-198. [ Links ]
SAGARPA. 2017. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Agenda técnica agrícola de Baja California Sur©. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Ciudad de México. Impreso en México. 160 p. [ Links ]
Shurtleff, M. and Averre, III, C. 2000. Diagnosing plant diseases caused by nematodes. Second edition. The American Phytopathological Society. Minnesota, USA. 187 p. [ Links ]
Southey, J. F. 1986. Laboratory methods for work with plant and soil nematodes. Her Majesty’s Stationery Office, London, UK. 202 pp. [ Links ]
Taylor, A. L. and Sasser, J. N. 1978. Biology, identification and control of rootknot nematodes (Meloidogyne species). North Carolina State University, USAID, NC Graphics, Raleigh. 110 pp. [ Links ]
Taylor, A. L.; Sasser, J. N. and Nelson, L. A. 1982. Relationship of climate and soil characteristics to geographical distribution of Meloidogyne species in agricultural soils. North Carolina State University Graphics. 65 p. [ Links ]
Taylor, A. y Sasser, J. 1983. Biología, identificación y control de los nemátodos de nódulo de la raíz. Raleigh, USA. Universidad del Estado de Carolina del Norte. 111 p. [ Links ]
Yang, B. J. and Eisenback, J. D. 1983. Meloidogyne enterolobii (Meloidogynidae), a root-knot nematode parasiting pacara earpod tree in China. J. Nematol. 15:381-391. [ Links ]
Zeng, J.; Zhang, Z.; Li, M.; Wu, X.; Zeng, Y. and Li, Y. 2017. Distribution and molecular identification of Meloidogynespp. parasiting Flue-cured Tobacco in Yunnan, China. Plant Protect. Sci. 1-7. Doi: 10.17221/82/2017-PPS. [ Links ]
Zijlstra, C.; Donkers, V. D. T. H. M. and Fargette, M. 2000. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterized amplified region (SCAR) based PCR assays. Nematology. 8:847-853. [ Links ]
Received: December 2018; Accepted: February 2019











 texto em
texto em