Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.10 no.1 Texcoco Jan./Fev. 2019
https://doi.org/10.29312/remexca.v10i1.570
Articles
Induced response to antioxidative enzymes in rice under stress due to lead and nickel
1Departamento de Biotecnología Agrícola-CIIDIR-Unidad Sinaloa-IPN. Guasave, Sinaloa, México. CP. 8110. (jefaturaba2015@gmail.com).
2Universidad Agraria de la Habana ‘Fructuoso Rodríguez Pérez’. San José de las Lajas, Mayabeque, Cuba. CP. 33200. (dcabezasmontero71@gmail.com).
3Centro de Investigación en Alimentación y Desarrollo AC-Coordinación Delicias. Fraccionamiento Vencedores del Desierto, Delicias, Chihuahua, México. CP. 33089.
4Facultad de Ingeniería Agronómica-Universidad Técnica de Manabí. Portoviejo, Ecuador. CP. EC130105. (johnvih@gmail.com; esteban@ciad.mx).
The effects of lead (PbAc2) and nickel (NiCl2) on the development of rice plants and the activity of antioxidant enzymes, under the same experimental conditions, were evaluated using four concentrations (0, 50, 100 and 300 mg kg-1) in two different organs of the plant (roots and leaves). The research was conducted from September 2010 to March 2011 at the Esalq in Brazil under greenhouse conditions. The activities of the enzymes superoxide dismutase (SOD) and glutathione reductase (GR) were determined by spectrophotocolorimetry and non-denaturing polyacrylamide gel electrophoresis (PAGE) was used to identify the SOD and GR isoenzymes. Rice plants with NiCl2 treatments grew less than those treated with PbAc2 at all concentrations used compared to the control. The highest specific activity of the SOD in the roots was obtained at 0 and 300 mg kg-1 of NiCl2 and 300 mg kg-1 of PbAc2. In leaves, the highest activity of this enzyme was detected with 0 mg kg-1 of NiCl2 and 0 and 300 mg kg-1 of PbAc2. The NiCl2 caused significant increases in GR activity in the roots at all concentrations with respect to the control, no significant differences were observed with respect to the control in any of the organs analyzed in the presence of PbAc2. Four isoenzymes of the SOD (two Mn and two Fe) and five GRs were found in both organs analyzed. Of the latter, the GR-V was identified as leaf tissue specific with the heavy metal Ni.
Keywords: GR; heavy metals; isoenzymes; oxidative stress; rice; SOD
Los efectos del plomo (PbAc2) y el níquel (NiCl2) sobre el desarrollo de plantas de arroz y la actividad de enzimas antioxidantes, bajo las mismas condiciones experimentales, se evaluó utilizando cuatro concentraciones (0, 50, 100 y 300 mg kg-1) en dos órganos diferentes de la planta (raíces y hojas). La investigación se realizó de septiembre de 2010 a marzo de 2011 en la Esalq en Brasil en condiciones de invernadero. Se determinaron por espectrofotocolorimetría las actividades de las enzimas superóxido dismutasa (SOD) y glutatión reductasa (GR) y se utilizó la electroforesis en gel de poliacrilamida (PAGE) no desnaturalizante para identificar las isoenzimas de SOD y GR. Las plantas de arroz con los tratamientos de NiCl2 crecieron menos que las tratadas con PbAc2 en todas las concentraciones utilizadas en comparación con el control. La mayor actividad específica de la SOD en las raíces se obtuvo a 0 y 300 mg kg-1 de NiCl2 y a 300 mg kg-1 de PbAc2. En las hojas la mayor actividad de esta enzima se detectó con 0 mg kg-1 de NiCl2 y a 0 y 300 mg kg-1 de PbAc2. El NiCl2 provocó incrementos significativos en la actividad de la GR en las raíces en todas las concentraciones con respecto al control, no se observaron diferencias significativas con respecto al control en ninguno de los órganos analizados en presencia de PbAc2. Se encontraron cuatro isoenzimas de la SOD (dos Mn y dos Fe) y cinco GR en ambos órganos analizados, de estas últimas, la GR-V se identificó como específica de los tejidos de las hojas con el metal pesado Ni.
Palabras claves: arroz; estrés oxidativo; GR; isoenzimas; metales pesados; SOD
Introduction
Rice is the most important cereal in the world, due to the extent of cultivation it occupies and its consumption by almost 3 billion people (Zhao and Mc Grath, 2009); however, it is reported as a hyperaccumulating plant of various heavy metals, the most harmful being cadmium, due to its mobility and toxicity and lead, although it is not a mobile metal, it is very toxic (Reeves et al., 2000).
Agricultural soils can be contaminated with heavy metals through industrial waste, gasoline, paint, mining waste, fertilizers, pesticides, animal fertilizers, irrigation of wastewater and other sources (Khan et al., 2008). The most commonly found heavy metals in contaminated sites are lead (Pb), cadmium (Cd), mercury (Hg), chromium (Cr), arsenic (As), zinc (Zn), copper (Cu) and nickel (Ni) (Su et al., 2014).
Heavy metals released to the environment by anthropogenic activities do not go through a process of chemical or biological (microbial) degradation (Kirpichtchikova et al., 2006) and their total concentration in soils persists for a long time after their introduction (Adriano, 2003). The critical level of toxicity in cultivated species is in the range of 10 μg g-1 of dry mass (MS) in sensitive plants and 50 μg g-1 of MS in moderately sensitive and tolerant plant species (Marschner, 1995).
Several authors have reported that the toxicity of Pb and Ni could produce oxidative cellular damage when generating reactive oxygen species (ROS) (Islam et al., 2011). The ROS are the result of oxidative stress, which in turn leads to several detrimental effects on plant cells (Islam et al., 2008) so that antioxidant enzymes can reduce or prevent the toxic effects of ROS induced by stress metal. These antioxidant enzymes are superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and glutathione reductase (GR). These enzymes can maintain the balance between the production and destruction of ROS (Hassan and Mansoor, 2014).
The antioxidant effectiveness under stress of heavy metals in roots and leaves has been studied, but reports on the antioxidant system in those organs due to the presence of Ni and Pb, which induce oxidative stress in the same experimental conditions, are limited. For this reason, the objective of this research was to evaluate the influence of Ni and Pb in the development and antioxidant system of rice, by determining the activities of superoxide dismutase (SOD) and glutathione reductase (GR) in roots and leaves in identical experimental conditions.
Materials and methods
Crop management and experimental design
The research was conducted from September 2010 to March 2011 at the Luiz de Queiroz Higher Agricultural School (ESALQ) in Brazil, in the Genetics laboratory. The 8092 rice line (O. sativa L.) was provided by the National Institute of Agricultural Sciences (INCA, Cuba). The seeds were sterilized with 5% sodium hypochlorite for 15 min, then rinsed with distilled water three times, planted in pots filled with distilled water and covered with a thin cloth to prevent evaporation of the water. The pots were placed in a greenhouse with a temperature of 35 °C and a relative humidity in a range of 32-35%.
After one week, the seedlings were transplanted into one liter pots with sand and Hoagland nutrient solution (600 ml), in four treatments of nickel chloride (NiCl2) and lead acetate (PbAc2). The experimental design was completely randomized, with four levels of NiCl2 and PbAc2 (0, 50, 100 and 300 mg kg-1). All the experiments were performed in triplicate.
The experiments were carried out in a greenhouse under natural light with temperatures in the range of 20-30 C. The Hoagland solution with Ni and Pb was changed weekly and the total volume was completed with water once a week.
Sampling
The plant samples were obtained 30 days after sowing. All samples of roots and leaves were taken in a state of physiological maturity. The material was disinfected with non-ionic detergent (1%), rinsed three times in distilled water and then transferred to filter paper. In each sample, fresh matter from the roots and leaves was used for the analysis of antioxidant enzymes and the isoenzyme profile.
Analysis of the samples
Development of plants
The development of the plants in the presence of the metals under study (NiCl2 and PbAc2) was evaluated, comparing them with a control without heavy metals.
Concentration of soluble total proteins
Bovine serum albumin (BSA) was used as a standard for the determination of protein concentration, by the method of Bradford (1976).
Extraction and analysis of antioxidant enzymes
Samples of roots or leaves were homogenized (volume of buffer: fresh mass 2:1) in a mortar with 100 mM potassium phosphate buffer pH 7.5 (1 mM ethylenediaminetetraacetic acid (EDTA), 3 mM DL-dithiothreitol and insoluble PVP 5% w/v). The homogenate was centrifuged at 10 000 × g for 30 min and the supernatant was stored in separate aliquots at -80 °C, before the GR and SOD analyzes. All steps of the extraction were carried out at 4 °C.
The activity of SOD was determined at 560 nm (Beyer and Fridovich, 1987), based on the inhibition of nitroblue tetrazolium (NBT) reduction. One unit of enzymatic activity was defined as the amount of SOD required for a 50% inhibition of the reduction of NBT. The SOD activity was expressed as μmol min-1 mg-1 protein.
The total GR activity was determined as described by Azevedo et al. (1998) at 412 nm for 2 min, analyzing the rate of reduction of oxidized glutathione (GSSG). The activity of GR was expressed as μmol min-1 mg-1 protein.
Determination of isozyme profiles of SOD and GR
The SOD and GR isoenzyme separation assays and the SOD isoenzyme classification were performed as described by Gomes-Junior et al. (2007).
Electrophoresis was carried out on 8% non-denaturing polyacrylamide gels (PAGE) for 4 h at 4 °C, with a constant current of 20 mA/gel with 60 mg of protein in each lane.
The SOD bands were classified using hydrogen peroxide and KCN-taking into account the inhibition patterns with both chemicals- such as Mn-SOD (SOD I-resistant to both inhibitors) and Fe-SOD (SOD II-inactivated by one of the inhibitors).
Statistical analysis
After checking the normality and homoscedasticity of the data (through the Kolmogorov-Smirnov and Bartlett tests, respectively), the data of the enzymatic activities were subjected to a simple variance analysis. The differences between the means of the treatments were compared using the Tukey test (p< 0.05). Statgraphics 5.1 (2001) statistical package was used for the analyzes.
Results
Development of plants
The development of the plants was affected by both metals (Ni and Pb) (Figure 1). In comparison with the control, the plants that grew in the presence of NiCl2 did not fully develop their leaves and the height of the plant was reduced in all the concentrations analyzed, especially those treated with 300 mg kg-1. The same happened with the plants to which PbAc2 was applied in the concentrations of 100 and 300 mg kg-1.
Analysis of antioxidant enzymes
Superoxide dismutase (SOD)
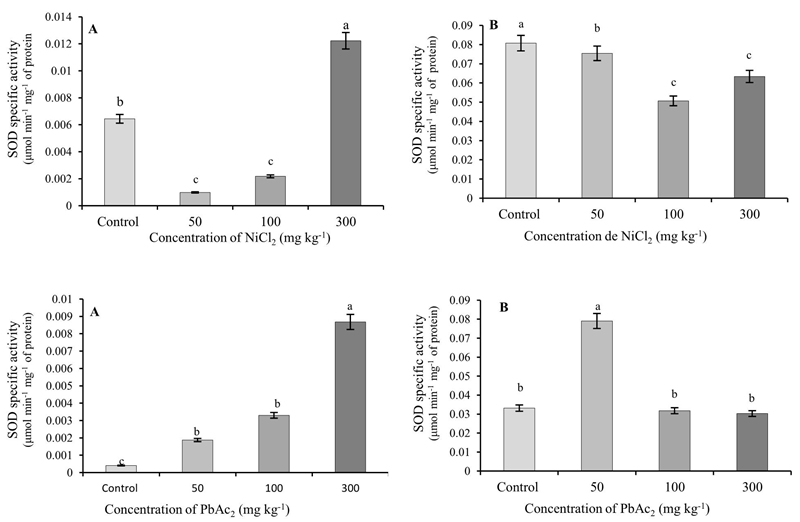
When NiCl2 was used, the specific activity of SOD in the roots decreased significantly with respect to the control in the concentrations of 50 and 100 mg kg-1 and increased significantly in the highest concentration (300 mg kg-1). When applying PbAc2 the activity of the enzyme was significantly higher than that of the control when using 50 and 100 mg kg-1, reaching the highest value with the concentration of 300 mg kg-1.
In the leaves of the plants that grew in the presence of NiCl2, the activity of the SOD decreased significantly with respect to the control, reaching the lowest values with 50 and 100 mg kg-1. The use of PbAc2 (50 mg kg-1) increased the enzymatic activity, obtaining statistically similar values to the control in the higher concentrations (Figure 2).
Glutathione reductase (GR)
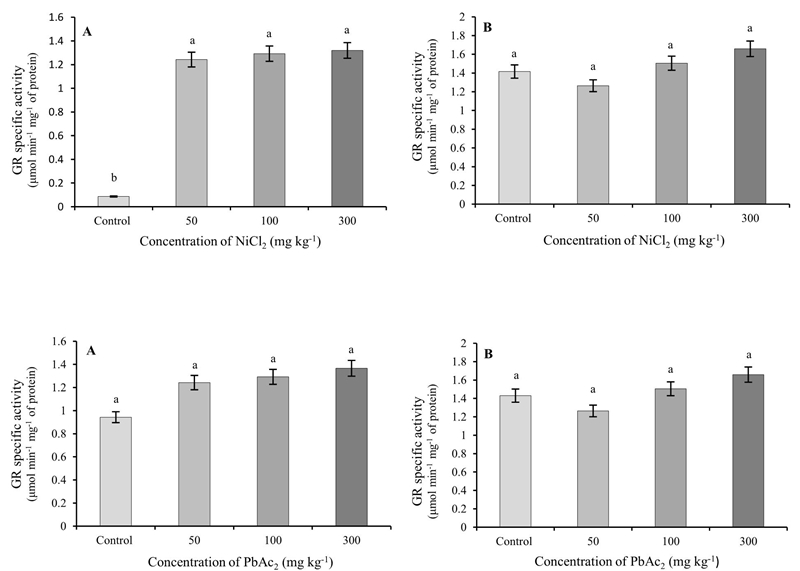
No significant differences were found in the activity of this enzyme in leaves and roots due to the use of PbAc2. In contrast, the application of NiCl2 in all the concentrations used caused significant increases in GR activity in the roots with respect to the control, without causing significant variations in the leaves (Figure 3).
Isozyme profiles
Superoxide dismutase (SOD)
The presence of SOD bands was similar to the control in all concentrations tested (Figure 4). On the other hand, some changes in the profile could be visualized, particularly in the leaves, where the Fe-SOD bands at 50 and 100 mg kg-1 of NiCl2 exhibited increases in their intensity. The treatment with PbAc2 showed in the roots at 0 mg kg-1 that the Mn-SOD2 was absent, while four bands were very intense in the leaves at 100 mg kg-1 and no band was visualized at 300 mg kg-1.
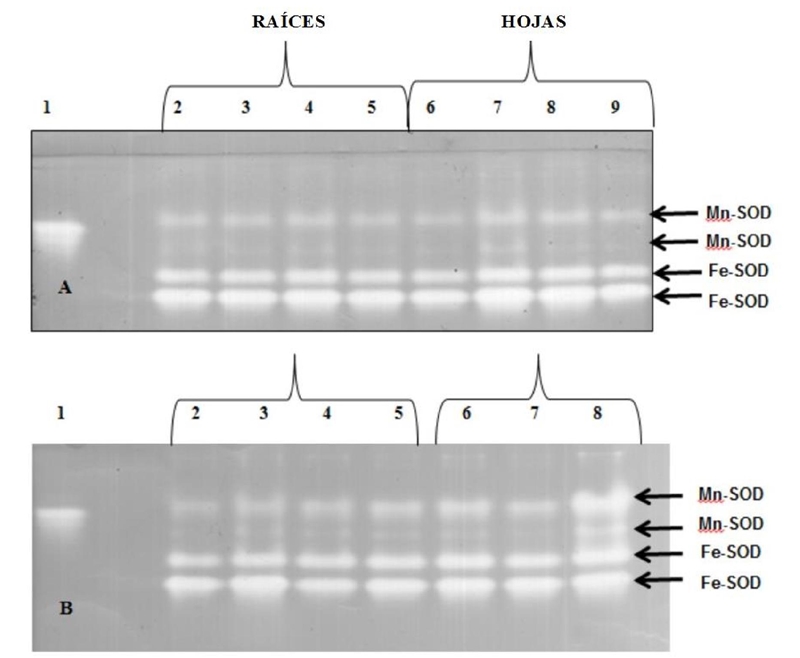
Figure 4 Isozyme profiles (SOD) in PAGE of rice plants (O. sativa L.) line 8092. A-nickel (NiCl2), B- lead (PbAc2). Lane 1= bovine serum albumin; Lane 2= control (0 mg kg-1); Lane 3= 50 mg kg-1; Lane 4= 100 mg kg-1; Lane 5= 300 mg kg-1; Lane 6= control (0 mg kg-1); Lane 7= 50 mg kg-1; Lane 8= 100 mg kg-1; Lane 9= 300 mg kg-1. The arrows indicate the SOD isoenzymes identified by staining the gels after incubation with KCN or H2O2 (5 mM in both cases).
Glutathione reductase (GR)
Five GR isoenzymes were identified (Figure 5). GR V did not appear in the roots of the plants treated with NiCl2, but it was present in the leaves, with greater intensity in the bands corresponding to the treatments with 50 and 100 mg kg-1, in these two, GR IV was also more intense at 300 mg kg-1 of NiCl2, GR IV and GR V exhibited low intensity bands in the leaf samples.
The treatment with 50 mg kg-1 of PbAc2 in the roots induced the suppression of GR V. In the leaves, the GR I was absent in all the treatments and also in the control, the use of 100 mg kg-1 reduced the intensity of GR IV and GR V, while the concentration of 300 mg kg-1 caused the suppression of all bands.
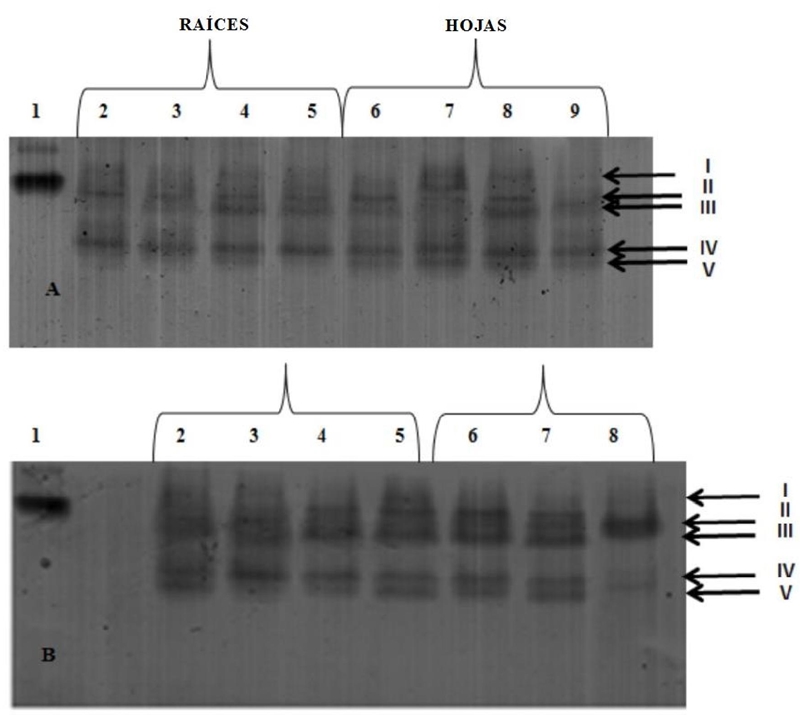
Figure 5 Isozyme profiles (GR) in PAGE of rice plants (O. sativa L.) line 8092. A-nickel (NiCl2), B-lead (PbAc2). Lane 1= GR pattern of Saccharomyces cerevisiae; Lane 2= control (0 mg kg-1); Lane 3= 50 mg kg-1; Lane 4= 100 mg kg-1; Lane 5= 300 mg kg-1; Lane 6= control (0 mg kg-1); Lane 7= 50 mg kg-1; Lane 8= 100 mg kg-1; Lane 9= 300 mg kg-1. The arrows indicate the GR isoenzymes identified.
Discussion
Heavy metals (Pb and Ni) at concentrations of 100 and 300 mg kg-1 caused a reduction in the normal development of rice plants, a common symptom that has been reported by several authors (Dogan et al., 2009; Maestri et al., 2010). This damage is related to the low activity of SOD caused by both metals in the highest concentrations (100 and 300 mg kg-1) in the leaves, which indicates that the defense system of rice plants is affected with these concentrations, showing weaknesses in the elimination pathway of superoxides and H2O2 due to stress due to Ni and Pb. It has been shown that SOD is the first line of defense against ROS, because this enzyme participates in its detoxification (Elstner, 1991).
Maheshwari and Dubey (2009) observed increases in the activities of all SOD isoforms in rice plants subjected to high concentrations of Ni (400 μM). The SOD in plants treated with Ni should confer defenses against oxidative damage, in this investigation increases in SOD activity were observed in the highest concentration of Ni and Pb (100 and 300 mg kg-1) in the roots, against what happens in wheat and pea (Gajewska and Sklodowska, 2008).
Cai-Lin et al. (2013) studied the changes in the activity of SOD with different concentrations of Cd2+, Cu2+ and Hg2+ in flowering plants of rice finding that this activity was higher in the roots, followed by the panicle and finally the leaves. In another study carried out on four rice cultivars (S17, HA63, T3028, S8258) using cadmium (50 and 250 μM), the activity of different antioxidant enzymes (POD-peroxidase, SOD, CAT-catalases, among others) was analyzed in the tissues of roots and leaves and an increase in the activity of the majority of enzymes was detected, among them the SOD in both tissues analyzed (Wang et al., 2013).
Three SOD isoenzymes according to their metallic cofactor have been reported (Mn-SOD, Fe-SOD and CuZn-SOD) in crops such as Olea europea L. (Corpas et al., 2006), Nicotiana tabacum L. (Pompeu et al., 2008), Lepidium sativum L. (Manaa et al., 2014) and others. These isoenzymes have a heterogeneous distribution among higher plant species, perhaps because their specific function depends on their location (Corpas et al., 2006).
In rice subjected to stress by cadmium, the intensity of the SOD isoforms increases in the lowest concentrations of the metal, but decreases as the concentration increases, especially if this factor is combined with thermal stress (Shah and Nahakpam, 2012).
The high level of SOD activity observed in the control may be indicative of inverse stress (due to the absence of Ni). This can be explained because Ni is an important metal in several metabolic processes, such as ureolysis, hydrogen metabolism and some others (Mulrooney and Hausinger, 2003).
In the treatments with PbAc2, the roots maintained a greater SOD activity than the leaves, especially at 50 mg kg-1. Verma and Dubey (2003) obtained similar results in growing rice plants, in which the SOD activity was higher in the roots than in the buds.
The Pb accumulates mainly in the tissues of the roots, because it is the first barrier for this translocation of metals (Blaylock and Huang, 2000), but it can concentrate in the tissues of the phloem and move through the xylem towards the leaves (Pinho and Ladeiro, 2012).
Alterations in the activity of SOD in rice, as in other species, are associated with abiotic stress of various types, including those caused by heavy metals such as manganese (Srivastava and Dubey, 2011), cadmium (Shah and Nahakpam, 2012), copper (Thounaojam et al., 2012), arsenic (Tripathi et al., 2013) and aluminum (Bhoomika et al., 2013).
At the roots, the intensity of the GR bands was approximately similar in all cases, regardless of the metal used. Ching-Lin and Ching-Hue (2005) found no effect of Ni application on GR activity in roots of rice seedlings. In this study, the Ni induced increases in the intensity of the bands of the leaf samples, particularly at 50 and 100 mg kg-1, indicating oxidative stress. Conversely, treatment with lead at 100 mg kg-1 caused a decrease in the intensity of the bands in the leaves and at 300 mg kg-1 no bands were observed.
The GR isoenzymes showed differences according to the organ of the analyzed plant and the concentration used. The isoenzymes GR III and IV were present in all the concentrations and organs of the plant analyzed. These two isoenzymes are responsible for the majority of GR activity (Pompeu et al., 2008).
The amount of GR isoenzymes varies according to the species; for example, in Arabidopsis two GRs were found (Xiang and Oliver, 1998) and in wheat five have been identified (Yannarelli et al., 2007) as in this investigation. Stress by cadmium in rice does not increase the expression of the genes that control GR except when the presence of the metal is associated with thermal stress (Chou et al., 2012). On the other hand, copper increases the activity of these enzymes (Thounaojam et al., 2012), which suggests that their expression can be selective depending on the metal present.
The GR staining revealed that GR-V is a specific isoform of leaves when Ni is present. In O. sativa, Wu et al. (2013) have described two chloroplast isoforms but Kaminaka et al. (1998) had purified and characterized the GR in this species, finding that although the enzyme is predominantly found in chloroplasts, it is also found in small amounts in mitochondria and cytosol.
Conclusions
The enzyme superoxide dismutase (SOD) showed different leaf and root behaviors for the treatments with the two heavy metals, whereas only the NiCl2 caused significant increases in the activity of glutatation reductase (GR) in the roots in all concentrations with respect to control. The PbAc2 did not cause differences in the activity of GR with respect to the control in any of the analyzed organs.
A specific isoform of glutathione reductase (GR-V) was found in the leaves of plants treated with NiCl2.
Literatura citada
Adriano, D. C. 2003. Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals. 2nd (Ed.). Springer, New York. 867 p. [ Links ]
Azevedo, R.; Alas, R.; Smith, R. and Lea, P. 1998. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild- type and a catalase deficient mutant of barley. Physiol. Plantarum. 104(2):280-292. [ Links ]
Beyer, W. F. and Fridovich, I. 1987. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical Biochem. 161(2):559-566. [ Links ]
Bhoomika, K.; Pyngrope, S. and Dubey, R. S. 2013. Differential responses of antioxidant enzymes to aluminum toxicity in two rice (Oryza sativa L.) cultivars with marked presence and elevated activity of Fe SOD and enhanced activities of Mn SOD and catalase in aluminum tolerant cultivar. Plant Growth Regulation. 71(3):235-252. [ Links ]
Blaylock, M. J. and Huang, J. W. 2000. Phytoextraction of metals. In: phytoremediation of toxic metals: using plants to clean up the environment. Raskin I, Ensley BD. JohnWiley and Sons. New York. 53-70 pp. [ Links ]
Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochem. 72(1- 2):248-254. [ Links ]
Cai-Lin, G. E.; Xiao-yong, Y. and Yang, J. 2013. Effect of heavy metal stress on different rice varieties of superoxide dismutase. Acta Agric. Nucleatae Sinica. 17(4):286-291. [ Links ]
Chou, T. S.; Chao, Y. Y. and Kao, C. H. 2012. Involvement of hydrogen peroxide in heat shock- and cadmium-induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J. Plant Physiol. 169(5):478-486. [ Links ]
Corpas, F. J.; Fernández-Ocaña, A.; Carreras, A.; Valderrama, R.; Luque, F.; Esteban, F. J.; Rodríguez-Serrano, M.; Chaki, M.; Pedrajas, J. R.; Sandalio, L. M.; del Río L. A. and Barroso, J. B. 2006. The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves. Plant Cell Physiol. 47(7):984-994. [ Links ]
Dogan, M.; Saygideger, S. D. and Colak, U. 2009. Effect of lead toxicity on aquatic macrophyte Elodea canadensis Michx. Bull. Environ. Contam. Toxicol. 83(2):249-254. [ Links ]
Elstner, E. F. 1991. Mechanism of oxygen activation in different compartments of plants cell. In: Pell EJ, Steffen KL, eds. Active oxygen/oxidative stress and pant metabolism. Rockville, MD. 13-25 pp. [ Links ]
Gajewska, E. and Skłodowska, M. 2008. Differential biochemical responses of wheat shoots and roots to nickel stress: antioxidative reactions and proline accumulation. Plant Growth Reg. 54(2):179-188. [ Links ]
Gomes-Junior, R. A.; Gratão, P. L.; Gaziola, A. S.; Mazzafera, P.; Lea, P. J. and Azevedo, R. A. 2007. Selenium-induced oxidative stress in coffee cell suspension cultures. Functional Plant Biol. 34(5):449-456. [ Links ]
Hassan, M. and Mansoor, S. 2014. Oxidative stress and antioxidant defense mechanism in mung bean seedlings after lead and cadmium treatments. Turkish J. Agric. For. 38:55-61. [ Links ]
Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Khan, M. A.; Mahmood, Q.; Hayat, Y. and Imtiaz, M. 2011. Effect of Pb toxicity on the growth and physiology of two ecotypes of Elsholtzia argyi and its alleviation by Zn. Environ. Toxicol. 26(4):403-416. [ Links ]
Islam, E.; Liu, D.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q.; Tian, S. and Li, J. 2008. Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazardous Materials. 154(1-3):914-926. [ Links ]
Kaminaka, H.; Morita, S.; Nakajimma, M.; Masumara, T. and Tanaka, K. 1998. Gene cloning and expression of cytosolic glutathione reductase in rice (Oryza sativa L.). Plant Cell Physiol. 39(12):1269-1280. [ Links ]
Khan, S.; Cao, Q.; Zheng, Y. M.; Huang, Y. Z. and Zhu, Y. G. 2008. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollution. 152(3):686-692. [ Links ]
Kirpichtchikova, T. A.; Manceau, A.; Spadini, L.; Panfili, F.; Marcus, M. A. and Jacquet, T. 2006. Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Geochimica et Cosmochimica Acta. 70(9):2163-2190. [ Links ]
Maestri, E.; Marmiroli, M.; Visioli, G. and Marmiroli, N. 2010. Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ. Exp. Bot. 68(1):1-13. [ Links ]
Maheshwari, R. and Dubey, E. R. S. 2009. Nickel-induced oxidative stress and the role of antioxidant defense in rice seedlings. Plant Growth Regulation. 59(1):37-49. [ Links ]
Manaa, A.; Mimounia, H.; Terrasa, A.; Chebila, F.; Wastia, S.; Gharbib, E. and Ahmeda, H. B. 2014. Superoxide dismutase isozyme activity and antioxidant responses of hydroponically cultured Lepidium sativum L. to NaCl stress. J. Plant Inter. 9(1):440-449. [ Links ]
Marschner, H. 1995. Mineral nutrition of higher plants. Academic Press. New York, USA. 672 p. [ Links ]
Mulrooney, S. B. and Hausinger, R. P. 2003. Nickel uptake and utilization by microorganisms. FEMS Microbiology Reviews. 27(2-3):239-261. [ Links ]
Pinho, S. and Ladeiro, B. 2012. Phytotoxicity by lead as heavy metal focus on oxidative stress. J. Bot. 2012:1-10. [ Links ]
Pompeu, G. B.; Gratão, P. L.; Vitorello, V. A. and Azevedo, R. A. 2008. Antioxidant isoenzyme responses tonickel-induced stress in tobacco cell suspension culture. Sci. Agric. 65(5):548-552. [ Links ]
Reeves, R.D.; Baker, A.J.; Raskin, I. and Ensley, B. D. 2000. Phytoremediation of toxic metals, In: Wiley (Eds.). New York. 193-229 pp. [ Links ]
Shah, K. and Nahakpam, S. 2012. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Biochem. 57:106-113. [ Links ]
Srivastava, S. and Dubey, R. S. 2011. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regulation. 64(1):1-16. [ Links ]
Statgraphics plus 5.1. 2001. Statistical graphics corp. http://www.statgraphics.com/. [ Links ]
Su, C. H.; Jiang, L. and Zhang, W. 2014. A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environ. Skeptics and Critics. 3(2):24-38. [ Links ]
Thounaojam, T. C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G. D. and Panda, S. K. 2012. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Phsyiol. Biochem. 53:33-39. [ Links ]
Tripathi, P.; Tripathi, R. D.; Singh, R. P.; Dwivedi, S.; Shri, M.; Trivedi, P. K. and Chakrabarty, D. 2013. Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol. Eng. 52:96-103. [ Links ]
Verma, S. and Dubey, R. S. 2003. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 164(4):645-655. [ Links ]
Wang, X.; Zhong-Wei, Zh.; Shi-Hua, T.; Wen-Qiang, F.; Xu, F.; Zhu, F.; Da-Wei, Zh.; Jun-Bo, D.; Yuan, Sh. and Hong-Hui, L. 2013. Comparative study of four rice cultivars with different levels of cadmium tolerance. Biologia. 68(1):74-81. [ Links ]
Wu, T. M.; Lin, W. R.; Kao, L. T.; Hsu, Y. T.; Yeh, C. H.; Hong, C. Y. and Kao, C. H. 2013. Identification and characterization of a novel chloroplast/mitochondria co-localized glutathione reductase three involved in salt stress response in rice. Plant Mol. Biol. 83(4- 5):379-390. [ Links ]
Xiang, C. and Oliver, D. J. 1998. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 10:1539-1550. [ Links ]
Yannarelli, G. G.; Fernández-Alvarez, A. J.; Santa-Cruz, D. M. and Tomaro, M. L. 2007. Glutathione reductase activity and isoforms in leaves and roots of wheat plants subjected to cadmium stress. Phytochemistry. 68(4):505-512. [ Links ]
Zhao, F. and Mcgrath, S. 2009. Biofortification and phytoremediation. Current Opinion in Plant Biology. 12(3):373-380. [ Links ]
Received: January 01, 2019; Accepted: February 01, 2019











 texto em
texto em