Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 n.8 Texcoco Nov./Dec. 2018 Epub Oct 06, 2020
https://doi.org/10.29312/remexca.v9i8.1030
Articles
Water preconditioning on germination and emergence of Capsicum chinense Jacq.
1University of Papaloapan Tuxtepec. Central Circuit 200, Industrial Park, San Juan Bautista Tuxtepec, Oaxaca. ZC. 68300. (oscarnzg@unpa.edu.mx; axavila@unpa.edu.mx).
2Institute of Chemical-Biological Research-Michoacán University of San Nicolás de Hidalgo, Morelia, Michoacán, Mexico. (eldabelt@umich.mx).
3Center for Biosciences and Biotechnology-State University of North Fluminense. Av. Alberto Lamego 2000, California Park, Campos dos Goytacazes, RJ Brazil. ZC. 28013-600. (elenir@uenf.br).
The objective of this work was to determine the effect of water pre-conditioning on the percentage of germination and emergence of Capsicum chinense. The seeds were cultivated during the productive cycle from December 2015 to February 2016. For the pre-conditioning, the seeds were imbibed with 2.5, 3.5, 5, 7.5 and 10 ml of water. The volume of 7.5 ml was used as a reference. Germination results indicate that the three best imbibition volumes were 2.5, 3.5 and 5 mL, with a Pearson correlation of -0.905 (p= 0.000) between the volume of water and the percentage of accumulated germination, as well as the decrease of the germination time 50. Subsequently, the same seeds were transplanted to evaluate the emergence. The best imbibition volumes for the emergency were, in that order, 3.5, 5 and 2.5 ml. In the emergency, the volume with the best percentage of germination (2.5 ml) occupied the third place, which modified the Pearson coefficient to -0.641 (p= 0.01). The results suggest that a certain degree of stress, generated by the decrease in water supply, favors germination and establishment, which could be related to the synthesis and accumulation of ethylene within the germination system. On the other hand, the increase in the diameter of the water film generates a decrease in the availability of oxygen. The contribution of the present work was to demonstrate that the correct hydration of the seeds influences later stages of germination, without adding growth regulators.
Keywords: Capsicum chinense; germination; water preconditioning
El objetivo de este trabajo, fue determinar el efecto del pre-acondicionamiento hídrico sobre el porcentaje de germinación y la emergencia de Capsicum chinense. Las semillas fueron cultivadas durante ciclo productivo diciembre 2015 a febrero 2016. Para el pre-acondicionamiento, las semillas se imbibieron con 2.5, 3.5, 5, 7.5 y 10 ml de agua. El volumen de 7.5 ml, se usó como referencia. Los resultados de germinación indican que, los tres mejores volúmenes de imbibición fueron 2.5, 3.5 y 5 mL, con una correlación de Pearson de -0.905 (p= 0.000) entre el volumen de agua y el porcentaje de germinación acumulada, así como la disminución del tiempo de germinación 50. Posteriormente, las mismas semillas, se trasplantaron para evaluar la emergencia. Los mejores volúmenes de imbibición para la emergencia fueron, en ese orden, 3.5, 5 y 2.5 ml. En la emergencia el volumen con el mejor porcentaje de germinación (2.5 ml), ocupó el tercer lugar, lo que modificó el coeficiente de Pearson a -0.641 (p= 0.01). Los resultados sugieren que, un cierto grado de estrés, generado por la disminución en el aporte de agua, favorece la germinación y el establecimiento, lo que podría estar relacionado con la síntesis y acumulación de etileno dentro del sistema de germinación. Por otro lado, el aumento en el diámetro de la película de agua genera disminución en la disponibilidad de oxígeno. La contribución del presente trabajo fue evidenciar que la correcta hidratación de las semillas influencia etapas posteriores de la germinación, sin adicionar reguladores del crecimiento.
Palabras clave: Capsicum chinense; germinación; pre-acondicionamiento hídrico
Introduction
Capsicum sp. cultivation has cultural and economic relevance in Mexico; in recent years, it has started to take on considerable economic importance worldwide. Even though, Mexico is considered one of the centers of origin and diversification of chilli, Montes et al. (2010), Perez-Castañeda et al., 2015) this crop is successfully cultivated in China, Turkey, Indonesia, Spain and USA; according to FAO (2014) these countries together with Mexico are the six largest producers of chilli worldwide. In 2013, China was the first producer country with 15 800 000 t followed by Mexico with a 2 294 400 t production, which equals to 56 and 8% of the world production, respectively FAO (2014). There are also local species that have commercial success and its cultivation is protected by designation of origin. In particular, for Capsicum chinense Jacq (best known as habanero chili), the agricultural sector of the Yucatan Peninsula, Mexico, obtained the denomination of origin in 2005 and in 2012, its annual production reached 2 615 t with a value of $28 487 070.00 mexican pesos (SIAP, 2012; Moo-Muñoz et al., 2016).
Unfortunately, C. chinense producers have reported important problems associated to its cultivation, such as the low quality seeds, viability loss due to storage time and as a result, high costs of the seeds that reflects on an increase on the production costs Garruña-Hernández (2014); Moo-Muñoz et al. (2016). Some authors attribute these problems to the physiological characteristics of C. chinenseGarruña-Hernández (2014), while others associate them with poor management during cultivation or drying and storage of the seeds (Moo-Muñoz et al., 2016). In order to overcome these disadvantages, different strategies have been explored: i) assurance of physiological quality during seed development by optimizing the drying process; ii) use of phytoregulators to enhance production of viable fruits and therefore, seeds viability Zarate-García et al. (2014) and iii) analyzing the effect of water preconditioning supplemented with gibberellic acid (GA3) and abscicic acid (ABA) to improve germination and decrease seed viability loss (Moo-Muñoz et al., 2016).
Research has shown that seed quality is genetically determined (conditioning physiological processes), although there are other factors involved such as nutrition and health of the parent plant and storage conditions. However, since the decrease on viability and loss of germination ability are still not clearly explained, it is interesting to investigate if this behavior can be reverted by treatments prior to sowing that reduce the time required to radicle protrusion (germination) and emergence of the seedlings and that together are called preconditioning (Nicasio-Arzeta et al., 2011). There are several preconditioning methods that can be classified in a) osmotic, b) matric, c) water stress (Heydeker et al., 1973; Taylor, 1981; Iqbaar y Ashraf, 2005; Artola et al., 2010; Hacisalhoglu y Ross, 2010; Mavi et al., 2010; Sánchez y Muñoz, 2010; Moo-Muñoz et al., 2016). In water stress preconditioning, water availability for germination is regulated by limiting the quantity and time of hydration (Nicasio-Arzeta et al., 2011).
These studies focused on one specific phenological stage and do not give details about the effect of the evaluated treatments on subsequent development stages, but only on germination, emergence and establishment (sprout of the first true leaf), which means that it has not been investigated if the effect of preconditioning treatments on plant development is lasting. Since an adequate imbibition allows the reactivation of metabolic processes such as enzyme activation, mechanisms for membrane, DNA and protein repair, cell elongation and radicle appearance, it has been widely reported that water availability is essential for seed germination Dubreocq et al. (2000); Maldonado et al. (2003); therefore, plants should synchronize their growth cycles with and adequate water supply (Foley and Fenhimoe, 1998; Maldonado et al., 2003). There is not information on water requirements for Capsicum chinense; the objective of this research was to determine if water preconditioning of Capsicum chinense Jacq var Jaguar is enough to improve germination and emergence percentages.
Materials and methods
Biological material
Capsicum. chinense, var. Jaguar mature seeds were obtained from the INIFAP experimental field Las Huastecas, Tampico, Tamaulipas, Mexico. Seeds were sown in the December 2015 - February 2016 productive cycle. Seeds were treated with PIRIMFOS and THIRAM 42% by the supplier and then, vacuum packed in a dark bag. In the laboratory, seeds were divided in subsets of 300mg of seeds, packed in 7 x 5 cm transparent polyethylene bags and stored at approximately 25°C.
Preconditioning experiment and germination evaluation
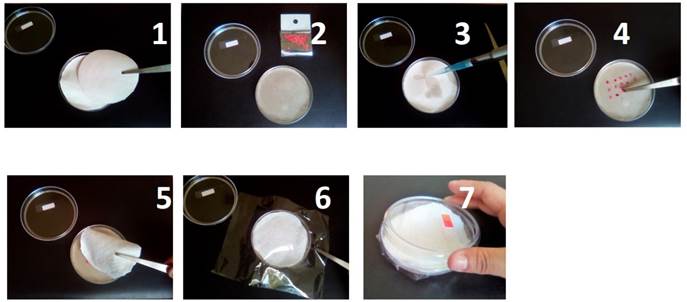
First, we performing the germination test using rolling paper, according ISTA criteria (2016, chapter 5, subsection 5.6.2.1.1, “Between paper”) and we noticed that, the maximum percentages of germination were very low. Additionally, when performing the morphometric evaluations, the roots tended to injure themselves and finally, it was difficult to control the amount of water supplied. Then, we opted for performed other ISTA method; “Top of paper” in Petri dishes, the sequence is illustrated in the Figure 1.
Preconditioning and germination were performed in 8.5 cm-diameter Petri dishes (ISTA, 2016 ch. 5 subsections 5.6.2.1.1), with the follow modifications: two circle of paper food grade (estraza) were placed inside (1), and moistened with the different volumes of water: 2.5, 3.5, 5, 7.5 and 10 ml (3), 20 seeds were distributed equidistant manner, on this circles of paper (4). Later, a layer of white flannel, previously washed three times with distilled water and dry, was placed above seeds (5). The water volume 7.5 mL was used as control. This water volume was adjusting to petri dish’s diameter employed (8.5 cm diameter), considered that Garruña-Hernández (2014) employed the volume of 8 mL in Petri dish’s 9 cm-diameter. Finally, the dishes were sealed with a layer of flexible food grade polyvinyl chloride film (EGAPACK®) (6) and incubated in a germinator chamber for 4 days at 29 ±0.5 °C, with photoperiod control at 12h light/12h dark (7). From day 4 after imbibition start (DAIS), the seal was opened quickly and carefully, to verify germination daily.
Four variables were evaluated in the preconditioning experiments: 1) cumulative germination percentage, 2) cumulative emergence percentage, 3) radicle length and 4) seed dormancy period (days passed until the first germinated seed).
Cumulative germination was registered daily until day 9 after imbibition start (9 DAIS) because in pilot experiment, when the percentage germination in 7.5 ml was similar to reported by Garruña-Hernandez (2014), the fast treatment has been completed the emergency inside Petri’s dish. Germination criteria was that the protruded root reached 1mm long. Germinated seeds at 9 DAIS were transplanted to polystyrene pots with 5 g of Miracle-Gro (The Scotts Lawn Company, Ohio USA), 0.21 N -0.11 P -0.16 K. Seeds were placed at a 2.5 cm depth and the pots were incubated in a growth chamber under the conditions previously described, watered daily with 3 ml of distilled water and observed for the following 10, 13, 14 and 15 DAIS, for registering the emergence of the cotyledons from the substrate. Three independent replicates with three repetitions each one were made for each evaluated volume (n= 9). Quantitative values were calculated as follows.
Total germination capacity or cumulative total germination percentage (CTG %).
Where: nFPR= final protruded radicle at 9 days after imbibition start (DAIS); n= number of seeds used per repetition.
Inferred time to reach 50% germination (GT50) and 90% germination (GT90). It was calculated by PROBIT test using the cumulative germination value.
Total emergence percentage or cumulative emergence percentage (CE%).
Where: nTSEC= number of total seedlings with emerged cotyledons at 15 DAIS; n= number of seeds used per repetition.
Time when 90% seedling has emerged (ET90). It was calculated by PROBIT test using the cumulative emergence values.
Cumulative radicle length (CRL).
Where:
Values of germinated seeds per Petri dish were converted to percentage considering 20 seeds as 100%. Values of cumulative germination percentage were graphed and analyzed in order to determine significant differences between treatments.
A completely randomized experimental design was used. Data were analyzed by one way ANOVA followed by Tukey test. Pearson’s correlation coefficient at 9 DAIS, p≤ 0.05, was calculated. All statistical analysis was performed in Minitab® software (Minitab Inc. 2007), version 15 for Windows, State College, Pennsylvania, USA. Graphs were made using Office Excel 2016 (ver. 1611)
Results and discussion
The set of seeds used for the study was evaluated according to ISTA criteria; all repetitions reached more than 70% germination; therefore, they were considered suitable for the assays Garruña-Hernandez et al. (2014). In the present work, we studied the effect of water preconditioning on seed germination and emergence; germination percentage was quantified in Petri dishes while emergence percentage was determined after the germinated seeds were transplanted in pots with substrate (Figure. 2).
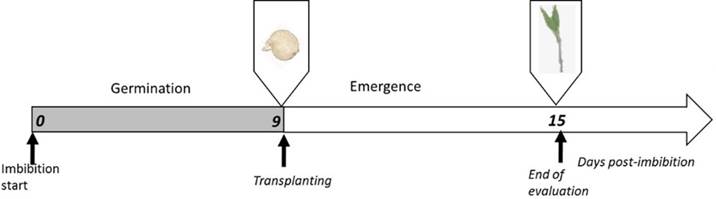
Figure 2 Experimental strategy. Timeline indicating the days after imbibition start (DAIS). Germination was evaluated daily until transplanting at 9 DAIS and emergence until 15 DAIS.
Germination was quantified until 9 DAIS, since in a pilot assay it was observed that at this time, seeds from two treatments reached at least 70% of germination. Results of cumulative germination percentage regarding time are shown in Figure 3a, where it can be observed that 2.5 and 3.5 ml of imbibition water significantly increased (p≤ 0.05) cumulative germination percentage compared to 7.5 ml (reference volume). This difference appears since the fourth day after imbibition start and except for this time, there were no significant differences between 2.5 and 3.5 ml (p˃ 0.05). Figure 3b shows the results of cumulative germination percentage in response to water conditioning with 5 and 10 ml compared to 7.5 ml (reference volume). Cumulative germination percentage increased only in the 5 ml water imbibition treatment since 7 DAIS (p≤ 0.05).
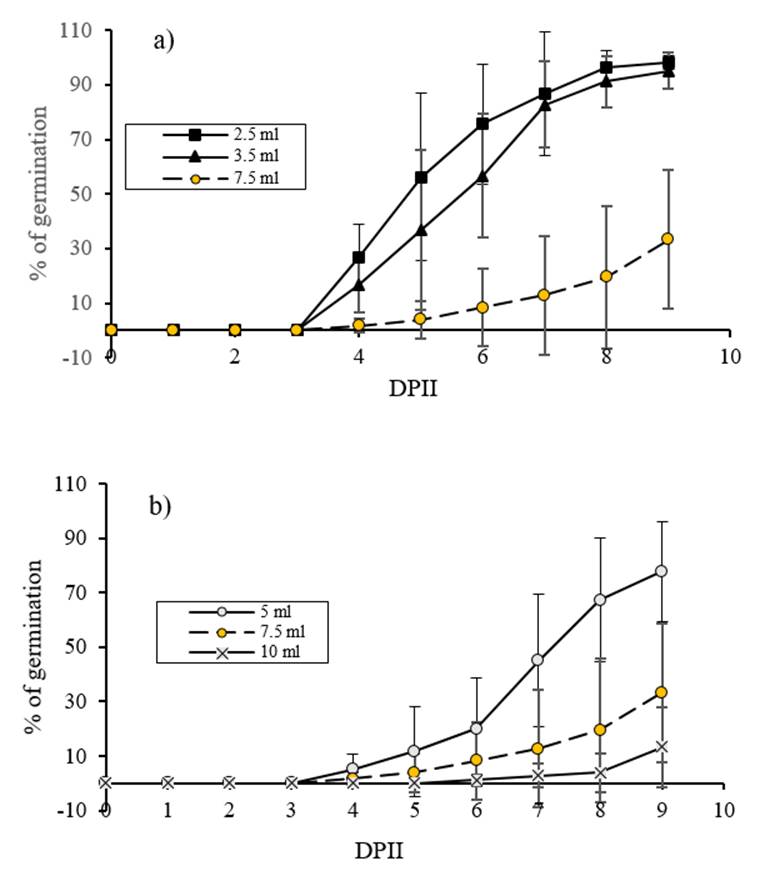
Figure 3 Effect of different water imbibition volumes on C. chinense germination. a) seeds were imbibed with 2.5 and 3.5 ml compared to the reference volume (7.5 ml). b) Seeds were imbibed with 5 and 10 ml compared to the reference volume (7.5 ml). Germination was evaluated daily after imbibition start.
Germination capacity or maximum cumulative germination percentage at 9 DAIS showed an inversely proportional relationship to the amount of water used for seed imbibition. Pearson correlation coefficient was -0.905, p= 0.000. There were no significant differences in the maximum cumulative germination percentage at 9 DAIS between treatments 2.5, 3.5 and 5 ml of water (p˃0.05), even though they showed different averages which can be due to the high biological variability inherent to Capsicum chinense seeds (SIPRUFO, 2009; Zárate-García y Sánchez-Azcorra, 2014; Garruña-Hernández et al., 2014; Moo-Muñoz et al., 2016). In addition, there were significant decrease in the maximum cumulative germination percentage in 10 ml with respect to 7.5 ml (the reference volume) at 9 DAIS (p≤ 0.05) (Table 1). In Figure 2, it can be observed that as the imbibition volume increases, the variability also increases which does not allow to demonstrate statistically the differences observed between all results.
Table 1 Germination capacity at 9 DAIS and dormancy period showing standard deviation, as well as pair Tukey test comparisons.
| Preconditioning Volume | N | Germination capacity or final germination percentage* | Dormancy period* | Inferred GT50 (DAIS)** |
|---|---|---|---|---|
| 2.5 | 9 | 98.3 ±2.5 A | 3.2 ±0.4 A | 5.19 ±1.4 A |
| 3.5 | 9 | 95 ±6.6 A | 3.7 ±2.5 A | 5.78 ±1.6 A |
| 5 | 9 | 78 ±18.3 A | 5 ±1.2 A | 7.35 ±1.8 AB |
| 7.5 | 9 | 33.3 ±25.5 B | 7 ±2.3 B | 9.78 ±2.3BC |
| 10 | 9 | 13.3 ±14.8 B | 8.7 ±1.9 B | 11.19 ±2.1C |
*= Means with different letters are significantly different, p≤ 0.05; **= Data inferred by PROBIT method using MINITAB.
Radicles of the seedlings were measured during imbibition showing similar results to germination pattern; stress for limited water, favored the increase in radicle length (Figure.4), with a Pearson correlation coefficient at 9 DAIS of -085, p= 0.001. However, in the treatment with 2.5 ml, there is a decrease in the length values at 9 DAIS, which can indicate that an excessive decrease of water availability may end up affecting growth. These results might be explained since roots are the key organ to drought adaptation Maldonado et al. (2003); Foolad, (2007); Florido y Fundora (2014) and therefore, its growth and architecture respond to the aerial organs needs Heydecker et al. (1973); Petruzelli et al. (2003). In other vegetal species, it has been determined than an early increase in root length is an indicator of stress resistance (Florido and Fundora, 1998; Farooq et al., 2008).
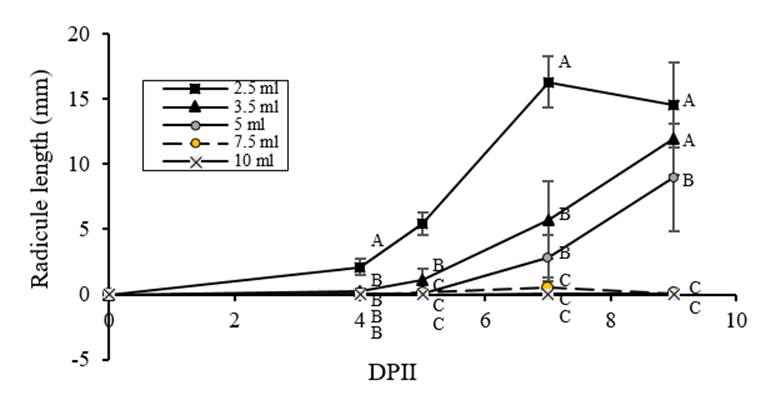
Figure 4 Effect of different water imbibition volumes on C. chinense radicle length. a) Seeds were imbibed with 2.5, 3.5, 5, 7.5 and 10 ml distilled water and radicle length was measured at 4, 5, 7 and 9 DAIS. Reference volume was 7.5 ml.
In order to determine the effect of water volume on germination rate, GT50 was calculated using the Probit method that allows to make inferences, assuming a normal distribution of the data. This approach was taken for two reasons: a) increasing the imbibition volume results in a higher variability in the data and b) germination percentage for 7.5 and 10 ml did not reach 50% germination in the time evaluated. The obtained results allowed calculating the time necessary to reach 50% germination for each imbibition treatment (Table 1). The reference volume (7.5 ml) had a GT50 of 9.78 days; volumes of 2.5 and 3.5 ml significantly decreased this time (5.19, and 5.78 days, respectively, p≤ 0.05), although there were no significant differences between them, while 5 ml did not show significant difference compared to 7.5 ml. On the contrary, 10 ml significantly increased the GT50 (11.19 days). These results show that stress for limited water, increases germination rate.
Furthermore, dormancy period in seeds imbibed in low volumes shortens significantly compared to the reference volume and 10 ml. The observed effect is similar to results obtained in others models, where seeds preconditioning increased germination rate and final percentage (Mc Donald, 2000; Foolad, 2007; Labate et al., 2007; Morandi-Dezfulli et al., 2008).
In previous works, it has been reported that stress conditions related with water availability favor germination in Capsicum chinense (Garruña-Hernández et al., 2014), maybe due to genetic characteristics that allow seeds adapting to a habitat with water limitations (Maldonado et al., 2003). In others models, such as some varieties of Zea mays and Helianthus annuus L., a controlled limitation of water entry favors germination rate both with osmotic and water preconditioning (Dubreuq et al., 2000; Mc Donald, 2000; Ramón y Mendoza, 2002; Rojo, 2005; Zhang et al., 2007; Farooq et al., 2008; Guan et al., 2009; FAOSTAT, 2014).
In general, the tendency of the evaluated parameters show that increasing the imbibition water increases germination time, dormancy period and GT50 (Table 1). In addition, other factors in the system should be considered; for example, oxygen, which is essential for seed aerobic metabolism (Prisco et al., 1992; Koorneef et al., 2002; Ramón and Mendoza, 2002) and that could be less available when increasing water volume, forming a thicker film which decreases diffusion of this gas. Also, it is important to consider some elements of the seed coat that when soaking in water make difficult the entry of oxygen to the embryo (Doria, 2010; Rojo, 2005).
Another important variable to consider is that the system used in the present work is sealed with a polyvinyl chloride film, and this should be also taken in account for accumulation of gases Mavi et al. (2010) that are products of seed metabolism such as ethylene gassemian, which is tightly related to germination when inhibited by ABA (Beaudoin et al., 2000). In this sense, it has been reported that ethylene promotes seed germination by antagonizing with ABA (Beaudoin et al., 2000; Limkies et al., 2009; Ghassemian et al., 2014). In addition, ethylene induces expression of genes codifying for enzymes related to radicle protrusion such as β-1,3-glucanase (Petruzzelli et al., 2003).
On another hand, regarding the average of cumulative emergence percentage (Figure 5), it can be observed that the highest values correspond to the lower volumes (3.5, 5 and 2.5 ml, respectively) compared to the reference volume (7.5 ml), while the lowest values correspond to 10 ml. It was also observed that when the imbibition volume increased, data dispersion also increased as occurred for germination. Due to this increase in dispersion, significant differences were only found at 13 DAIS. The Pearson correlation coefficient was -0.641, p =0.01 for the distribution of the values of emergence percentage versus imbibition volumes. It can be observed that treatments with 3.5 and 5 ml are distributed above the regression line, while data from 2.5 ml treatment are distributed below the regression line. Data from the reference volume (7.5 ml) and 10 ml treatment had a big dispersion.
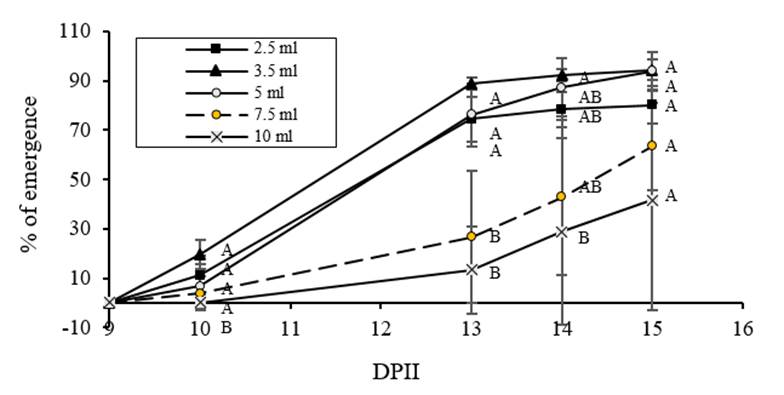
Figure 5 Effect of imbibition volume on C. chinense emergence. Seeds imbibed with 2.5, 3.5, 5 and 10 ml of water and the reference volume (7.5 ml) were transplanted to soil and plant emergence was evaluated 1, 4, 5 and 6 days after transplanting.
It is important to notice that even though 2.5 ml was the best treatment for germination, when analyzing emergence this treatment occupied the third place. This behavior may suggest that even though, limited water stress, favor germination, when it is excessive, it has a negative effect on the emergence (Heydecker et al., 1973; Sánchez et al., 2001; Rojo, 2005; Stepunh y Raney, 2005; Moradi-Dezfulli et al., 2008; Doria et al., 2010; Nicasio-Arzeta et al., 2011; Florido and Fundora, 2014).
Finally, GT90 and ET90 were calculated by the Probit method using the MINITAB software. A stacked bar graph was plotted with these values, in order to compare the sum of the total estimated times at which 90% emergence would be reached after transplanting seeds with 90 % germination (Figure 6). Total time for seeds imbibed with the reference volume (7.5 ml) was 22.6 days, while for 3.5 ml was 13 days, which correspond to approximately half of the time (0.59 times). Summation of GT90 and ET90 of seeds imbibed with 2.5, 5 and 10 ml were 15, 16 and 24 days, respectively and correspond to 0.68, 0.72 and 1.09 times the sum of the seeds treated with the reference volume. Results show that treatments with 3.5 and 5 ml, in that order, favor emergence and even though 2.5 ml was the best treatment for stimulating germination, it occupied the third place for emergence. Therefore, it can be concluded that a higher germination rate does not necessarily results in an improvement on emergence percentage.
Conclusions
Water preconditioning in C. chinense seeds was enough for stimulating germination and decreasing GT50 and the dormancy time in an inversely proportional way, i.e. the highest germination percentage and rate were obtained with the lowest water imbibition volumes. However, when C. chinense seeds were transplanted, in order to analyses the emergence, was not there a higher inverse correlation between imbibition volume and emergence percentage, suggesting that a higher germination rate induced by low volumes of imbibition water (2.5 mL), does not necessarily result in an improvement on emergence.
The contribution of the present work was to evidence that the correct seed hydration has an effect on later stages to germination, even, without adding growth regulators or inducers. Also, it was observed that water preconditioning decreases the erratic behavior (great standard deviations) of the analyzed parameters during germination.
We suggest that in future studies on C. chinense seedlings development, water preconditioning should be considered as the first option for optimizing production technologies and GT90 and ET90 used to predict optimal germination and emergence times. In order to be able to adequately advice producers, it is necessary to analyze more phenological stages.
Literatura citada
Artola, A.; Carrillo, C. G. and De los Santos, G. 2010. Hydropriming: a strategy to increase Lotus corniculatus L. seed vigor. Seed Sci. Technol. 31(2):455-463. [ Links ]
Beaudoin, N.; Serizet, C.; Gosti, F. and Giraudat, J. 2000. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 12(7):1103-1116. [ Links ]
Doria, J. 2010. Generalidades sobre las semillas: su producción, conservación y almacenamiento. Cultivos Tropicales. 31(1):74-85. [ Links ]
Dubreucq, B.; Berger, N.; Vincent, E.; Boisson, M.; Caboche, M. and Lepiniec, I. 2000. The Arabidopsis Atepr1 extensin-like gene is specifically expressed in endosperm during seed germination. J. Plant. 23(5):643-652. [ Links ]
FAOSTAT. 2014. FAOSTAT http://www.fao.org/faostat/en/#rankings/countries-by-commodity. [ Links ]
Farooq, M.; Aziz, T.; Basra, A.; Cheema, M. A. and Rehman, H. 2008. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop. Sci. 194(2):161-168. [ Links ]
Farooq, M.; Wahid, A.; Ahmad, N. and Asad, S. P. 2010. Comparative efficacy of surface drying and re-drying seed priming in rice: changes in emergence, seedling growth and associated metabolic events. Paddy Water Environ. 8(1):15-22. [ Links ]
Florido, M. and Fundora, L. 2014. Tolerancia a estrés por déficit hídrico en tomate (Solanum lycopersicum L.) Cultivos Tropicales . 35(3):70-88. [ Links ]
Foley, M. E. and Fennimore, S. A. 1998. Genetic basis for seed dormancy. Seed Sci. Res. 8(2):173-182. [ Links ]
Foolad, M. 2007. Tolerance to abiotic stresses. In: Razdan, M. K. y Matoo, A. K. (Eds.). Genetic improvement of solanaceous crops: tomato. Science Publishers Enfield. 2:521-590. [ Links ]
Garruña, H. R.; LaTournerie, M. L.; Ayala, G. O.; Santamaría, J. y Pinzón, L. L. 2014. Acondicionamiento pre-siembra: una opción para incrementar la germinación de semillas de chile habanero (Capsicum chinense jacq). Agrociencia. 48(4):413-423. [ Links ]
Ghassemian, M.; Nambara, E.; Cutler, S.; Kawaide, H.; Kamiya, Y. and McCourt, P. 2000. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell . 12(7):1117-1126. [ Links ]
Guan, Y. J.; Hu, J.; Wang, X. J. and Shao, Ch. J. 2009. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejianq Univ. Sci. B. 10(6):427-433. [ Links ]
Hacisalihoglu, G. and Ross, Z. 2010. The influence of priming on germination and soil emergence of non-aged and aged annual ryegrass seeds. Seed Sci. Technol . 38(6):214-217. [ Links ]
Heydecker, W.; Higgins, J. and Gulliver, R. L. 1973. Accelerated germination by osmotic seed treatment. Nature. 246(2):42-46. [ Links ]
Iqbar, M. and Ashraf, M. 2005. Changes in growth, photosynthetic capacity and ionic relations in spring wheat (Triticum aestivum L.) due to pre-sowing seed treatment with polyamines. Plant Growth Regul. 46(1):19-30. [ Links ]
ISTA. 2017. International rules for seed testing. International rules for seed testing. http://www.seedtest.org/en/ista-rules-for-2017--content---1--1448.html. [ Links ]
Koornneef, M.; Bentsink, L. and Hilhorst, H. 2002. Seed dormancy and germination. Curr. Opin. Plant Biol. 5(1):3-36. [ Links ]
Labate, J.; Grandillo, S.; Fulton, T.; Munos, S.; Caicedo, A.; Peralta, I.; Ji, Y.; Chetelat, R.; Scott, J.; Gonzalo, M. 2007. Tomato In: genome mapping and molecular breeding in planets genome mapping. Springer International Publishing AG. NY. USA. Mol Breed. 5:1-125. [ Links ]
Linkies, A.; Muller, K.; Morris, K.; Tureckova, V.; Wenk, M.; Cadman, C. S. C.; Corbineau, F.; Strnad, M.; Lynn, J. R.; Finch-Savage, W. E. and Leubner-Metzger, G. 2009. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell . 21 (12):3803-3822. [ Links ]
Maldonado, C.; Pujado, E. and Squeo, F. A. 2003. Phenotypic response of Lycopersicum chilense to water deficit. Rev. Chil. His. Nat. 76 (2):129-137. [ Links ]
Mavi, K., Light, M. E.; Demir, I.; Staden, V. and Yasar, F. 2010. Positive effect of smoke-derived butenolide priming on melon seedling emergence and growth. J. Crop Hort. 38(2):147-155. [ Links ]
McDonald, M. B. 2000. Seed primin In: seed technology and its biological basis. Black, M. and Bewley, D. (Eds). Academic Press, London. 286-32 pp. http://www.seedbiology.de/ seedtechnology.asp. [ Links ]
Montes, S.; López, P.; Hernández, S. y Ramírez, M. Recopilación y análisis de la información existente de las especies del género Capsicum que crecen y se cultivan en México. INIFAP- bajío. http://www.biodiversidad.gob.mx/genes/centrosOrigen/Capsicum/ 1er-Informe/Primer% 20informe%20Capsicum.pdf. [ Links ]
Moo, M. A. J.; Ayala, G. O. J.; Latournerie, M. L.; Tzec, M. Y. A. and Pinzón, L. L. L. 2016. Effect of maturity and drying of Capsicum chinense jacq. seeds. Agroprod. 9(1):63-67. [ Links ]
Moradi, D. P.; Sharif, Z. F.; Janmohammadi, M. 2008. Influence of priming techniques on seed germination behavior of maize inbred lines. ARPN. J. Agric. Biol. Sci. 3(3):22-25. [ Links ]
Nicasio, A. S.; Sánchez, C. M. E.; Orozco, S. A. y Gamboa, de B. A. 2011. Efecto del preacondicionamiento y el sustrato salino en la germinación y el crecimiento de plántulas de maíz, (Zea mays) raza Chalqueño. Agrociencia . 45(2):195-205. [ Links ]
Pérez-Castañeda, L. M.; Castañon, N. G.; Ramírez, M. M. y Mayek, P. N. 2015. Avances y perspectivas sobre el estudio del origen y la diversidad genética de Capsicum sp. Ecos. Rec. Agrop. 2(4):117-128. [ Links ]
Petruzzelli, L.; Muller, K.; Hermann, K. and Leubner, M. G. 2003. Distinct expression patterns of ß-1,3-glucanases and chitinases during the germination of Solanaceous seeds. Seed Sci. Res . 13(2):139-153. [ Links ]
Prisco, J. T.; Haddad, C. R. and Bastos, J. L. P. 1992. Hydration-dehydration seed pre-treatment and its effects on seed germination under water stress conditions. Rev. Brasil. Bot. 15(1):31-36. [ Links ]
Ramón, M. y Mendoza, C. 2002. Efecto del deterioro post-corte sobre la germinación de la semilla asexual de cinco variedades de caña de azúcar. Rev. Fac. Agron. 19(4):264-272. http://www.scielo.org.ve/scielo.php?script=sci-arttext&pid=S0378-78182002000400002. [ Links ]
Rojo, C. 2005. Acondicionamiento osmótico de simientes de girasol (Helianthus annuus L.) para el avance de la germinación en siembras precoces para zonas áridas. Tesis Doctoral. Universidad Politécnica de Madrid, Escuela Técnica Superior de Ingenieros Agrónomos. Madrid. España. [ Links ]
Sánchez, A. J.; Orta, R. y Muñoz, B. 2001. Tratamientos pre germinativos de hidratación-deshidratación de las semillas y sus efectos en plantas de interés agrícola. Agr. Cost. 25(1):67-92. [ Links ]
SIAP. 2012. Chile habanero de la península de Yucatán http://www.siap.gob.mx/index.Php?option=com-content&view=article&id=338chilehabanero-de-la-peninsula-deyucatan&catid=91:infogramas&itemid=570:. [ Links ]
SIFUPRO. 2009. Paquete tecnológico de chile habanero. Sistema de Información de Fundaciones Produce. Coordinadora Nacional de las Fundaciones Produce, AC. [ Links ]
Stepuhn, H. and Raney, J. 2005. Emergence, height, and yield of canola and barley grown in saline root zones. Canadian J. Plant Sci. 85(4):815-827. [ Links ]
Taylor, G. B. 1981. Effect of constant temperature treatments followed by fluctuating temperatures on the softening of hard seeds of T. subterraneum L. Aust. J. Plant Phys. 8(6):547-558. [ Links ]
Zárate, G. A. y Sánchez, A. P. S. 2014. Efecto de reguladores de crecimiento y desarrollo sobre la producción de semillas en chile habanero. In: informe final de residencia profesional. Ingeniería en Agronomía. Instituto Tecnológico de la Zona Maya. México. [ Links ]
Zhang, C. F.; Hu, J.; Lou, J.; Zhang, Y. and Hu, W. M. 2007. Sand priming in relation to physiological changes. Seed germination and seedling growth of waxy maize under high salt stress. Seed Sci. Technol . 35(3):733-738. [ Links ]
Received: August 2018; Accepted: October 2018











 text in
text in