Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 spe 20 Texcoco Abr./Mai. 2018
https://doi.org/10.29312/remexca.v0i20.998
Articles
Inoculation of nitrogen-fixing halobacteria in the contribution to tolerance to salt stress in bean tepary
1Department of Research and Graduate Studies in Food-University of Sonora, Hermosillo, Sonora, Mexico CP 83000. (prabhaharan.renganathan@gmail.com; Jborboaf@guayacan.uson.mx; erosass@guayacan.uson.mx; jecardena@guayacan.uson .mx).
2Center for Biological Research of the Northwest SC. National Polytechnic Institute num. 195, Col. Playa Palo of Santa Rita Sur, La Paz, Baja California Sur. CP 23096, Mexico. (bemurillo04@cibnor.mx).
3Department of Biological and Agricultural Chemistry Sciences-University of Sonora. Caborca, Sonora, Mexico. CP 83690. (jesus.ortega@caborca.uson.mx).
4Department of Agriculture and Livestock-University of Sonora, Hermosillo, Sonora, Mexico. CP. 83000. (erueda04@santana.uson.mx).
Drought tolerant tepary bean (Phaseolus acutifolius A. Gray) is a microbiologically unexplored culture in arid regions of Mexico where it is consumed as a source of dietary protein. However, soil salinity hinders the productivity of this crop. The isolation of specific halotolerant nitrogen-fixing bacteria (BFN) could be a novel practice to improve salt tolerance and improve crop productivity. In the present study, 24 BFN were isolated from seeds of three tepary ecotypes exposed to high salinities (0, 0.25 and 0.5 M NaCl) in vitro. Nitrogen-fixing halotolerant strains were characterized. Among the halotolerant isolates, only one showed high nitrogenous activity of 6.97 ±1.1 nmol of culture h-1. From the 16S analysis of rRNA, the halotolerant microorganism exhibited 99% sequence homology with the known bacterium Bacillus amyloliquefaciens. The results show that the inoculation with the identified halotolerant isolate had stimulating effects on growth parameters, seed germination, seed emergence, shoot length, root length, biomass, foliar chlorophyll and protein content, ash and crude fibers.
Keywords: Azospirillum halopraeferens; Bacillus amyloliquefaciens; Phaseolus acutifolius; nitrogen fixation; saline stress
El frijol tepary tolerante a la sequía (Phaseolus acutifolius A. Gray) es un cultivo microbiológicamente inexplorado en regiones áridas de México donde se consume como fuente de proteína dietética. Sin embargo, la salinidad del suelo dificulta la productividad de este cultivo. El aislamiento de bacterias fijadoras de nitrógeno halotolerantes específicas (BFN) podría ser una práctica novedosa para mejorar la tolerancia a la sal y mejorar la productividad de los cultivos. En el presente estudio, se aislaron 24 BFN de semillas de tres ecotipos de tepary expuestos a altas salinidades (0, 0.25 y 0.5 M de NaCl) in vitro. Se caracterizaron cepas halotolerantes fijadoras de Nitrógeno. Entre los aislados halotolerantes, sólo uno mostró alta actividad nitrogenada de 6.97 ±1.1 nmol de cultivo h-1. A partir del análisis 16S de rRNA, el microorganismo halotolerante exhibió una homología de secuencia de 99% con la bacteria conocida Bacillus amyloliquefaciens. Los resultados muestran que la inoculación con el aislado halotolerante identificado, tuvo efectos estimulantes sobre los parámetros de crecimiento, germinación de semillas, emergencia de semillas, longitud de brotes, longitud de raíz, biomasa, clorofila foliar y contenido de proteínas, cenizas y fibras brutas.
Palabras clave: Azospirillum halopraeferens; Bacillus amyloliquefaciens; Phaseolus acutifolius; fijación de nitrógeno; stress salino
Introduction
The growing problem of the increase of salts in the areas of cultivation together with the low rainfall and high temperatures of arid-saline environments, requires integral solutions for their productivity (Ungar, 1982). The tepary bean (Phaseolus acutifolius A. Gray) is a legume adapted to the high temperatures and droughts of the Sonoran Desert (Southwest of the United States of America and Northwest of Mexico).
The seeds of this species are characterized by a high content of dietary protein (21.1 to 32.49%) compared to other Phaseolus species; for example, P. vulgaris (19.1-29.7%), P. lunatus (19.7-24.9%), P. coccineus (20-27.4%), P. polyanthus (21.6-25.6%), P. filiformis (24.2%) and P. Angustissimus (25.9%) (Nabhan, 1985). In general, tepary yields have been estimated between 200 and 900 kg ha-1. Yields of up to 2000 kg ha-1 have been reported (Hamama and Bhardwaj, 2002; Ahmad et al., 2013) depending on the sowing season and the appropriate supplementation of nitrogen fertilizers (N).
In northwestern Mexico, large amounts of chemical fertilizers are applied to the soils to compensate for N deficiency and to increase the yields of tepary beans, unfortunately, the excessive and continuous use of these fertilizers increases salinity, affecting the physical and soil chemistry, which as a whole alter microbial activities, which could be beneficial for crops (Rueda-Puente et al., 2003, 2007, 2010). To reduce inputs of nitrogen fertilizers in order to slow or stop further increases in soil salinity and reduce fertilizer costs, rhizobacteria that promote plant growth, particularly N-fixing bacteria (RPCP-BFN), are an attractive and sustainable alternative for the cultivation of tepary beans in saline soils of semi-arid and arid regions, such as those located in the state of Sonora, Mexico.
Studies on bacteria associated with tepary bean yields are scarce. Studies related to RPCP-BFN have shown the biopromotor and bioprotective effect against environmental stress in plants, for example, drought, osmotic stress and flood (Mayak et al., 2004), extreme temperatures (Terre et al., 2007), nutrient deficiency (Cassan et al., 2009) and toxic metals (Sandhya et al., 2010; Rokhzadi and Toashih, 2011).
During the last decades, the RPCP-BFN have been isolated and cultivated as a promising alternative (Pathak and Keharia, 2013). Based on the above described, the hypothesis that arises is whether the inoculation with RPCP-BFN, especially those of the halotolerant type (HBFN) contribute to improve the effect of salinity stress in crops such as tepary beans. Therefore, the objectives of the present study were: i) to isolate a nitrogen-fixing halotolerant bacteria from the rhizosphere of P. acutifolius; and ii) to identify the contribution of the isolate (Bacillus amyloliquefaciens RP22) on tolerance to stress by salinity in seeds and seedlings of P. acutifolius.
Materials and methods
Seeds
The seeds evaluated in the present study were those ecotypes of San Judas (SJ), Indio Yumi (IY) and Elena-Mora Property (EMP), which were collected from the Sierra de Sonora-Chihuaha (29° 05’ north latitude 110° 57’ west longitude) in the state of Sonora.
Halobacteria nitrogen fixers
Isolation
For the isolation of bacteria, the technique of Rueda et al. (2003). The seeds were crushed with mortar and pestle to obtain the final weight of 4 g and serial dilutions (up to 10-6) in 0.85% sterile saline were seeded on OAB free of N in agar medium according to (Bashan et al., 1993) with three different concentrations of NaCl (0, 0.25 and 0.5 M) (Rueda et al., 2003), agar plates were incubated at 30 °C for four days, obtaining 24 individual colonies on agar, based on the phenotype (color, brightness, shape, elevation and margin) according to Smibert and Krieg (1994), later the purified isolates were stored at -80 ºC (15% glycerol).
Nitrogen fixation (acetylene reduction technique)
The acetylene reduction assay (ERA) to the selected strains was performed as described by Reinhold et al. (1987). The halotolerant isolates were cultured in serum flasks containing 25 ml of liquid medium OAB free of N and 0.5 M NaCl. The results were compared with a known halobacteria (HBFN) Azospirillium halopraeferens AU10 (Reinhold et al., 1987). The isolate that showed the highest acetylene reduction activity was selected for genetic identification and for further evaluation with inoculation tests.
Identification
The selected halotolerant bacterium was identified at the genus level by partial sequencing of 16S rRNA genes by Acculab, Inc. (USA). The sequence obtained was compared with sequences in the GenBank database of the National Center for Biotechnology Information (NCBI) using the BLAST program ( http://blast.ncbi.nlm.nih.gov/; http://www.megasoftware.net/) and then it was deposited in the GenBank under the access number KM652480. In addition, a phylogenetic tree was built using MEGA6 freeware (http://www.megasoftware.net/).
Inoculation tests-experiment design
The inoculation tests were carried out in a growth chamber and inside a greenhouse. Both trials were randomized in a complete block design with a three-dimensional design (3 × 3 × 3)= 27 treatments with n repetitions; 3 bean ecotypes (San Judas= SJ =, Indian Yumi= IY = and Elena-Mora Property= EMP=; 3 levels of NaCl (0, 0.25 and 0.5 M) and 3 levels of bacteria (Ba= Bacillus amyloliquefaciens, Ah= Azospirillium halopraeferens AU10, U= the non-inoculated ones (Table 3) Once seeds were sterilized, they were inoculated with the HBFN isolated and characterized in this study, using the air vacuum pump technique described by Carrillo et al. (1998).
Table 1 Colony forming units of HBFN associated with seed of different ecotypes of Phaseolus acutifolius on agar OAB free N supplemented with different concentrations of NaCl.
| Seeds | Ecotypes | Count (×106 UFC g-1 seed) | |||
| 0 M NaCl | 0.25 M NaCl | 0.5 M NaCl | 0.75 M NaCl | ||
| with seed coat | SJ | 9.3 ±1.2a* | 8.3 ±0.6ab | 5 ±0.5bc | 3.1 ±0.5a |
| IY | 8.6 ±2.6a | 8 ±1.1a | 5 ±1.1ab | 2.6 ±0.8ab | |
| EMP | 12.6 ±2a | 8.6 ±0.6b | 3.6 ±0.3c | 2.3 ±0.3b | |
| without seed coat (endosperm) | SJ | 8.3 ±0.4a | 6.7 ±0.3b | 3.7 ±0.9c | 2.4 ±0.3b |
| IY | 7.6 ±1.2a | 7.4 ±1.2a | 5 ±0.6ab | 3.3 ±0.4a | |
| EMP | 10.6 ±0.9a | 7.6 ±0.4b | 4.4 ±0.3c | 2.3 ±0.7b | |
*= Values represent an average ± standard error (average of three repetitions). Different letter in the same line denotes significant difference (p≤ 0.05, Tukey test). Ecotypes SJ = San Judas; IY = Indian Yumi; EMP = Elena-Mora Property.
Table 2 Seed germination and emergence rates of three Phaseolus acutifolius ecotypes under growth chambers and greenhouse trials.
| NaCl treatment | Num. inoculated (control) | B. amyloliquefaciens | A. halopraeferens AU10 |
| Germination chamber of growth | |||
| 0 M | 46.7-86.7 | 93.3-100 | 86.7-100 |
| 0.25 M | 53.3-66.7 | 73.3-100 | 53.3-80 |
| 0.5 M | 0-13.3 | 20-26.7 | 6.7-20 |
| Greenhouse emergency | |||
| 0 M | 55.6-66.7 | 100 | 100 |
| 0.25 M | 22.2-33.3 | 33.3-44.4 | 33.3-44.4 |
| 0.5 M | 11.1-22.2 | 11.1-33.3 | 11.1-33.3 |
Table 3 Effect of NaCl concentrations and bacterial inoculation on the total content of chlorophyll, protein, ash and crude fiber in Phaseolus acutifolius seedlings under greenhouse conditions.
| Ecotypes | NaCl treatments | Inoculants | Total chlorophyll | Protein (%) | Ashes (%) | Crude fiber (%) |
| SJ | 0 M | U | 31.5 ±0.08b | 11.9 ±0.07b | 5.5 ±0.03b | 17.8 ±0.5b |
| Ba | 34.9 ±0.1a | 14.4 ±0.07a | 7.6 ±0.2a | 21.9 ±0.9a | ||
| Ah | 34.1 ±0.03a | 14 ±0.2a | 6.5 ±0.2a | 22.9 ±0.5a | ||
| 0.25 M | U | 8.9 ±8.9b | 3.1 ±3.1b | 1.4±1.4b | 4.7 ±4.7b | |
| Ba | 29.4 ±0.4a | 12.3 ±0.06a | 7.2 ±0.2a | 21.1 ±1a | ||
| Ah | 29 ±0.02a | 11.8 ±0.2a | 6.4 ±0.2a | 22.85 ±0.3a | ||
| 0.5 M | U | 4.9 ±4.9c | 2.3 ±2.3b | 1 ±1b | 4.1 ±4.1b | |
| Ba | 12.7 ±6.4a | 5.1 ±2.5a | 3.2 ±1.6a | 8.4 ±4.2a | ||
| Ah | 6.6 ±6.6b | 2.5 ±2.5b | 1.9±1.9b | 4.1 ±4.1b | ||
| IY | 0 M | U | 31.2 ±0.2b | 11.29 ±0.04c | 5.3 ±0.09b | 17.5±0.9b |
| Ba | 34.9 ±0.8a | 14.3 ±0.09a | 7.6 ±0.3a | 23.3 ±0.05a | ||
| Ah | 34.3 ±0.1a | 13.66 ±0.2b | 6 ±0.1b | 23.1 ±0.2a | ||
| 0.25 M | U | 8.9 ±8.9c | 2.6 ±2.6c | 1.3 ±1.3c | 5.2 ±5.2c | |
| Ba | 30.3 ±0.8a | 12.2 ±0.07a | 7 ±0.05a | 23.7 ±0.6a | ||
| Ah | 19.6 ±9.8b | 7.8 ±3.9b | 4.2 ±2.1b | 15.1 ±7.5b | ||
| 0.5 M | U | 4.5 ±4.5b | 2.5 ±2.5a | 1 ±1a | 3.7 ±3.7a | |
| Ba | 6.6 ±6.6a | 2.4 ±2.4a | 1.7 ±1.7a | 4.1 ±4.1a | ||
| Ah | 6 ±6a | 2.6 ±2.6a | 1.9 ±1.9a | 4.4±4.4a | ||
| EMP | 0 M | U | 32.5 ±0.06b | 11.5 ±0.01c | 5.6 ±0.03c | 22.4 ±0.03a |
| Ba | 35.2 ±0.4a | 15 ±0.2a | 8.6 ±0.4a | 23.6 ±0.03a | ||
| Ah | 34.1 ±0.03a | 14.6 ±0.4b | 6.2 ±0.06b | 23.5 ±0.19a | ||
| 0.25 M | U | 9.2 ±9.2b | 3.2 ±3.2b | 1.5 ±1.5b | 7.2 ±7.2b | |
| Ba | 29.9 ±0.2a | 12.7 ±0.2a | 7 ±0.06a | 21.8 ±0.2a | ||
| Ah | 29 ±0.02a | 12.1 ±0.03a | 6.2 ±0.2a | 22.1 ±0.1a | ||
| 0.5 M | U | 5.8 ±5.8b | 2.5 ±2.5b | 1 ±1b | 4.4 ±4.4b | |
| Ba | 18.6 ±0.61a | 7.9 ±0.03a | 5.8 ±0.4a | 13.2 ±0.6a | ||
| Ah | 19.3 ±0.6a | 7.7 ±0.1a | 5 ±0.5a | 12.6 ±0.3a |
Tests in growth chamber
The germination of 675 seeds (25 seeds per treatment) was performed in sterilized Petri dishes, sealed with paraffin tape to maintain humidity conditions and placed in a growth chamber with a 16-hour light photoperiod (32 ±0.5 °C) and 8 h at night (25 ±0.5 °C) with a relative humidity of 36 ±1% (HR). After 7 days, the germination rates of the seed, the growth (length of stem and root) and the dry weights were recorded.
Greenhouse tests
For the tests inside the greenhouse, the substrate used in the plastic pots was sterile vermiculite without nutrients. The 540 seeds (20 seeds per treatment) were planted in pots and the emergence rates were recorded at day 9. The seedlings were cultivated under control conditions with a photoperiod 16 h day (32 ±0.5 ºC) and 8 h night (25 ±0.5 ºC) and with 36 ±1% HR. The plants were irrigated every third day, considering spilling the water to prevent an increase in salinity with the corresponding concentrations of NaCl and maintained until 21 days.
Variables evaluated
Germination and emergency seed rates were calculated using the formula described by Maguire (1962): M= n1/t1 + n2/t2 + ... n7/t7 ... n9/t9, where n1, n2,... ., n7 ..., n9 are; 1 are the number of germinated seeds; t1, t2, ..., t7 ..., t9 is the time in days. The chlorophyll foliar content was measured with a chlorophyll meter (SPAD-502, Minolta, Japan). Root and shoot lengths were measured separately with a digital calibrator (General No. 143, General Tools Manufacturing Co., Inc., New York, USA). The plants were dried at 110 °C for 36 h to estimate the dry weight. The micro-kjeldahl method was used to determine the total N and crude protein (N×6.25), while the crude fiber and the ash contents were determined gravimetrically (Snedecor, 1956).
Results and discussion
Halobacteria nitrogen fixer
The total counts of nitrogen-fixing bacteria (BFN) are shown in Table 1. It could be seen that the isolates on control agar plates (N-free OAB agar medium without NaCl) were 8.6 to 12.6×106 forming units of colonies (UFC g-1 and from 7.6 to 10.6×106 UFC g-1 for seeds with and without seed coat, respectively.) Isolates in the SJ and EMP ecotypes decreased significantly (p≤ 0.05) in agar plates supplemented with NaCl 0.5 and 0.75 M. In the case of control seeds in the SJ ecotype (8.3-9.3×106 CFU g-1), the counts showed a NaCl tolerance of 45-54% (3.7-5×106 CFU g-1 seeds) and 29-33% (2.4-3.1×106 CFU g-1 seeds) of total culturable BFN at 0.5 and 0.75 M NaCl, respectively.
Similarly, counts in the EMP ecotype showed a salt tolerance of 28-41% (3.6-4.4×106 CFU g-1 seeds) and 18-22% (2.3×106 CFU g-1 seeds) of BFN total arable to 0.5 and 0.75 M NaCl, while in the control the values were 10.6-12.6×106 CFU g-1.
In relation to the capacity of N fixation (acetylene reduction), the strain encoded as RP22 had the highest acetylene reduction activity (6.97 ±1.1 nmol culture h-1), which is similar to reference A. halopraeferens AU10 (7.83 ±1.3 nmol of culture h-1). This result shows that growth improved by 28 and 43% with bacteria isolated at NaCl concentrations between 0.5 M and 0.75 M NaCl.
Analogously, Rueda et al. (2003) proposed that 5% of the halotolerant strains of saline-flat areas reduced acetylene ~ 6.26 ±0.56 nmol of culture h-1 (Klebsiella pneumoniae) with the control A. halopraeferens, which produced a value of 7.1 ±1.7 nmol-1 h-1. This could be attributed to the codependency of other bacteria, a phenomenon that is quite common among microorganisms (Diby and Harshad, 2015). The genetic identification of the bacterial isolate in the present study, based on the 16S rRNA gene sequence, showed a high similarity (99%) with the plant growth promoting bacterium Bacillus amyloliquefaciens (Figure 1).
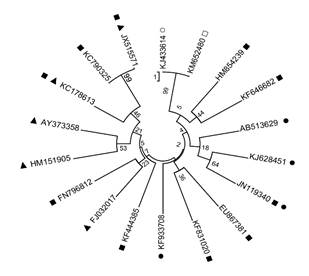
Figure 1 Phylogenetic tree showing the similarity with the isolate Bacillus amyloliquefaciens RP22 (□) and the close relationship with Bacillus amyloliquefaciens (○) and its relationship with the halotolerant (●) fixative of N (▲) and endophyte (■) desert bacteria. Alignment and close relationship with the tree was done using MEGA6 (http://www.megasoftware.net/). The bars indicate 10% divergence; a bootstrap analysis was developed with 1000 trials. KJ433614, Bacillus amyloliquefaciens strain IARI-AR25; KM652480, Bacillus amyloliquefaciens strain RP22; HM854239, Bacillus safensis strain KLH-14; KF646682, Bacillus tequilensis strain DH-10; AB513629, Bacillus megaterium; KJ628451, Bacillus subtilis strain HPCAQRKSM106; JN119340, Halobacillus sp. KLBMP 2429; EU867381, Bacillus pumilus strain CCGE2028; KF831020, Bacillus firmus strain L-4; KF933708, Bacillus baekryungensis strain QD56; KF444385, Bacillus aryabhattai strain HYR8(1); FJ032017, Bacillus pumilus strain ES4; FN796812, Bacillus cereus strain GP17; HM151905, Bacillus subtilis strain DAZ26; AY373358.1, Bacillus megaterium strain c5; KC178613, Bacillus flexus strain DNEB39; KC790325, Bacillus methylotrophicus strain SY33; JX515571, Bacillus subtilis strain H171.
The phylogenetic analysis based on the 16S rRNA gene isolated from the NFB halotolerant showed 99% similarity with a B. amyloliquefaciens (accession number KJ433614), a bacterium isolated from the cold desert of the northwest Himalayas by PGPR analyzes. As far as is known, the species B. amyloliquefaciens has never been reported for P. acutifolius, which is indicative of the association of beneficial bacteria and the plant under study. Other species of the genus Bacillus have been isolated and identified in other leguminous plants (B. pumilus, B. cepacia, B. japonicum, B. vallismortis, B. mojavensis, B. atrophaeus, B. megaterium and B. Stearothermophilus) (Bashan et al., 1993; Dobbelaere and Okon, 2007a b).
Inoculation tests
When the seeds were inoculated with bacterial strains, the negative effects of 2% NaOCl on germination of the seeds decreased (Table 2). Thus, higher germination rates were obtained under growth chamber conditions with inoculated seeds (53.3-100%) than with non-inoculated seeds (46.7 - 86.7%) in the three ecotypes of tepary beans treated with 0 M NaCl and 0.25 M. In addition, the germination rate was strongly affected when 0.50 M NaCl was applied, showing ranges of 0-13.3% and 6.7-26.7% for the non-inoculated and inoculated seeds, respectively (Table 2).
Regarding the parameters measured in growth chamber conditions, treatments of bean tepary in the variables of shoots and roots in seedlings, the maximum effects of bacterial inoculation with B. amyloliquefaciens RP22, as well as A. halopraeferens AU10 were for the ecotype SJ, showing significant results with p≤ 0.05 to 0 M NaCl (8.9 and 10.5 cm of plant-1), between 6.9 and 8.7cm plant-1 for B. amyloliquefaciens and A. halopraeferens when adding 0.25 M NaCl, in comparison with the non-inoculated seedlings that were lower: (7.8 and 8.5 and 5.8 and 6.7 cm plant-1 for 0 and 0.25 M NaCl (Figure 2).
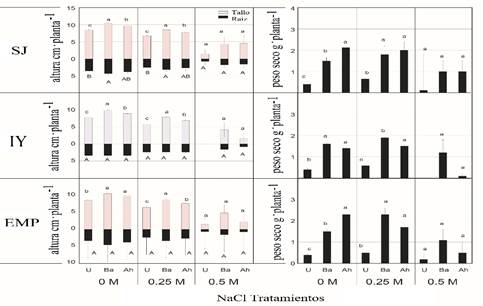
Figure 2 Effect of NaCl concentrations and bacterial inoculation on the height and dry weight of Phaseolus acutifolius seedlings under growth chamber conditions. The values represent the mean ± standard error (n= 3). Different letter indicates significant differences (p≤ 0.05, Tukey test) of the inoculated plants (Ba= Bacillus amyloliquefaciens, Ah= Azospirillium halopraeferens AU10) in relation to the non-inoculated controls (U). Ecotypes SJ= San Judas; IY= Indian Yumi; EMP= Elena-Mora Property.
Root lengths showed significant statistical differences (p≤ 0.05) between the inoculated and non-inoculated seedlings in IY and EMP ecotypes, considering all the salinities, except the SJ ecotype where the plants were inoculated with B. amyloliquefaciens RP22, (4.4 and 2.7 cm plant-1 to 0 and 2.5 M NaCl (p≤ 0.05) (Figure 2).
The production of dry biomass according to the effect of the treatments were statistically different (p≤ 0.05); the increases in dry biomass of the plants inoculated with respect to those not inoculated were 17 and 38%, respectively. Coincident with the growth chamber test, greenhouse test results (Figure 3) showed lower emergence seed rates and seedling biomass and growth values of the three ecotypes of tepary beans irrigated with 0.25 and 0.5 M NaCl with NaCl at 0 M, this assay also confirms the growth promoting effect of the plants on seeds and seedlings of both inoculated bacterial strains, which reaffirms the possibility of proposing beneficial microorganisms as biofertilizing prospects (Stefana et al., 2013; Diby and Harshad, 2015).
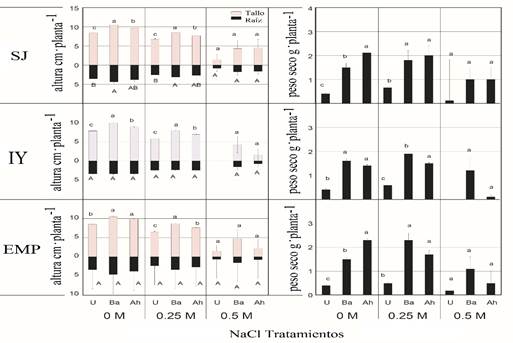
Figure 3 Effect of NaCl concentrations and bacterial inoculation on the growth and dry weight of Phaseolus acutifolius seedlings under greenhouse conditions. The values represent the mean ± standard error (n= 3). Different letters indicate significant differences (p≤ 0.05) of the inoculated plants (Ba= Bacillus amyloliquefaciens, Ah= Azospirillium halopraeferens AU10) in relation to the non-inoculated controls (U). Ecotypes SJ= San Judas; IY= Indian Yumi; EMP= Elena-Mora Property.
Similar results indicate that the positive effects of this type of bacteria apparently are due to the production of growth promoting substances as reported in other studies (Yadav, 2011; Rugheim et al., 2012).
Regarding the variables of growth and biomass in greenhouse conditions, significant differences were observed (p≤ 0.05) in lengths of shoots in the SJ ecotype, considering the inoculation with B. amyloliquefaciens RP22 (70 cm plant-1), as well as A. halopraeferens AU10 (58 cm plant-1), in relation to the non-inoculated controls (U) (35 cm plant-1) in NaCl M (Figure 3).
Regarding the dry weight, the maximum values were recorded in inoculated seedlings in the three ecotypes at 0, 0.25 and 0.5 M NaCl in relation to the non-inoculated controls (U). However, it should be mentioned that salinity reduces dry weight, while it increases in all treatments (Figure 3). In particular, the values of germination, emergence, growth and biomass were higher in the seedlings inoculated with B. amyloliquefaciens RP22 than in those inoculated with A. halopraeferens AU10, particularly in the ecotype IY treated with NaCl 0 and 0.25 M (Figures 2 and 3).
Likewise, the analysis of foliar chlorophyll and the contents of proteins, ashes and crude fiber confirm the effect of NaCl treatments and bacterial inoculation in relation to controls (not inoculated) on the growth and biomass of the seedlings (Cuadro 3).
Conclusions
The present study represents a first report of Bacillus amyloliquefaciens as a nitrogen-fixing bacterium associated with the leguminous Phaseolus acutifolius. Likewise, it is a first approximation in the first stages under salinity conditions, specifically with tepary beans, evaluating the isolated and halotolerant microorganism compared to a biological control Azospirillum halopraeferens AU10.
The halobacteriae B. amyloliquefaciens, isolated from ecotype tepary seeds, is able to resist high salt concentrations (0.75 M NaCl) and may facilitate the promotion of plant growth in the presence of levels of inhibition of salinity growth of soil that exceed 0.25 M NaCl.
Inoculation based on beneficial bacteria such as nitrogen fixers is a biological and reliable method to help maintain or improve the fertility of the soils that support the tepary fields. However, it is suggested to develop more evaluations in more advanced phenological stages and in other ecotypes in order to better understand the mechanisms of action and the particular reactions with each ecotype before recommending the association of bacteria at the field level.
Gratefullness
The present study was supported by the Institution: National Forestry Commission (CONAFOR) (Project No. 14651). The authors express their gratitude to the University of Sonora (UNISON) for the infrastructure and equipment provided and the partial financial grant granted by the National Council of Science and Technology (CONACYT) during the doctoral studies.
REFERENCES
Ahmad, M.; Zahir, Z. A.; Khalid, M.; Nazli, F. and Arshad, M. 2013. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 63(1):170-176. [ Links ]
Bashan, Y.; Holguin, G. and Lifshitz, R. 1993. Isolation and characterization of plant growth-promoting rhizobacteria. In: Glick, B. R. and Thompson, J. E. (Eds.). Methods in plant molecular biology and biotechnology. CRC Press, Boca Raton. 331-350 pp. [ Links ]
Carrillo, A.; Puente, M.; Castellanos, T. and Bashan, Y. 1998. Aplicaciones biotecnológicas de ecología microbiana. Manual de Laboratorio, Pontificia Universidad Javeriana, Santa Fe de Bogotá, Colombia and Centro de Investigaciones Biologicas del Noroeste. Manual de Laboratorio. La Paz, Mexico. 15-20 pp. [ Links ]
Cassan, F.; Maiale, S.; Masciarelli, O.; Vidal, A.; Luna, V. and Ruiz, O. 2009. Cadaverine production by Azospirillum brasiliense and its possible role in plant growth promotion and osmotic stress mitigation. Eur. J. Soil Biol. 45(1):12-19 [ Links ]
Diby P. and Harshad, L. 2015. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron. Sustainable Develop. 34(4):737-752. [ Links ]
Dobbelaere, S. and Okon, Y. 2007a. The plant growth promoting effects and plant responses. In: nitrogen fixation: origins, applications and research progress. Newton, W. (Ed.). Vol V: associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Elmerich, C. and Newton, W. E. (Eds.). Springer, Heidelberg. 145-170 p. [ Links ]
Dobbelaere, S. and Okon, Y. 2007b. The plant growth promoting effect and plant responses. In: Elmerich, C. and Newton, W. E. (Eds.). Associative and endophytic nitrogen-fixing bacteria and cyanobacterial associations. Kluwer Academic Publishers. The Netherlands. 1-26 pp. [ Links ]
Hamama, A. A. and Bhardwaj, H. L. 2002. Tepary bean: a short duration summer crop in Virginia. In: Janick, J. and Whipkey, A. (Eds.). Trends in new crops and new uses. ASHS Press, Alexandria. 429-431 pp. [ Links ]
Stefana, M.; Neculai, M.; Vasile, S.; Marius, M. and Lucian, H. 2013. Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L). Sci. Hortic. 151(28):22-29. [ Links ]
Mayak, S.; Tirosh, T. and Glick, B. R. 2004. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem . 42(6):565-572. [ Links ]
Nabhan, G. P. 1985. Gathering the Desert. University of Arizona Press, Tucson, USA. 24-35 pp. ISBN 13: 9780816509355. [ Links ]
Pathak, K. V. and Keharia, H. 2013. Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). Biotech. 4(1):41-48. [ Links ]
Reinhold, B.; Hurek, T.; Fendrik, I.; Pot, B.; Gillis, M.; Kersters, K.; Thielmans, S. and De Ley, J. 1987. Azospirillum halopraeferens sp. novo, a nitrogen-fixing organism associated with roots of Kallar grass (Leptochloa fusca L. Kunth). Inter. J. System. Bacteriol. 37(1):43-51. [ Links ]
Rokhzadi, A. and Toashih, V. 2011. Nutrient uptake and yield of chickpea (Cicer arietinum L.) inoculated with plant growth-promoting rhizobacteria. Austr. J. Cop Scci. 5(4):44-48. [ Links ]
Rueda, P. E.; Castellanos, T.; Troyo, D. E.; Díaz de León, Á. J. and Murillo, A. B. 2003. Effects of a nitrogen-fixing indigenous bacterium Klebsiella pneumoniae on the growth and development of the halophyte Salicornia bigelovii as a new crop for saline environments. J. Agron. Crop Sci. 189(5):323-332. [ Links ]
Rueda, P. E. O.; García, H. J. L.; Preciado, R. P.; Murillo, A. B.; Tarazón, H. M. A. and Flores, H. A. 2007. Germination of Salicornia bigelovii ecotypes under stressing conditions of temperature and salinity and ameliorative effects of plant growth-promoting bacteria. J. Agron. Crop Sci . 193(3):167-176. [ Links ]
Rueda, P. E. O.; Murillo, A. B.; Castellanos, C. T.; García, H. J. L.; Tarazòn, H. M. A.; Moreno, M. S. and Gerlach, B. L. E. 2010. Effects of plant growth promoting bacteria and mycorrhizal on Capsicum annuum L. var. aviculare ([Dierbach] D’Arcy and Eshbaugh) germination under stressing abiotic conditions. Plant Physiol. Biochem . 48(8):724-730. [ Links ]
Rugheim, A. M. E. and Abdelgani, M. 2012. Effects of microbial and chemical fertilization on yield and seed quality of faba bean (Vicia faba). Int. Food Res. J. 19(3):417-422. [ Links ]
Sandhya, V.; Ali, S. Z.; Grover, M.; Reddy, G. and Venkateswarlu, B. 2010. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regulation. 62(4):21-30. [ Links ]
Smibert, R. M. and Krieg, N. R. 1994. Phenotypic characterization. In: Gerhardt, P.; Murray, R.; Wood, W. and Krieg, N. (Ed.). Methods for general and molecular bacteriology. ASM Press, Washington, DC. 615 p. [ Links ]
Snedecor, G. 1956. Statistical methods applied to experiments in agriculture and biology. The Iowa State College Press, Ames, Iowa, USA. 237-290 pp. [ Links ]
Terré, S.; Asch, F.; Padham, J.; Sikora, R. A. and Becker, M. 2007. Influence of root zone bacteria on root iron plaque formation in rice subjected to iron toxicity. In: Tielkes, E. (Ed.). Utilisation of diversity in land use systems: sustainable and organic approaches to meet human needs. Tropentag, Witzenhausen, Germany. 446 p. [ Links ]
Ungar, I. 1982. Germination ecology of halophytes. In: Ungar, I. (Ed.). Tasks for vegetation science, Kluwer Academic Publishers, Dordrecht, The Netherlands. 143-154 pp. [ Links ]
Yadav, B. K. 2011. Interaction effect of phosphorus and sulphur on yield and quality of cluster bean in typic Haplustept. World J. Agric. Sci. 7(1):556-560. [ Links ]
Received: January 2018; Accepted: March 2018











 texto em
texto em 


