Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 spe 20 Texcoco abr./may. 2018
https://doi.org/10.29312/remexca.v0i20.1005
Articles
Effect of phenolic acids in tomato plants (Lycopersicon esculentum Mill.) inoculated with Clavibacter michiganensis
1Department of Horticulture-Autonomous Agrarian University Antonio Narro. Saltillo, Coahuila, Mexico.
2CONACyT-Department of Horticulture Chairs. Antonio Narro Agrarian Autonomous University, Saltillo, Coahuila, Mexico.
3Department of Plant Breeding-Antonio Narro Autonomous Agrarian University, Saltillo, Coahuila, Mexico.
4Department of Food-Antonio Narro Autonomous Agrarian University. Saltillo, Coahuila, Mexico.
5Department of Botany-Antonio Narro Autonomous Agrarian University, Saltillo, Coahuila, Mexico.
In order to evaluate the effect of exogenous applications of phenolic acids in the tomato crop, four treatments were established: 1) an absolute control; 2) plants inoculated with Clavibacter michiganensis subsp. Michiganensis (Cmm); 3) plants inoculated with Clavibacter michiganensis subsp. michiganensis and with application of phenolic acids; and 4) plants only with application of phenolic acids. The applications were made with intervals of one week to accumulate a total of 10 applications during the crop cycle. The results indicate that the application of phenolic acids did not promote effects in the agronomic variables of the crop, however, if a significant decrease in severity of Cmm was observed. In addition, it was demonstrated that the application of phenolic acids modifies the density and stomatal index and in combination with biotic stress induces a smaller size of stomata. In the histological variables, it was found that with the application of phenolic acids the palisane palisade decreased its length, also induced a smaller number and area of xylem vessels in leaf and root respectively and a greater length of cortex in the root. The results showed that the application of phenolic acids can be a viable alternative for the control of Cmm.
Keywords: histological changes; stomata; incidence; severity
Con el objetivo de evaluar el efecto de aplicaciones exógenas de ácidos fenólicos en el cultivo de tomate, se establecieron cuatro tratamientos: 1) un testigo absoluto; 2) plantas inoculadas con Clavibacter michiganensis subesp. Michiganensis (Cmm); 3) plantas inoculadas con Clavibacter michiganensis subesp. michiganensis y con aplicación de ácidos fenólicos; y 4) plantas solo con aplicación de ácidos fenólicos. Las aplicaciones se realizaron con intervalos de una semana hasta acumular un total de 10 aplicaciones durante el ciclo de cultivo. Los resultados indican que la aplicación de ácidos fenólicos no promovió efectos en las variables agronómicas del cultivo; sin embargo, si se observó una disminución significativa en severidad de Cmm. Además, se demostró que la aplicación de ácidos fenólicos modifica la densidad e índice estomático y en combinación con estrés biótico induce un menor tamaño de estomas. En las variables histológicas se encontró que con la aplicación de ácidos fenólicos el parénquima empalizada disminuyó su longitud, también indujo un menor número y área de vasos de xilema en hoja y raíz respectivamente y una mayor longitud de córtex en la raíz. Los resultados demostraron que la aplicación de ácidos fenólicos puede ser una alternativa viable para el control de Cmm.
Palabras clave: cambios histológicos; estomas; incidencia; severidad
Introduction
Tomato (Solanum lycopersicum L.) is a crop that in economic terms represents 72% of the value of the production of fresh vegetables worldwide (Hanssen et al., 2010). It is one of the most cultivated vegetables with the highest economic value in the world, since its demand increases steadily (Ortega-Martínez et al., 2010). However, the development of this crop can be affected by different factors that can be biotic or abiotic. Among the biotic factors are viruses, bacteria, fungi and nematodes that cause devastating diseases causing great economic losses in the tomato crop (Arshad et al., 2014).
Particularly bacterial diseases are a problem of great relevance in the production both in greenhouse and in the open field (Balestra et al., 2009). The bacterium Clavibacter michiganensis subsp. michiganensis (Cmm), which causes bacterial cancer, considerably affects the tomato crop (Cooksey, 1990; Hadas et al., 2005; Yuliar et al., 2015), and is present in all the producing areas of the world, and is considered as severe (Chang et al., 1991). The Cmm epidemics have been reported in tomato crops in several countries such as Israel, Japan, Spain and Mexico, causing production losses between 80 and 100% (Borboa-Flores et al., 2009; EPPO, 2010; De Leon et al., 2011). This causes a need to reduce the effects caused by pathogens, which together with the interest of increasing productivity and agricultural quality, has led to an excessive use of chemical products, creating problems such as environmental pollution (Arredondo, 2017).
For their part, the plants have generated defense mechanisms that allow them to tolerate the damages caused by pathogens (Harman et al., 2004; Nawrocka and Malolepsza, 2013). One of these mechanisms is the systemic defense that includes the generation of secondary metabolites, which are compounds that do not have an apparent function within the primary metabolism, but that have an important role in biotic interactions such as chemical defense against herbivores and pathogens (Neilson, 2013).
Phenolic compounds are necessary for the survival of plants in situations of stress imposed by both biotic and abiotic factors (Broun, 2005; do Nascimento, 2010). It is known that phenolic compounds have protective functions against pathogenic microorganisms (Osorio et al., 2010). There are reports on the use of phenolic compounds as inhibitors of different pathogens, for example, Mendoza (2013) reports the potential of these compounds against Botrytis cinerea. In the same way, isolated Veratrum album compounds were reported as active compounds against the fungi Erysiphe necator, Plasmopara viticola and B. cinerea in the vine culture (Alonso-Villaverde et al., 2011; Adrian and Jeandet, 2012; Wu et al., 2013).
This shows that phenolic compounds can be a potential alternative to chemical control for the management of phytopathogenic bacteria, and that the development of natural antibacterials will help to diminish the negative effects of chemical control (Riviera et al., 2014) which will impact positively to the environment.
The objective of the work was to evaluate the foliar application of phenolic acids in the tomato crop and its effect on tolerance to Clavibacter michiganesis subsp. michiganensis, as well as to determine the changes in the agronomic, physiological and histological characteristics.
Materials and methods
Establishment of the crop
The crop was established in a tunnel-type greenhouse with dimensions of 20 x 8 m with 30% shade from the Horticulture Department of the Antonio Narro Autonomous Agrarian University. Tomato seed of the variety “Rio Grande” type saladete and of determined growth was used. It was transplanted in black polyethylene bags with a capacity of 10 liters. As substrate, a mixture of perlite: peat moss ratio 1:1 (v:v) was used. The crop was handled to a single stem with the help of tutoring. Nutrition was administered through a directed irrigation system using the Steiner solution (Steiner, 1961 ), which was applied at different concentrations: 25% in vegetative stage, 50% in flowering, 75% in fruit mooring, and 100 % in fruit filling and harvest.
Application of treatments
The treatments consisted of the application of phenolic acids using as a source the Defense Gr® product (10 000 ppm phenolic acids in the form of soluble granules). A total of four treatments were applied, which are described below: 1) an absolute control (T0); 2) plants inoculated with Clavibacter michiganensis subsp. michiganensis (Cmm); 3) plants inoculated with Cmm and application of phenolic acids (Cmm + AF); and 4) plants only with application of phenolic acids (AF).
The applications of the phenolic acids were at a dose of 1 kg ha, starting seven days after the transplant (DDT), a total of 10 applications were made during the cultivation cycle every seven days.
Inoculation of Clavibacter michiganensis subsp. Michiganensis
The bacterium Clavibacter michiganensis subsp. michiganensis was isolated from tomato plants with symptoms attributable to it. Sap was obtained from the collected plants by means of a macerated porcelain mortar. The sap was seeded in Petri dishes with NBY culture medium and incubated at 29 °C for 48 h. Later they were reseeded for purification. The colonies obtained were subjected to morphological and biochemical tests for their identification.
The bacterium was increased in Petri dishes with NBY culture medium (nutritive broth 0.8%, yeast extract 0.2%, K2HPO4 0.2 %, KH2PO4 0.025%, agar 1.5%) (Borboa, 2009). The Petri dishes were incubated at 28 °C for 48 h, after which a bacterial growth wash was carried out, adjusting to the concentration and volume required, this was done in the Physiology Laboratory of the Department of Horticulture.
The plants corresponding to the treatments with biotic stress were inoculated with Clavibacter michiganensis subsp. michiganensis (Cmm) at 21 DDT, and using a concentration of 1X106 colony forming units (UFC) per milliliter. For the inoculation of the tomato plants with Cmm, slices were made in the leaves and they were submerged in 30 mL of bacterial solution for 5 min, the remainder was sprinkled on the foliage.
Agronomic analyzes
To determine the effect on the vigor of tomato plants the following agronomic variables were studied: plant height (AP), number of leaves (NH), stem diameter (DT), number of leaves (NH), number of bunches (R) at 75 days after transplantation (DDT), fresh weight aerial part (PFPA) and fresh root weight (PFR) at 85 DDT. After drying in a drying oven at a constant temperature of 80 °C for 72 h, the aerial part dry weight (PSPA) and the root dry weight (PSR) were obtained. In addition, the number of harvested fruits (NFC) and weight of harvested fruits (PFC) per plant was quantified.
Analysis in leaves and fruits
In the leaves SPAD (US) units were measured three times throughout the crop, at 30, 75 and 105 DDT, for which a SPAD chlorophyll meter (Minolta 502) was used. The measurement was made on the first physiologically mature leaf. The content of Vitamin C (VC) was also determined by the titration method with 2,6-dichlorophenolindophenol (Padayatt et al., 2001). The results are expressed in mg 100 g-1 fresh weight.
For these variables evaluated in fruit a total of five tomatoes were taken per treatment, with a degree of maturity 5 (Light red): red to pink color between 60-90% of the fruit, according to the color chart proposed by Boyette (1997) with a scale of 1-6 (1) Green, 2) Breakers, 3) Turning, 4) Pink, 5) Light red and 5) red). In these fruits the total soluble solids (TSS) were determined with a manual Atago refractometer (Master-T) and the titratable acidity (AT) was determined according to the methodology of the AOAC (2000). The electrical conductivity (CE) was obtained with a HI 98130 potentiometer (Hanna Instruments) and the measurement was made in 10 g of completely macerated fruit.
The firmness (F) (kg cm-2) was determined with a FDK-20 manual penetrometer (Wagner Instruments), for these three measurements were taken per fruit. The maturity index (IM) was calculated by means of the relation total soluble solids/titrable acidity (Casierra-Posada, 2008). The content of Lycopene (L) was determined by the methodology of Fish et al., (2002). The hydrogen potential (pH) was determined with a HI 98130 potentiometer (Hanna Instruments) in 10 g of completely macerated fruit. The content of vitamin C (VC) was determined by the titration method with 2,6-dichlorophenolindophenol (Padayatt et al., 2001). The equatorial diameter (DE) and polar diameter (DP) were determined with a digital vernier. These variables were determined in the Physiology Laboratory of the Department of Horticulture.
Incidence and severity of Clavibacter michiganensis subsp. Michiganensis
The incidence of Cmm was determined at 30, 45, 60, 75, 90 days after inoculation (DDI) and was expressed as the percentage of plants that presented symptoms (Anfoka, 2000). While the visual scale of Baysal et al. (2003). This scale has values of 0-5: (0: the leaves show no signs of wilting, 1: 1-10% of the leaves show mild marginal wilting, 2: 11-25% of the leaves withered, 3: 26-49 % of leaves have wilt associated with chlorosis, wilt in sectorized form, 4: 50-74% of leaves show wilting, excessive leaf fall, and 5: all wilted leaves). On the other hand, the severity index was calculated using the formula described by Raupach et al., (1996) as follows:
Where: IS= severity index; NC= classification number; NPC= number of plants in the classification; NTP= total number of plants; CMA= highest classification.
Stoma analysis
To determine changes in the stomata, a sampling was made at 75 DDT, where six plants were used per treatment. A fully expanded leaf of the same and with the same orientation was taken per plant. From the middle part of this sheet an epidermal impression of the beam (adaxial) and another of the lower surface (abaxial) was taken using polystyrene-xylol in liquid form, which was applied on the leaf surface with a brush. Once the film dried, it was removed with clear tape and placed on a slide. For each impression, three 100X microscopic fields were observed at random, in which the number of stomata and epidermal cells was determined. Three microphotographs were taken for each impression and the width and length (μm) of the occlusive cells of two stomata were measured for each one. The stomatal density was obtained in the following way:
Where: AC= 0.0254 mm2.
While the following formula was used to obtain the stomatal index (Wilkinson, 1979):
A compound microscope (Carl Zeiss) with a digital camera (PixeraWinder Pro) was used to take the photographs and processed in the AxionVision Rel. 4.8 measuring software.
Histological analysis
To verify the changes in the structures of the tomato plants, a histological analysis was also carried out. For this, a sampling of three plants per treatment was carried out at 90 DDT, from each plant the following structures were taken: a) stem segments of 1 cm in length taken 3 cm from the base of the stem; b) root 1 cm long taken at 5 cm from the base of the root; and c) a 1 cm2 foliole fragment taken from the middle part of the fourth leaf. To stop the cellular metabolism of the tissues, the fixation was made in FAA, which is composed as follows: 5 ml of formaldehyde at 36-40%, 90 ml of ethyl alcohol at 70% and 5 ml of glacial acetic acid.
They were then dehydrated at one hour intervals in 60%, 70%, 85% and 96% alcohol, and in absolute alcohol-xylol mixtures in 3:1, 1:1 and 1:3 ratios. After this the samples were included in paraffin, in aluminum molds with the following dimensions 8.2 x 9.4 of base and 5 cm of height. The 20 μm thick sections were made with a rotating microtome and adhered to a slide with Haupt adhesive. The tissues were stained with safranin and fast green (Rivero et al., 2007). A compound microscope (Carl Zeiss) with a digital camera (PixeraWinder Pro) was used for its observation, and microphotographs of three fields were taken by cutting the stem, root and leaf in 10 X and 40 X magnification. The measurements of the tissue images were made with the Axion Vision Rel 4.8 software.
For its part, the description of the anatomical characters was made according to the terminology of Evert (2006). The variables that were determined were the following: upper leaf epidermis (ESH), lower leaf epidermis (EIH), palisaw leaf palisach (PEH), number of xylem vessels in leaf (NVXH), root cortex (CR), number of xylem vessels in root (NVXR), root xylem vessel area (AVXR), stem epidermis (ET), number of stem xylem vessels (NVXT) and stem xylem vessel area (AVXT ). This work was carried out in the Cytogenetics Laboratory of the Plant Breeding Department.
Statistical analysis
The experimental design for the field work was randomized complete blocks. While for the rest of the analyzes a completely random design was used. An analysis of variance and a comparison test of means were performed according to the Fisher LSD test (p≤ 0.05) with the InfoStat program, version 2016.
Results and discussion
In the Table 1 shows the results obtained from the agronomic variables evaluated. The application of phenolic acids with or without stress did not cause changes in the agronomic variables, since no statistical differences were found between treatments. However, a trend of lower values is observed in the treatment with biotic stress, which may be due to the fact that the plant activates the defense mechanisms against stress (Conrath, 2006) and synthesizes secondary metabolites, which translates into an expense energy (Brown, 2003, Cipollini, 2003, Lattanzio et al., 2013). Villanueva-Couoh (2009) observed in chrysanthemum plants sprinkled with phenolic compounds higher height, larger stem diameter and higher biomass production (San Miguel et al., 2003), results that are different from those observed in this work.
Table 1 Agronomic variables evaluated in tomato plants.
| Trat | AP | NH | DT | R | PFPA | PFR | PSPA | PSR | NFC | PFC |
| T0 | 104 a | 17.6 a | 10.13 a | 14.67 a | 304.6 a | 51.4 a | 83.8 a | 12.3 a | 28 a | 1461.1 a |
| Cmm | 102.8 a | 19.27 a | 10.2 a | 11.03 a | 234 a | 55.6 a | 75.4 a | 12.1 a | 28.1 a | 1633.3 a |
| Cmm+AF | 100.4 a | 18.2 a | 9.87 a | 16.23 a | 294.7 a | 62 a | 82.9 a | 13.9 a | 27.4 a | 1542.7 a |
| AF | 98.53 a | 16.87 a | 10.13 a | 13.73 a | 275 a | 57.8 a | 66.9 a | 12.9 a | 28.6 a | 1692.8 a |
| CV (%) | 11.88 | 11.74 | 7.17 | 40.75 | 28.1 | 23.66 | 15.78 | 13.46 | 10.6 | 14.9 |
T0= control; Cmm= C. michiganensis; AF= phenolic acids; AP= plant height (cm); NH= number of leaves; DT= stem diameter (mm); R= clusters; PFPA= fresh weight aerial part (g); PFR= fresh root weight (g); PSPA= dry weight aerial part (g); PSR= root dry weight (g); NFC= number of fruits harvested per plant; PFC= weight of harvested fruits per plant (g). Different letters per column indicate statistical differences according to Fisher LSD (p≤ 0.05).
The foliar application of phenolic compounds increased the biomass of soybean plants (Gutiérrez-Coronado et al., 1998) and wheat yield (López-Tejeda et al., 1998). In addition, it extends the banana shelf life (Srivastava and Dwivedi, 2000). In the tomatillo, almost all the variables responded negatively with the application of benzoic acid and salicylic acid so they do not show a response pattern (Valdez, 2015). These results show that the effect of phenolic compound applications will not always be positive.
The results obtained from the measurements made in the leaves are in Table 2. In the measurement of SPAD units that were made at 30, 75 and 105 DDT, no statistical difference was found by the application of phenolic acids. However, the treatment with phenolic acids (AF) induced between 1 to 5% more units SPAD with respect to T0, contrary to what happens in the treatment AF+Cmm since it has lower values with respect to T0 which range from 1 to 9%.
Table 2 Measurements made on the leaves of tomato plants.
| Treatment | US (30 ddt) | US (75 ddt) | US (105 ddt) | VC (90 ddt) |
| T0 | 49.25 a | 51.19 a | 44.51 a | 17.43 a |
| Cmm | 47.15 a | 47.43 a | 44.75 a | 17.3 a |
| Cmm+AF | 49.29 a | 46.75 a | 44.37 a | 15.83 a |
| AF | 48.80 a | 51.89 a | 46.74 a | 17.43 a |
| CV (%) | 8.19 | 18.3 | 13.47 | 11.08 |
T0= control; Cmm= C. michiganensis; AF= phenolic acids; US= SPAD units; VC= vitamin C (mg 100 g-1). Different letters per column indicate statistical differences according to Fisher LSD (p≤ 0.05).
The results of the variables evaluated in the tomato fruits are presented in Table 3. There was a difference between treatments in the variables of titratable acidity, lycopene and vitamin c. while in the variables, electrical conductivity, firmness, maturity index, hydrogen potential, equatorial and polar diameter, no differences were observed between treatments.
Table 3 Fruit quality variables of tomato plants.
| Trat | SST | AT | CE | F | IM | L | pH | VC | DE | DP |
| T0 | 3.6 a | 0.12 ab | 2.2 a | 4.9 a | 30.9 a | 4.9 b | 4 a | 21.9 bc | 43.1 a | 52.7 a |
| Cmm | 4.1 a | 0.15 a | 2.7 a | 4.3 a | 28 a | 3.9 b | 3.9 a | 28.2 a | 46.6 a | 60.3 a |
| Cmm+AF | 3.7 a | 0.12 ab | 2.2 a | 4.8 a | 30.3 a | 5.3 b | 4 a | 23.2 b | 46.1 a | 59.9 a |
| AF | 3.8 a | 0.11 b | 2.8 a | 4.8 a | 34.8 a | 12.2 a | 4 a | 19.7 c | 47.4 a | 59.2 a |
| CV (%) | 8.87 | 13.35 | 30.78 | 8 | 16.05 | 48.31 | 6.8 | 5.93 | 6.37 | 6.62 |
T0= witness; Cmm= C. michiganensis; AF= phenolic acids; SST (°Brix)= total soluble solids; AT (%)= titratable acidity; CE (mS cm-1)= electrical conductivity; F (kg cm-1)= firmness; IM= maturity index; L (mg kg-1)= lycopene; pH= hydrogen potential; VC (mg 100 g-1)= vitamin C; DE (mm)= equatorial diameter and DP (mm)= polar diameter. Different letters per column indicate statistical differences according to Fisher LSD (p≤ 0.05).
In the obtained results, it is observed that the treatment with Cmm (plants with biotic stress) had the highest concentration of total soluble solids, titratable acidity, electrical conductivity and vitamin C, being higher than T0 by 13.8, 25, 22.7 and 28.7% respectively. Under conditions of biotic stress, plants as a defense mechanism synthesize a greater amount of antioxidants, in this sense Muñoz (2007) reports a greater antioxidant capacity in different fruits that contained a higher amount of phenolic compounds. Antioxidants are increased under stress conditions due to the crucial role they play in inactivating reactive oxygen species, in addition to influencing gene expression associated with biotic and abiotic stress responses (Tokunaga et al., 2005).
In relation to the results of Incidence and Severity of Cmm, these are presented in Figures 1a and 1b. The incidence of Cmm can be seen in Figure 1a, in the measurements made at 50, 65 and 80 days the AF+Cmm treatment had a lower incidence compared to the Cmm treatment, since it reduced the incidence of Cmm up to 12%. Figure 1b shows the results of Cmm severity, for this variable the AF+Cmm treatment registered up to 36% lower severity of the disease with respect to the Cmm treatment, this at 95 DDT. It has been reported that some phenolic compounds play a key role in the responses of plants to the attack of pathogens or insects (Kutchan et al., 2005; Muthuswamy et al., 2007; Lattanzio et al., 2008), which corresponds to the result obtained in the present study where this effect is clearly observed.
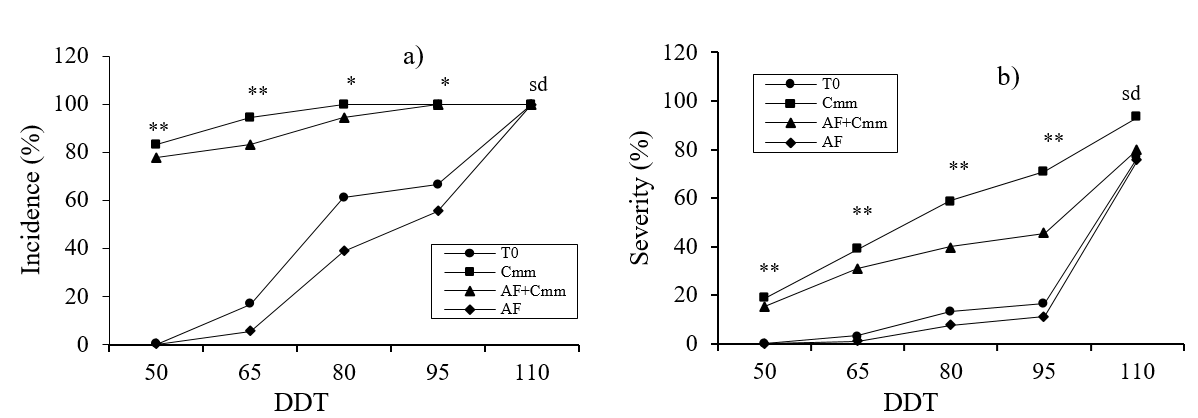
Figure 1 a) incidence and b) severity of Clavibacter michiganensis subsp. michiganensis in tomato plants. T0= witness; Cmm= C. michiganensis; AF= phenolic acids. Statistical differences according to Fisher LSD; *= p< 0.05, **= p< 0.01.
The results obtained show that the application of phenolic acids in a foliar way reduces the incidence and severity of Cmm (Figure 1a and 1b), as mentioned by Cornell (2003) and Bhattacharya (2010), who highlight its role as protective agents. In addition, the use of phenolic acids as natural antimicrobial agents has been recommended for showing a degree of inhibition of the growth of bacteria and fungi (Sivakamim, 2007).
In the Table 4 shows the results of the variables evaluated in the stoma analysis. It can be observed that in the density of stomata and tabloid cells, as well as in the stomatal index, no statistical difference was found between treatments. However, the application of phenolic acids (AF) induced on the adaxial surface lower stomatal density and stomatal index in 13 and 28% with respect to T0. In addition, this same treatment but on the abaxial surface caused lower cell density 86% with respect to T0.
Table 4 Evaluated variables related to stomata.
| Trat | DEAd | DCAd | DEAb | DCAb | IEAd | IEAb | LEAd | AEAd | LEAb | AEAb |
| T0 | 118 a | 884.6 a | 263.3 a | 697.6 a | 11 a | 27.6 a | 32.3 a | 17.3 a | 35.6 a | 22.3 a |
| Cmm | 111.3 a | 859.6 a | 250.6 a | 681.3 a | 11.3 a | 27.3 a | 32 a | 16.6 a | 35.3 a | 21 a |
| Cmm+AF | 117.3 a | 940.3 a | 260.3 a | 684.6 a | 11.3 a | 27.6 a | 31.3 a | 17.3 a | 33.6 a | 21.6 a |
| AF | 85.3 a | 915.6 a | 289.6 a | 99.6 a | 9.6 a | 29.6 a | 33.6 a | 17.6 a | 37 a | 22.3 a |
| CV (%) | 29.43 | 27.01 | 16.78 | 10.76 | 19.15 | 13.16 | 11.77 | 7.91 | 9.28 | 7.20 |
T0= witness; Cmm= C. michiganensis; AF= phenolic acids; DEAd (Num mm-2)= adaxial stoma density; DCAd (Num mm-2)= adaxial cell density; DEAb (Num mm-2)= abaxial stoma density; DCAb (Num mm-2)= abaxial cell density; IEAd= adaxial stomatal index; IEAb= abaxial stomatal index; LEAd (μm)= adaxial stomatal length; AEAd (μm)= adaxial stomatal width; LEAb (μm)= length of abaxial stomata; AEAb (μm)= width of abaxial stomata. Different letters per column indicate statistical differences according to Fisher LSD (p≤ 0.05).
The treatment to which phenolic acids + Cmm were applied had shorter stomata on both surfaces. The Cmm treatment presented stomata of smaller width in both surfaces, without presenting statistical difference with respect to the other treatments. The above is an important feature to be highlighted since the bacteria penetrates the vascular tissues through wounds, stomata, trichomes and leaf hydatids (Gleason et al., 1993; Ramirez and Sainz, 2006). Particularly the size of stomata influences the entrance of the bacteria to the plant, larger size will facilitate the entry and vice versa. The Cmm and AF treatments had a lower number of stomata, which ultimately resulted in a lower probability of access for the bacteria.
Conclusions
The application of phenolic acids has no effect on the morphology and physiology of the crop since no significant statistical differences were observed between the treatments evaluated.
However, the above phenolic acids significantly decrease the incidence and severity of Cmm, so the use of these compounds can be an alternative for the management of Clavibacter michiganensis in tomato cultivation.
In histological variables, it was clearly observed that the application of phenolic acids induces changes in the different structures of the plant, which can increase the tolerance to attack of pathogens such as Clavibacter michiganensis.
We propose the study of phenolic acids in the control of other pathogens in different crops.
Literatura citada
Anaya, A. L. 2003. Ecología Química. Plaza y Valdés Editores. Instituto de Ecología, UNAM, México. 349 p. [ Links ]
Anfoka, G. H. 2000. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester induces systemic resistance in tomato (Lycopersicon esculentum. Mill cv. Vollendung) to cucumber mosaic virus. Crop Protection. 19:401-405. [ Links ]
AOAC. 1990. Official Methods of Analysis. Association of Official Analytical Chemists. 15th (Ed.). Arlington, Virginia, USA. 384 Pp. [ Links ]
Arredondo, V. R.; Hernández, C. F. D.; Anguiano, C. J. C.; Ochoa, F. Y. M.; Gallegos, M. G.; Jasso, C. D. and Cristóbal, N. A. 2017. Review of antibacterial activity of plant extracts and growth-promoting microorganism (GPM) against phytopathogenic bacterial tomato crop. Eur. J. Biotechnol. Gen. Eng. 4(1):11-36. [ Links ]
Arshad, W.; Haq, I.; Waheed, T. M.; Mysore, K. S. and Mirza, B. 2014. Agrobacterium-mediated transformation of tomato with a rolb gene results in enhancement of fruit quality and foliar resistance against fungal pathogens. PLoS One. 9(5):e96979. [ Links ]
Balestra, G. M.; Heydari, A.; Ceccarelli, D.; Ovidi, E. and Quattrucci, A. 2009. Antibacterial effect of Allium sativum and Ficus carica extracts on tomato bacterial pathogens. Crop Protec. 28:807-811. [ Links ]
Baysal, Ö.; Soylu, E. M. and Soylu, S. 2003. Induction of defence-related enzymes and resistance by the plant activator acibenzolar-Smethyl in tomato seedlings against bacterial canker caused by Clavibacter michiganensis ssp. michiganensis. Reino Unido. Plant Pathol. 52(6):747-753. [ Links ]
Bhattacharya, A.; Sood, P. and Citovsky, V. 2010. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant. Pathol. 11:705-719. DOI: 10.1111/j.1364-3703.2010.00625.x. [ Links ]
Borboa, F. J.; Rueda, P. E. O.; Acedo, F. E.; Ponce, J. F.; Cruz, M.; Grimaldo, J. O. y García, O. A. M. 2009. Detección de Clavibacter michiganensis subespecie michiganensis en el tomate del estado de Sonora, México. Rev. Fitotec. Mex. 32(4):319-326. [ Links ]
Boyette, M. and Estes, E. 1997. Postharvest cooling and handling of field- and greenhouse-grown tomatoes. Maintaining the quality of aparat. Carolina Fresh Produce. 3:1-8. [ Links ]
Broun, P. 2005. Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr Opin Plant Biol. 8: 272-279. DOI: 10.1016/j.pbi.2005.03.006. [ Links ]
Brown, J. K. M. 2003. A cost of disease resistance: paradigm or peculiarity? Trends Genet. 19:667-671. DOI: http://dx.doi.org/10.1016/j.tig.2003.10.008. [ Links ]
Casierra, P. F. y Aguilar, A. O. E. 2008. Calidad en frutos de tomate (Solanum lycopersicum L.) cosechados en diferentes estados de madurez. Agron. Colomb. 26(2):300-307. [ Links ]
Cipollini, D.; Purrington, C. B. and Bergelson, J. 2003. Costs of induced responses in plants. Basic Appl Ecol. 4:79-85. [ Links ]
Conrath, U.; Beckers, G.; Flors, V.; García, A. P.; Jakab, G.; Mauch, F.; Newman, M. A.; Pieterse, C.; Poinssot, B.; Pozo, M. J.; Pugin, A.; Schaffrath, U.; Ton, J.; Wendehenne, D.; Zimmerli, L. and Mauch-Mani, B. 2006. Priming: getting ready for battle. Mol Plant-Microbe Interact. 19:1062-1071. [ Links ]
Cooksey, D. A. 1990. Genetics of bactericide resistance in plantpathogenic bacteria. Annual Rev. Phytopathol. 28:201-219. DOI: 10.1007/s10658-016-1035-2. [ Links ]
Cornell, H. V. and Hawkins, B. A. 2003. Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am Nat. 161:507-522. DOI:10.1086/368346. [ Links ]
Dangl, J. L. and Jones, J. D. G. 2001. Plant pathogens and integrated defence responses to infection. Nature. 411:826-833. DOI:10.1038/35081161. [ Links ]
De León, L.; Siverio, F.; López, M. M. and Rodríguez, A. 2011. Clavibacter michiganensis subsp. michiganensis a seedborne tomato pathogen: healthy seeds are still the goal. Plant dis. 95(11):1328-1338. [ Links ]
do Nascimentom, N. C. and Fett-Neto, A. G. 2010. Plant secondary metabolism and challenges in modifying its operation: an overview. In: Fett-Neto, A. G. (Ed.). Plant secondary metabolism engineering - methods and application, methods in molecular biology. Humana Press, New York. 643:1-13. [ Links ]
Durrant, W. E. and Dong, X. 2004. Systematic acquired resistance. Annu. Rev. Phytopathol. 42:185-209. [ Links ]
EPPO. 2010. Diagnostics Clavibacter michiganensis subsp. insidiosus. Bulletin. 40:353-364. [ Links ]
Evert, R. F. 2006. Esau’s plant anatomy: meristems, cells and tissues of the plant body: their structure, function and development, Hoboken. 3rd . Ed. Wiley-Interscience. 624 p. [ Links ]
Fish, W. W.; Perkins, V. P. and Collins, J. K. 2002. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Composit. Ann. 15:309-317. [ Links ]
Gleason, M.; Braun, E. J. and Peterson, R. H. 1993. Survival and dissemination of Clavibacter michiganensis subsp. michiganensis in tomatoes. Phytopathology. 1:1519-1523. [ Links ]
Gutiérrez, C. M. A.; Trejo, L. C. and Larqué, S, A. 1998. Effect of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 36(8):563-565. [ Links ]
Hadas, R.; Kritzman, G.; Klietman, F.; Gefen, T. and Manulis, S. 2005.Comparison of extraction procedures and determination of thedetection threshold for Clavibacter michiganensis ssp. michiganensis intomato seeds. Plant Pathol. 54:643-9. DOI: 10.1111/ppa.12454. [ Links ]
Hanssen, I.; Lapidot, M.; and Thomma, B. 2010. Emerging viral diseases of tomato crops. The Am. Phytopathol. Soc. 23(5):539-548. doi: 10.1094/MPMI-23-5-0539. [ Links ]
Harman, G. E.; Howell, C. R.; Viterbo, A.; Chet, I. and Lorito, M. 2004. Trichoderma spp., opportunistic avirulent plant symbionts. Nature Microbiology Review. 2:43-56. [ Links ]
Kutchan, T. and Dixon, R. A. 2005. Physiology and metabolism - Secondary metabolism: nature’s chemical reservoir under deconvolution. Curr. Opin. Plant Biol. 8(3):227-229. doi: 10.1016/j.pbi.2005.03.016 [ Links ]
Lattanzio, V. 2013. Phenolic compounds: Introduction. In: Nat. Prod. (Ramawat, K. G. and Mérillon, J. -M. (Eds.). Springer Berlin Heidelberg.1543-1580 pp. doi: 10.1007/978-3-642-22144-6-57 [ Links ]
Lattanzio, V.; Kroon, P. A.; Quideau, S. and Treutter, D. 2008. phenolics-secondary metabolites with diverse functions. In: Daayf, F. and Lattanzio, V. (Eds.). Recent advances in polyphenol research, Wiley-Blackwell, Oxford. 1.1-35 doi: 10.1002/9781444302400.ch1. [ Links ]
López, T. R.; Camacho, R. V. y Gutiérrez, C. M. A. 1998. Aplicación de ácido salicílico para incrementar el rendimiento agronómico en tres variedades de trigo. Terra Latinoam. 16(1):43-48. [ Links ]
Lu, L.; Wang, J.; Zhu, R.; Lu, H.; Zheng, X.; and Yu, T. 2015. Transcript profiling analysis of Rhodosporidium paludigenum mediated signalling pathways and defense responses in mandarin orange. Food Chem. 172:603-612. [ Links ]
Muñoz, J. A. M.; Ramos-Escudero, F. D.; Alvarado-Ortiz, U. C. and Castañeda, C. B. 2007. Evaluación de la capacidad antioxidante y contenido de compuestos fenólicos en recursos vegetales promisorios. Rev. Soc. Quím. Perú. 73(3):142-149. [ Links ]
Muthuswamy, S. and Rupasinghe, H. P. V. 2007. Fruit phenolics as natural antimicrobial agents: selective antimicrobial activity of catechin, chlorogenic acid and phloridzin. Food Agric Environ. 5(3-4):81-5. [ Links ]
Nawrocka, J. and Malolepsza, U. 2013. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control. 67:149-156. [ Links ]
Neilson, E. H.; Goodger, J. Q. D.; Woodrow, I. E. and Møller, B. L. 2013. Plant chemical defense: at what cost? Trends in Plant Sci. 18:250-258. doi:10.1016/j.tplants.2013.01.001. [ Links ]
Ortega, M. L. D.; Sánchez, O. J.; Díaz, R. R. y Ocampo, M. J. 2010. Efecto de diferentes sustratos en el crecimiento de plántulas de tomate (Lycopersicum esculentum Mill). Ra Ximhai. 6:365-372. [ Links ]
Osorio, E.; Flores, M.; Hernández, D.; Ventura, J.; Rodríguez, R. and Aguilar, C. 2010. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind .Crop Prod. 31(1):153-157. [ Links ]
Padayatt, S. J. M.; Daruwala, R.; Wang, Y.; Eck, P. K.; Song, J.; Koh, W. S. and Levine, M. 2001. Vitamin C: from molecular actions to optimum intake. In: Cadenas, E. and Packer, L. (Eds.). Handbook of antioxidants. CRC Press. Washington, DC, USA. 117-145 pp. [ Links ]
Ramírez, V. J. y Sáinz, R. R. 2006. Manejo integrado de las enfermedades del tomate. 1ra .. Ed. Once Ríos. México. 19-160 pp. [ Links ]
Raupach, G. S.; Liu, L.; Murphy, J. F.; Tuzun, S. and Kloepper, J. W. 1996. Induced systemic resistance in cucumber and tomato against cucumber mosaic cucumovirus using plant growth-promoting Rhizobacteria (PGPR). Plant Dis. 80:891-894. [ Links ]
Richard, A. D. 2001. Natural products and plant disease resistance. Nature .411:843-847. DOI:10.1038/35081178. [ Links ]
Rivero, M. G. C.; Quirós, G.; Sánchez, U. A. B. y Sanabria, M. E. 2007. Morfoanatomía de sépalos y pedúnculo del fruto de Psidium guajava L., estructuras de preferencia del ácaro Brevipalpus phoenicis (Geijskes) (Acari: Tenuipalpidae). Venezuela. Rev. Fac. Agron. 24(1):135-140. [ Links ]
Riviera, S. E. V.; Escobar, S. M. A.; Morales, D.; Noé, A. C. y Rodríguez, H. R. 2014. Synergistic effects of ethanolic plant extract mixtures against food-borne pathogen bacteria. African J. Biotechnol. 13(5):669-704. DOI:10.5897/AJB2013.12273. [ Links ]
San Miguel, R.; Gutiérrez, M. and Larqué, S. A. 2003. Salicylic acid increases the biomass accumulation of Pinus patula. Southern J. Appl. Fores. 27:52-54. [ Links ]
Sivakamim, M. and Vasantha, R. H. P. 2007. Fruit phenolics as natural antimicrobial agents: Selective antimicrobial activity of catechin, chlorogenic acid and phloridzin. J. Food, Agric. Environ. 5 (3-4):81-85. [ Links ]
Srivastava, M. K. and Dwivedi, U. N. 2000. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 158(1-2):87-96. [ Links ]
Steiner, A. A. 1961. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 15:134-154. [ Links ]
Tancos, M. A.; Chalupowicz, L.; Barash, I.; Manulis, S. S. and Smart, C. D. 2013. Tomato fruit and seed colonization by C. michiganensis subsp. michiganensis through external and internal routes. Appl. Environ. Microbiol. 79:6948-6957. [ Links ]
Tokunaga, T.; Miyahara, K.; Tabata, K. and Esaka, M. 2005. Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for L-galactono-1,4-lactone dehydrogenase. Planta. 220:854-863. [ Links ]
United Nations Food and Agricultural Organization. 2016. FAOSTAT. Available from world wide Web. [ Links ]
Valdez, S. L.; González, M. S.; Valdez, A. L. A.; Ramírez, G. F y Benavides, M. A. 2015. Efecto de la aplicación exógena de ácido benzoico y salicílico en el crecimiento de plántulas de tomate, tomatillo y pimiento. Rev. Mex. Cienc. Agríc. 12:2331-2343. [ Links ]
Villanueva, C. E.; Alcántar, G. G.; Sánchez, G. P.; Soria, F. M. y Larque, S. A. 2009. Efecto del ácido salicílico y dimetilsulfóxido en la floración de [Chrysanthemum morifolium(Ramat) Kitamura] en Yucatán. Rev. Chapingo Ser. Hortic. 15(2):25-31. [ Links ]
Wilkinson, H. P. 1979. The plant surface (mainly leaf). En: Metcalfe, C. R. y Chalk, L. Eds. Anatomy of the Dicotyledons, 2nd (Ed.). Clarendon Press, Oxford. 1. 97-165 pp. [ Links ]
Yuliar, N. Y. A. and Toyota, K. 2015. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia Solanacearum. Microbes. Environ. 30(1):1-11. doi:10.1264/jsme2.ME14144. [ Links ]
Received: January 2018; Accepted: March 2018











 texto en
texto en 


