Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 no.1 Texcoco ene./feb. 2019
https://doi.org/10.29312/remexca.v10i1.1730
Articles
Distribution of fungi associated with chickpea root rot
1Instituto Nacional de Investigaciones Forestales, Agrícolas y PecuariasCampo Experimental Costa de Hermosillo. Pascual Encinas núm. 21, Col. La Manga, Hermosillo, Sonora. CP. 83220. Tel. 01(55) 38718700. (fierros.gustavo@inifap.gob.mx; ortegampedro@gmail.com).
2Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Campo Experimental Bajío. Carretera Celaya-San Miguel de Allende km 6.5. Celaya, Guanajuato. CP. 38110. Tel. 01(55) 38718700.
3 Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Campo Experimental Norman E. Borlaug. Calle Norman E. Borlaug km 12, Valle del Yaqui, Cd. Obregón, Sonora. CP. 85000. Tel. 01(55) 38718700. (padilla.valenzuela@inifap.gob.mx).
4Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Campo Experimental Santiago Ixcuintla. Carretera Internacional México-Nogales km 6, Santiago Ixcuintla, Nayarit. CP. 63300. Tel. 01(55) 38718700, ext. 84420. (alvarez.arturo@inifap.gob.mx).
5Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Campo Experimental Valle de Culiacán. Carretera a El Dorado km 16.5, Culiacán, Sinaloa. CP. 80430. Tel. 01(55) 38718700. (ramirez.milagros@inifap.gob.mx; velarde.sixto@inifap.gob.mx).
In northwestern Mexico, one of the constraints for the production of chickpeas are root diseases caused by a complex of soil fungi. Identifying and determining the distribution of these fungi in the areas used to plant chick-pea will allow defining the lowest risk of damage to the crop. During the autumn-winter 2012-13 cycle, 46 fields planted with chick-pea were sampled and geographed in the coast of Hermosillo. The 221 plant samples with symptoms of root rot were collected, and 197 fungal isolates were obtained in the laboratory. After their purification and classification, the individual isolates showed the typical morphological characteristics of Fusarium oxysporum f. sp. ciceris, Fusarium solani, Macrophomina phaseolina and Rhizoctonia solani. A risk index for soil pathogens was elaborated, defined by the number of species causing the disease in each site sampled, and distribution mapping of each pathogen. In 34 142 potential hectares of the agricultural area (22.5%) the risk index was low with a different species isolated through the sampling sites; 62 238 ha (41%) were detected with two different species and considered as medium risk areas. As high risk areas, where a complex of three pathogens was found in an area of 50 628 ha (33.3%) and with four pathogens considered as very high risk areas, 4 813 ha (3.2%). The species with the highest frequency of appearance in the coast of Hermosillo was Macrophomina phaseolina with 41% in the samples and in 77% of the sampled fields.
Keywords: Fusarium oxysporum f. sp. ciceris; Fusarium solani; Macrophomina phaseolina; Rhizoctonia solani; pathogenic potential
En el noroeste de México, una de las limitantes para la producción de garbanzo son las enfermedades de la raíz ocasionadas por hongos del suelo. El identificar y determinar la distribución de esos hongos en áreas para sembrar garbanzo permitirá definir las de menor riesgo para el cultivo. Durante el ciclo otoño-invierno 2012-13 se muestrearon y geoposicionaron en la Costa de Hermosillo 46 campos sembrados con garbanzo, se colectaron 221 muestras de plantas con síntomas de pudriciones de raíz, obteniéndose en el laboratorio 197 aislamientos fúngicos. Después de su purificación y clasificación, los aislamientos individuales presentaron las características morfológicas típicas de Fusarium oxysporum f. sp. ciceris, Fusarium solani, Macrophomina phaseolina y Rhizoctonia solani. Se elaboró un índice de riesgo por patógenos del suelo definido por el número de especies causando la enfermedad en cada sitio muestreado, y mapeo de distribución de cada patógeno. En 34 142 hectáreas potenciales del área agrícola (22.5%) el índice de riesgo fue bajo con una especie diferente aislada a través de los sitios de muestreo; con dos especies diferentes y consideradas como zonas de riesgo medio se detectaron 62 238 ha (41%). Como áreas de alto riesgo, en donde se encontró un complejo de tres patógenos en una superficie de 50 628 ha (33.3%) y con cuatro patógenos consideradas como áreas de muy alto riesgo 4 813 ha (3.2%). La especie de mayor frecuencia de aparición en la Costa de Hermosillo fue M. phaseolina con 41% en las muestras y en 77% de los campos muestreados.
Palabras clave: Fusarium oxysporum f. sp. ciceris; Fusarium solani; Macrophomina phaseolina; Rhizoctonia solani; potencial patogénico
Introduction
The white-creamy white chickpea for export is grown mainly in northwestern Mexico in the states of Sonora, Sinaloa and Baja California Sur (Morales and Duron, 2004), mainly in the irrigated areas. This legume has positioned itself as a strategic crop within the agriculture of northwestern Mexico thanks to the production of large grain (caliber 40-44, number of grains in 30 g), white- creamy color, with export quality.
However, this favorable scenario for the crop, there are problems that threaten its permanence as a profitable crop in the region and internationally competitive, among the main production constraints of the chickpea is Fusarium oxysporum Schlechtend.: Fr. f. sp. ciceris (Padwick) Matuo & K. Sato (Foc) and root rot that are caused by a complex of fungi such as, Fusarium solani Mart. Amend Sacc., Macrophomina phaseolina (Tassi) Goid., Sclerotium rolfsii Sacc. and Rhizoctonia solani Kühn. In the coast of Hermosillo have been reported to F. solani, M. phaseolina and R. solani, with the genus Fusarium the predominant (Ramírez, 1975).
These diseases are controlled with the use of resistant varieties; however, it has been observed that by extensively using a resistant cultivar, the rest of the pathogens in the soil gradually increase (Mathur and Sarbhoy, 1978). Crop rotation can also modify the population of pathogens. For example, rotating with grasses discourages the presence of Fusarium spp; but it benefits M. phaseolina, which attacks numerous crops (Muñoz-Cabañas et al., 2005), increasing its presence in greater proportion.
Worldwide, the vascular fusariosis disease of the chickpea caused by Fusarium oxysporum f. sp. ciceris is the most common causative agent of the wilt of the garbanzo (Trapero and Jiménez, 1985, Meyer et al., 1997, Sharma et al., 2005), this makes it the most studied species and with sources of genetic resistance identified. However, the regional outlook is complicated because four species have been identified causing root rot in chickpeas with different levels of distribution in the areas of the region. Therefore, the objective of this study was to identify and determine the distribution and potential risk for the cultivation of fungi associated with vascular fusariosis and chickpea root rot, based on their distribution in the agricultural areas of the coast of Hermosillo, Sonora, Mexico.
Materials and methods
Description of the study area
The agricultural region of the coast of Hermosillo is a coastal plain with a lower elevation of 100 meters above sea level, with a warm climate (BWh) prevailing, with a medium-textured Haplic Yermosol soil. The main crops are cereals, fruit trees and in the autumn-winter cycle, white- creamy-chickpea beans are produced for export.
Methodology of sampling in fields with diseased plants
The coast of Hermosillo was sampled during the fall-winter cycle of 2012-13. A directed sampling was carried out. Plants were collected that showed the typical symptoms of vascular fusariosis (yellowing and wilting), necrosis, root and stem rots.
The sampling carried out was mixed (Casal and Mateu, 2003), when combining a random sampling by conglomerates, dividing each field in groups with plants that showed similar symptoms. The phenological stage where the sampling was carried out was from grain formation to grain filling. The sampling points were geographically referenced (GPS brand Garmin model Oregon 450). A database was developed integrating to a geographic information system (SIG) in the commercial application ESRI ArcMap Version 10, where the layer of sampling plots, land use, bodies of water, roads, a digital model of elevation were combined, as well as the type of climate and soil.
Using the spatial analyzer incorporated in ArcMap V10, interpolations were made for the information of each pathogen with the weighted function of the inverse of the distance (IDW) between sampling points. Additionally, a fifth interpolation called ‘severity index’ was created the classification can be seen in Table 1. Due to the uniformity of the environment of the study area and the presence of the chickpea monoculture, it was deemed unnecessary to verify the statistical significance of the interpolations.
Table 1 Keys for the risk index of the presence of pathogens associated with vascular fusariosis and chickpea root rot in the coast of Hermosillo, Sonora, Mexico.
| Criterion | Colour | Color |
| Plot with an identified pathogenic | Low | Green |
| Plot with two identified pathogenic species | Medium | Yellow |
| Plots with three identified pathogenic species | High | Orange |
| Plot with the four pathogenic species identified in the site | Very high | Red |
Collection and identification of pathogens present
The collected samples were placed in polyethylene bags and taken to the biotechnology laboratory of the Valle de Culiacán Experimental Field (CEVACU) of the National Institute of Forestry, Agriculture and Livestock Research (INIFAP). The plants were washed with abundant distilled water with the help of a soft brush. The 0.5 cm portions of diseased and apparently healthy tissues were cut and disinfected with 2% sodium hypochlorite for one minute; samples were washed with sterile distilled water to remove excess sodium hypochlorite, and placed on sterile filter paper to remove excess moisture. Five approximately 2 mm fragments were placed in Petri dishes with acidified potato dextrose agar (PDA) medium, and incubated at a temperature of 25 °C. The boxes were examined under the compound microscope (Olympus C x 31, using the 4 x objective) at 24, 48 and 72 h to observe growth of fungi arising from the fragments, from these hyphae tips and groups of hyphae were extracted. transferred to new Petri dishes with PDA (Komada, 1975). The isolates were purified by monoconidial cultures or hyphal tips, depending on the isolated fungus.
Once fungal growth was detected, the strains were purified to confirm their identity; the identification at the gender level was carried out using the keys of Barnet and Hunter (1998). From the growth of the fragments, pathogens that cause wilting were selected, among them; Fusarium sp, Macrophomina phaseolina and Rhizoctonia solani. For Fusarium sp., from the mycelium grown in PDA medium, a fragment was cut, which was suspended in 1 mL of sterile distilled water.
The amount of spores with a hematocytometer was estimated and monosporic cultures were obtained by serial dilutions (Hernández-Martínez and Rangel-Montoya, 2011). The cultures were observed under a compound microscope to locate individual germinated spores, the site was marked on the agar and a fragment of this with the germinated and selected spore was transferred to a new Petri dish with PDA medium supplemented with PCNB and chloramphenicol, and incubated under conditions suitable for growth.
The identification of pathogens at the species level was done by observing the structures under a microscope and comparing them with the description corresponding to each one. For example, the identification of Fusarium was made based on the morphology of the mycelium and microconidia in the phialides, according to the proposal by Leslie and Summerell (2006). In a previous study Velarde et al. (2015), verified the ability of isolates to cause the symptoms of F. oxysporum f. sp. ciceris in susceptible genotypes and, if appropriate, identify the race of the pathogen through differential varieties and associated molecular markers. As for M. phaseolina, the isolates were purified by transferring portions of culture medium with fungal growth in fresh potato-dextrose- agar (PDA) culture medium, acidified with 1 mL L-1 of lactic acid and incubated at 30 °C for five to seven days to obtain pure isolation (Gil-Langarica et al., 2008).
Categorization of properties and elaboration of figures
With the information on the number of species that cause root rot, the categorization of the farms and areas established with chickpea was carried out according to the keys presented in Table 1. For example, to categorize a farm or area with a risk index relatively low, were those in which a single species was identified, any of those described below; while in the farms and areas considered high risk the four species were identified causing damage to the crop. It is convenient to clarify that this classification of risk is relative because the abundant presence of a single species and a susceptible variety without protection, can result in high damage. For data processing and mapping, the incidence of pathogens in each sampling point was related by means of data tables with the sampling points obtaining risk indexes based on the number of species of pathogens identified and from these generated a combined table, interpolations were made for each pathogen by using the IDW interpolator, that is, the risk indexes were generated spatially for the different agricultural areas of the coast of Hermosillo, classified according to the species found in each point in the index risk.
Results and discussion
Collection of plants with symptoms of root rot
The 221 samples of chickpea plants were collected in 46 agricultural fields of the coast of Hermosillo with characteristic symptoms of vascular fusariosis, root rot, chlorosis, achaping, brown xylem and wilt. This complex of pathogens causing root rot is favored by warm climates and sandy soils. Plants are susceptible at any stage of development. The symptoms are more evident in stages after flowering. Forty-two of the fields were positive for fungi, of which 197 isolates were obtained.
During the development of the crop favorable temperatures occurred to the different pathogens responsible for the disease (Figure 1) according to the temperature requirements of each species; for example, M. phaseolina is aggressive under high temperatures and dry soil during the reproductive stage of the crop (Gupta and Sharma, 2015). In addition, this pathogenic fungus attacks a wide spectrum of species (Muñoz-Cabañas et al., 2005), which makes control and eradication difficult.
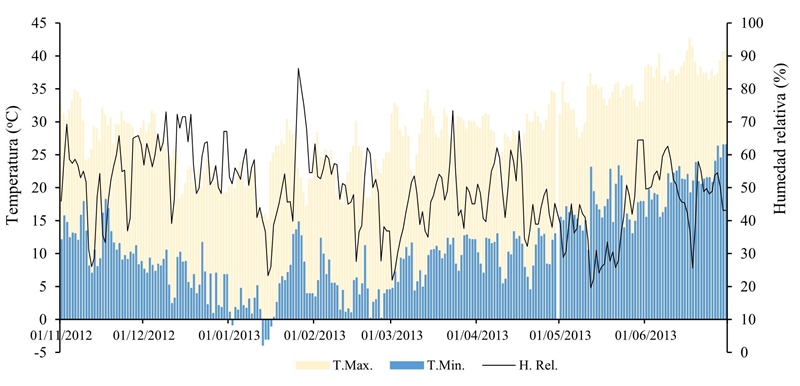
Figure 1 Maximum, minimum temperature and daily relative humidity during the development cycle of the chickpea crop in the Experimental Field coast of Hermosillo, representative site of the region.
Regarding Foc, it has been investigated that at a temperature around 25 °C and an inoculum amount of 104-105g-1 of soil, of micro or macroconidia, are the optimal conditions to develop the disease in chickpea (Landa et al., 2001). Likewise, several authors mention that under controlled conditions of the degree of temperature and density of the inoculum of Fusarium sp., as well as of the soil moisture, the potential risk of the diseases can be estimated, as well as the resistance and susceptibility of the genotype of chickpea (Bhatti and Kraft, 1992a, 1992b; Sharma and Muehlbauer, 2007; Navas et al., 2007; Andrabi et al., 2009). The improved varieties developed for the coast of Hermosillo region have resistance to Fusarium sp.; however, the presence of different races of F. oxysporum f. sp. ciceris and the use of grain as seed make their control difficult (Velarde et al., 2015).
Distribution of species in the study region
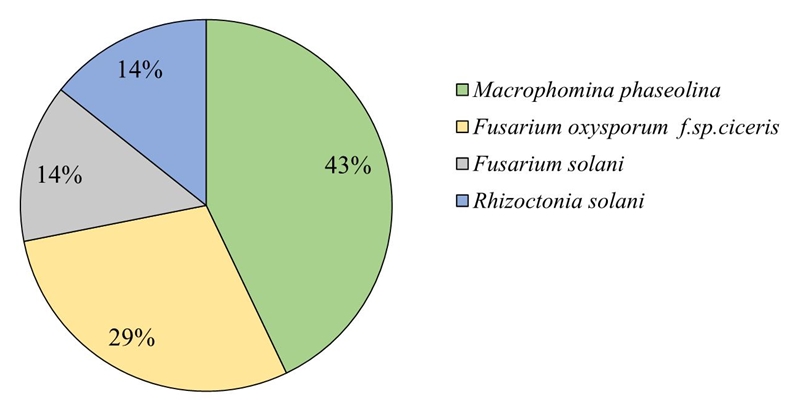
The results indicate that the species with greater distribution in the study area was M. phaseolina with 41% frequency of appearance in the total of the samples and 77% of the sites; Foc with presence in 27% of the samples and in 65% of the sites; F. solani was detected in 13% of the samples and in 41% of the fields and R. Solani in 14% of the samples and with presence in 27% of the sampled sites (Figure 2). In a similar study conducted in the state of Sinaloa, it was detected that the main genus was Fusarium sp. with 48% of the total samples (Ramírez et al., 2012). Ghosh et al. (2013) sampled in Central and Southern India and pointed out the presence of various pathogens causing wilt and root rot in chickpea; concluded that Sclerotium rolsfii and Macrophomina phaseolina were widely distributed in the region studied. Likewise, in a sampling work carried out in southern Spain, Trapero and Jimenez (1985) found in 99% of 108 fields inspected the presence of F. oxysporum f. sp. ciceris, F. solani and M. phaseolina
Mapping distribution of pathogens causing wilt and root rot in chickpea
The sites of low risk, with at least one identified species, not necessarily the same, corresponded to 22.5%, representing 34 142 ha of a potential of 151 820 ha (assuming that the total area of the coast of Hermosillo was planted with chickpea), these areas being located in a strip that extends from the northeast to the west of the region. These zones are theoretically the zones with less presence of pathogens associated with the drying and rotting of the chickpea root, and in which the cultivation should preferably be established.
Areas of medium severity with two identified species (not necessarily the same through localities) represent 41% of the surface corresponding to 62 236 ha located most in the central region of the agricultural area and in some areas north of this region (Figure 3). Those of high severity, with at least three species of pathogens detected, in 50 628 ha corresponding to 33.3% of the agricultural surface basically located to the south and an area north of the Hermosillo-Bay of Kino highway. Very high severity, with the four pathogens mentioned above, present in only 3.2% of the study area corresponding to 4 813 ha that theoretically would be the highest risk associated with pathogens that cause vascular fusariosis and chickpea root rot.
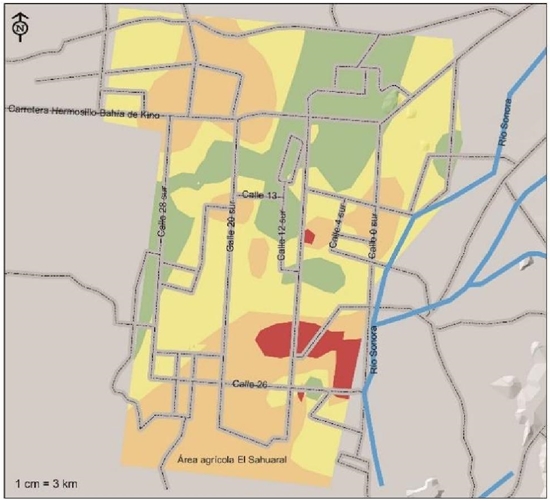
Figure 3 Risk indices based on the presence of pathogens associated with chickpea root rots, isolated and identified in the coast of Hermosillo, Sonora. In green, the low-risk areas, in yellow, are considered medium risk and the high-risk and high-risk areas are orange and red, respectively.
This surface is located in the form of isolated foci to the south of the agricultural region between streets 4, 12 and 26 and two small centers on 12th Street south and 26th Street (Figure 3). The maintenance of the production systems depends to a great extent on the protection of the plant. The simplest solution would be to avoid the planting of chickpea and crop rotation the time necessary for the inoculum to decrease in the soil. Another possible medium- and long-term solution is to pyramidate different resistance genes against the four different pathogens; While developing these cultivars, the use of clean seeds, antagonistic microorganisms (Paredes et al., 2009) and adequate crop nutrition (Huber and Haneklaus, 2007), can maintain the production and productivity of the chickpea in the region. (The typeface was checked and corrected).
In Figure 4, we can observe the agricultural areas of the coast of Hermosillo where M. phaseolina was isolated and identified; this pathogen was the most distributed with high risk index in the agricultural areas of the coast of Hermosillo, with potential presence in 105 572 ha representing 69.5% of the total. This species represents a strong health problem in case environmental conditions occur so that this fungus manifests itself by damaging the chickpea crop. Nagama et al. (2015) mention that the use of agrochemicals to control this fungus is difficult due to the wide range of host species.
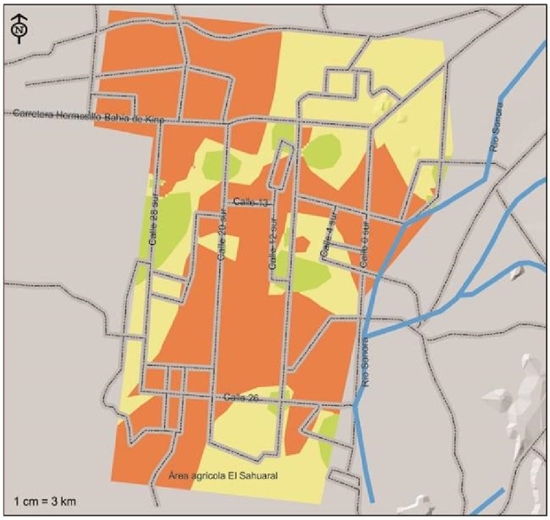
Figure 4 Indices of severity of presence of Macrophomina phaseolina, a pathogen associated with chickpea root rot, isolated and identified in the coast of Hermosillo, Sonora.
Its presence extends from north to south and from east to west, agricultural areas were defined with moderate severity indexes corresponding to 37 192 ha, representing 24.5% of the total area, being located towards the southwest of the region extending southward. east, two sub-regions with this same index of severity were located towards the center of 20th street south and another one to the north-west, one sub region down 28th street south and another one to the center of the agricultural region of the coast of Hermosillo. The areas where M. phaseolina was detected with intensity of moderate severity are located on the south on 12th street and on the west on 28th street, where theoretically the presence of this pathogen would have less drastic consequences due to having a lower frequency of appearance and perhaps due to very particular weather conditions. The presence of this fungus had greater impact in this cycle due to the fact that the maximum daily temperature was above 32 ºC more frequently (Figure 2). It has been mentioned that M. phaseolina has greater virulence under conditions of drought stress coupled with hot weather conditions (Gupta and Sharma, 2015, Mayek et al., 2002). The Argentine National Pest Surveillance and Monitoring System mentions that the infection of this pathogen is favored by conditions that cause plant stress, in general by high temperatures (28 to 35 ºC) and low soil humidity or drought, especially during the period reproductive of plants.
Regarding the risk indexes of F. oxysporum, this together with M. phaseolina are the most distributed in the soils of this region, with a high presence in around 86 698 hectares that represent 57.1% of the total agricultural area of the coast of Hermosillo (Figure 5), with distribution from the southwest to the east affecting the central part of the region, in addition to high presence in the northwest of the region and north of the highway Hermosillo-Bay of Kino, was located within from the moderate range around 53 857 ha (35.5%), in zones located to the east, southeast and northeast of the region and 7.4% of the surface corresponding to 11 267 has, were located in the low level located basically in the center and west of the region.
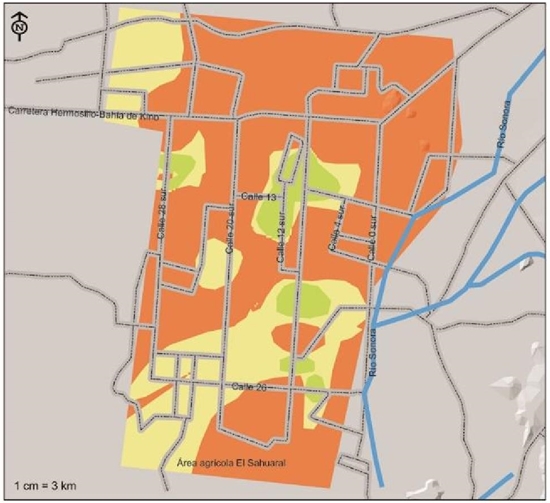
Figure 5 Severity indices of presence of Fusarium oxisporum f.sp. ciceris, pathogen associated with chickpea root rot, isolated and identified in the coast of Hermosillo, Sonora.
In a similar work carried out in the agricultural region of Maharashtra, India in which 756 thousand ha are sown with chickpea and taking samples of both native and improved varieties, Nikam et al. (2007), obtained results that indicated the presence of F. oxysporum causing damage to the crop through diverse localities from 9.15 to 16.5% of the sampled fields. Pande et al. (2015) noted that chickpea wilt caused by F. oxysporum is the most widespread and destructive disease in India; whereas, in Spain, Landa et al. (2001) mentioned that the development of this disease is greater than 25 ºC compared to 20 and 30 ºC.
In Figure 6, the distribution of F. solani in the agricultural region of the coast of Hermosillo is observed, according to the weighted risk indexes, this pathogen is less distributed compared to M. phaseolina and F. oxysporum, the areas with greater presence are located south southwest of the coast of Hermosillo and the Sahuaral region, between 4th and 28th streets, and some foci distributed around 13th Street, from east to west. They are considered within the high severity index, with 29.5% of the surface corresponding to approximately 44 765 ha; in 41 093 ha corresponding to 27.1% of the surface, these are considered with moderate risk index where the incidence of the disease would be expected to be less severe, are the areas that comprise the center region between 0 and 28 streets south, in addition to a region comprised to the southeast within the Sahuaral region. Soils with a low severity index were located practically from the center of the region to the north, comprising a total area of 65 962 ha (43.4%).
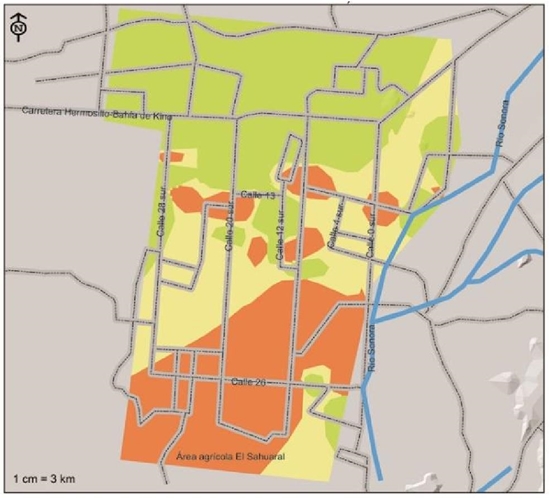
Figure 6 Severity indices of presence of Fusarium solani, pathogen associated with chickpea root rot isolated and identified in the coast of Hermosillo, Sonora.
Rhizoctonia solani, according to the risk indexes shown in Figure 7, this pathogen is located with a high risk index in the southwest of the region on 26th street between 12th and 4th streets, and northwest on the road to Kino Bay between streets 20 and 36 north, this distribution corresponding to an area of 34 106 ha that is equivalent to 22.5% of the total. With a moderate risk index, 25.1% of the soils corresponding to an area of 38 129 ha were detected, located basically in the south of the region until they entered the Sahuaral region. A low risk index was detected in the soils of a strip that runs from west on 28th street to northeast of 4th street passing through the center in a greater area of 79 585 ha that represents 52.4%.
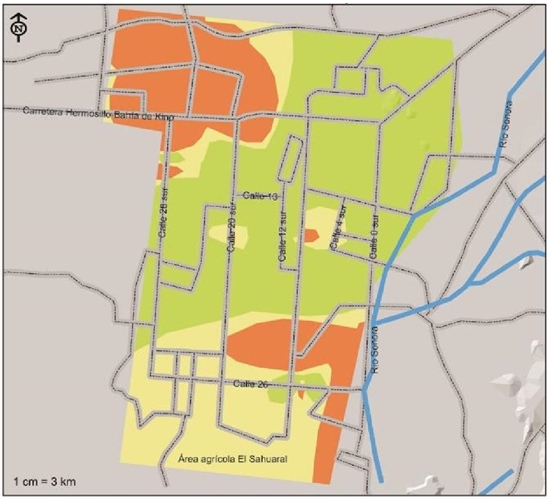
Figure 7 Indices of severity of presence of Rhizoctonia solani, pathogen associated with chickpea root rot isolated and identified in the coast of Hermosillo, Sonora.
The areas with the presence of only one or two species of fungi associated with vascular fusariosis and root rot, would be the most suitable for planting chickpea considering: the species present, the resistance of the variety to be used, and the opportunity and cost of a cultural control. The nutrition of the plant determines to a great extent its resistance or susceptibility to diseases, its histological and morphological structure or properties, and the virulence or ability of pathogens to survive (Huber and Haneklaus, 2007); thus, an adequate nutrition for the crop can contribute to diminish the risk of damage by diseases. In the areas with greater number and density of pathogens that cause root rot, crop rotation, preferably with cereals that are not affected by these, for three or four years would be an appropriate practice. When taking into account that one of the most frequent pathogens was M. phaseolina, whose damage is accentuated by drought conditions and high temperatures (Mayek et al., 2002; Gupta and Sharma, 2015), sowing dates should be considered. varieties with a short crop cycle that avoid the coincidence of the reproductive stage of the crop with the presence of these environmental stresses. At present, in this region most of the plantings are carried out in the month of January with a high probability of occurrence of temperatures above 30 °C since the end of march.
Conclusions
During the autumn-winter 2012-13 cycle, in different proportions and combinations, four species of pathogens were identified causing damage to the chickpea root in the coast of Hermosillo, in order of abundance these were: Macrophomina phaseolina, Fusarium oxysporum f. sp. ciceris, Fusarium solani and Rhizoctonia solani, the last two in the same proportion.
In 74.3% of the agricultural area of the coast of Hermosillo, which corresponds to 112 866 ha, the species were isolated and identified Macrophomina phaseolina and Fusarium oxysporum f. sp. ciceris, with a pathogenic potential of medium to high risk of causing damage to the chickpea crop.
The pathogen with the greatest potential distribution in terms of surface in the coast of Hermosillo region was Macrophomina phaseolina in 105 572 hectares that represent 69.5% of the surface area of the study area.
Literatura citada
Andrabi, M.; Vaid. A. and Razdan, V. K. 2009. Reaction of chickpea genotypes against wilt complex pathogens under greenhouse conditions. Journal of Research, SKUAST-J. 8(1):112-115. [ Links ]
Barnett, H. L. and Hunter, B. B. (1998). Illustrated Genera of Imperfect Fungi. 4th Edition, APS Press, St. Paul. 218 p. [ Links ]
Bhatti, M. A. and Kraft, J. M. 1992(a). Effects of inoculums density and temperature on root rot and wilt of chickpea. Plant Disease. 76:50-54. DOI: 10.1094/PD-76-0050. [ Links ]
Bhatti, M. A. and Kraft, J. M. 1992 (b). Influence of soil moisture on root rot and wilt of chickpea. Plant Disease. 76:1259-1262. DOI: 10.1094/PD-76-1259. [ Links ]
Casal, J. y Mateu, E. 2003. Tipos de muestreo. Rev. Epidem. Med. Prev. 1:3-7. (Documento en línea): http://minnie.uab.es/~veteri/21216/Tipos Muestreo 1. [ Links ]
Gil-Langarica, H. R.; Maldonado-Moreno, N.; Pecina-Quintero, V.; y Mayek-Pérez, N. 2008. Reacción de Germoplasma Mejorado de Soya [Glycine max (L.) Merr.] a Macrophomina phaseolina (Tassi) Goidanich y Déficit Hídrico. Revista Mexicana de Fitopatología. 26(2):105-113. [ Links ]
Gupta, O. and Sharma, M. 2015. Dry root rot of chickpea: An overview. J. Food Legumes. 28(4):267-276. [ Links ]
Ghosh, R.; Sharma, M. Telangre, R. and Pande, S. 2013. Ocurrence and distribution of chickpea diseases in Central and Southern parts of India. Amer. J. Plant Sces. 4:940-944. [ Links ]
Hernández-Martínez, R. y Rangel-Montoya, E. A. 2011. Búsqueda de cepas de Trichoderma antagonistas a hongos causantes de marchitez vascular en tomate. Revista Electrónica de Divulgación de la Investigación. 2:1-9. [ Links ]
Huber, D. M. and Haneklaus, S. 2007 Managing nutrition to control plant disease. Landbauforschung Völkenrode. 4(57):313-322. [ Links ]
Komada, H. 1975. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev. Plant. Prot. Res. 8(13):114-124. [ Links ]
Landa, B. B. Navas, C. J. A. Hervás, A. and Jiménez, D.R.M. 2001. Influence of temperature and inoculum density of Fusarium oxysporum f. sp. ciceris on suppression of Fusarium wilt of chickpea by rhizosphere bacteria. Phytopathology. 91(8):807-811. [ Links ]
Leslie, J. F. and Summerell, B. A. 2006. The Fusarium laboratory manual. Blackwell Publishing Ltd. 387 p. [ Links ]
Mathur, S. B. and Sarbhoy, A. K. 1978. Biological control of Sclerotium root of sugar beet. Indian Phytopatology. 31(3):366-367. [ Links ]
Mayek, P. N.; García, R. López, C.C.; Acosta, G. J. A. and Simpson, J. 2002. Water relations, histopathology and growth of common bean (Phaseolus vulgaris) during pathogenesis of Macrophomina phaseolina under drought stress. Physiological and Molecular Plant Pathology. 60(2):185-195. . [ Links ]
Meyer, M. S.; Tullu, C. J. A.; Simon, J.; Kumar, W. J.; Kaiser, J. M. K. and Muehlbauer F. J. 1997. Development of a DNA marker for Fusarium wilt resistance in chickpea. Crop Science. 37(5):1625-1629. doi:10.2135/cropsci1997.0011183X003700050036x. [ Links ]
Morales, G. J. A. y Durón, L. J. 2004. Aspectos generales del garbanzo. In. El cultivo de garbanzo blanco en Sonora. Libro técnico número 6. INIFAP-CIRNO-CECH. 11-12 pp. [ Links ]
Muñoz-Cabañas, R. M.; Hernández-Delgado, S. y Mayek-Pérez, N. 2005. Análisis patogénico y genético de Macrophomina phaseolina (Tassi) Goid. en diferentes hospedantes. Revista Mexicana de Fitopatología. 23(1):11-18. [ Links ]
Nagamma, G.; Saifulla, M.; Sab, J. and Pavitra, S. 2015. Screening of chickpea genotypes against dry root rot caused by Macrophomina phaseolina (tassi) goid. The Bioscan. 10(4):1795-1800. [ Links ]
Navas, C. J. A.; Landa, B. B.; Méndez, R. M. A. and Jiménez, D. R. M. 2007. Quantitative modeling of the effects of temperature and inoculum density of Fusarium oxysporum f. sp. ciceris races 0 and 5 on development of Fusarium wilt in chickpea cultivars. Phytopathology. 97:564-573. doi:10.1094/ PHYTO-97-5-0564. [ Links ]
Nikam, P. S.; Jagtap, G. P. and Sontakke, P. L. 2007. Management of chickpea wilt caused by Fusarium oxysporum f.sp. ciceris. Afric. J. Agric. Res. 2(12):692-697. [ Links ]
Pande, S.; Sharma, M.; Nagavardhini, A. and Rameshwar, T. 2012. High Throughput Phenotyping of Chickpea Diseases: Stepwise identification of host plant resistance. Information Bulletin No. 92. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics. 56 p. [ Links ]
Ramírez, V. J. 1975. Pruebas de patogenicidad de los organismos aislados de raíz de garbanzo Cicer arietinum, en el Valle de Culiacán. Tesis de licenciatura. Universidad Autónoma de Sinaloa. [ Links ]
Ramírez, S. M.; Álvarez, B. A.; Valenzuela, H.V.; Ortega, M. P. F. y Fierros, L. G. A.; Padilla. V. 2012. Detección de hongos asociados a la “rabia del garbanzo” , en la zona norte de Sinaloa. Memorias XV Congreso Internacional de Ciencias Agrícolas, 25 y 26 Octubre 2012; Universidad Autónoma de Baja California, Mexicali. 707-713 pp. [ Links ]
Sharma, K. D.; Chen, F. J. and Muehlbauer, F. J. 2005. Genetics of chickpea resistance to five races of Fusarium wilt and a concise set of race differentials for Fusarium oxysporum f. sp. ciceris. Plant Disease. 89:385-390. DOI: 10.1094/PD-89-0385. [ Links ]
Sharma, K. D. and Muehlbauer, F. J. 2007. Fusarium wilt of chickpea: physiological specialization, genetics of resistance and resistance gene tagging. Euphytica. 157:1-14. DOI 10.1007/s10681-007-9401-y. [ Links ]
Trapero, C. and Jiménez, D. R. M. 1985. Fungal wilt and root rot diseases of chickpea in southern Spain. Phytopathology. 75:1146-115. http://doi.org/10.1094/phyto-75-1146. [ Links ]
Velarde, F. S.; Ortega, M. P. F.; Fierros, L. G. A.; Padilla, V. I.; Gutiérres, P. E.; Rodríguez, C. F. G.; López, V. J. A.; Acosta, G. J. A. y Garzón, T. J. A. 2015. Identificación molecular y biológica de las razas 0 y 5 de Fusarium oxysporum Schlechtend: Fr f. sp. ciceris (Padwick) Matuo & K. Sato del garbanzo en el noroeste de México. Rev. Mex. Cienc. Agríc. 6(4):735-7. [ Links ]
Received: January 01, 2019; Accepted: February 01, 2019











 texto en
texto en