Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 spe 20 Texcoco Abr./Mai. 2018
https://doi.org/10.29312/remexca.v0i20.990
Articles
Age, benefit and gibberellic acid affect the germination and production of piquín pepper plant
2Center for Training and Development of Seed Technology of the Antonio Narro Autonomous Agrarian University. Saltillo, Coahuila, Mexico. CP. 25315. Tel. 01 (844) 4110303.
To obtain pepper or wild pepper seeds, the way of collecting or producing, the extraction technique, the selection, the packing, the form and time of storage and the conditioning prior to sowing, affect the germination and viability of the seed and the production of seedlings. The above, added to the natural latency, represent an important obstacle for the domestication and commercial production of this species. Therefore, the objective of this study was to evaluate germination and seedling production in relation to age, benefit and conditioning prior to sowing. Seeds of fresh red fruits of an ecotype of the region of Linares, Nuevo León, Mexico of 0, 2, 4 and 12 months of age after extraction were used. The benefit consisted of drying the seed with forced air up to 9% humidity, standardize by weight, pack in aluminized polyethylene bag and hermetically sealed and store at 15 °C. The conditioning was immersed with gibberellic acid at 5 000 mgL-1 for 12 h before planting. Germination, hydration rate, tetrazolium staining and seedling production were evaluated. The results show that the newly harvested seeds do not germinate due to immaturity of the embryo; after two months of rest the seed germinates, the benefit maintains its viability for more than a year, conditioning with gibberellic acid prior to sowing, increases the percentage of germination and the production of seedlings.
Keywords: Capsicum; wild peppers; seeds
Para obtener semillas de chile piquín o silvestre, la forma de colectar o producir, la técnica de extracción, la selección, el empaque, la forma y tiempo de almacenamiento y el acondicionamiento previo a la siembra, afectan la germinación y viabilidad de la semilla y la producción de plántulas. Lo anterior sumado a la latencia natural, representan un obstáculo importante para la domesticación y producción comercial de esta especie. Por lo anterior, el objetivo de este estudio fue evaluar la germinación y la producción de plántulas en relación a la edad, el beneficio y acondicionamiento previo a la siembra. Se utilizaron semillas de frutos rojos frescos de un ecotipo de la región de Linares, Nuevo León, México de 0, 2, 4 y 12 meses de edad después de extraída. El beneficio consistió en secar la semilla con aire forzado hasta 9% de humedad, uniformizar por peso, empacar en bolsa de polietileno aluminizado y sellada herméticamente y almacenar a 15 °C. El acondicionamiento fue inmersión con ácido giberélico a 5 000 mg L-1 por 12 h antes de la siembra. Se evaluó germinación, velocidad de hidratación, tinción con tetrazolio y producción de plántula. Los resultados muestran que las semillas recién cosechadas no germinan por inmadurez del embrión; después de dos meses de reposo la semilla germina, el beneficio mantiene su viabilidad por más de un año, el acondicionamiento con ácido giberélico previo a la siembra, aumenta el porcentaje de germinación y la producción de plántulas.
Palabras clave: Capsicum; chiles silvestres; semillas
Introduction
The demand for “piquín” or “del monte” wild peppers (Capsicum annuum var., aviculare, Dierb D'Arcy and Eshbaugh or glabriusculum) is on the increase, as traditional selling of fresh and dried fruit on the street and markets regional, the commercialization of derivative products such as pickles, sauces and dehydrated in commercial stores chains is added. There is no evidence of commercial production, only its backyard cultivation for self-consumption (Latournerie et al., 2002; Pedraza and Gómez, 2008). Therefore, this market is supplied almost entirely by the collection of wild fruits (Medina et al., 2002; Bran et al., 2007).
This situation has made the collection of this species more intense and aggressive, because collectors that, in their eagerness to harvest a greater quantity, do not collect only the fruits but cut the productive branches and even the whole plant, limiting their possibilities of regeneration. This has caused the disappearance of the species in some regions, especially those close to population centers. If this situation continues, it is put at risk if not the species, if important ecotypes (Bañuelos et al., 2008; Araiza-Lizarde et al., 2008). Therefore, it is necessary to look for options to conserve the existing wild populations and it is considered that one of the strategies is to discourage the collection of fruits of wild plants, through the domestication and agronomic production of these peppers, using the technology that is currently used they produce varieties of commercial peppers. However, for the establishment of these production programs, the problem of germination of the seed must first be solved, which has germination rates ranging from 5 to 80% (Ramírez-Meraz, 2001; Rodríguez Del Bosque et al., 2003).
The low and variable germination rate is an important impediment to having certified seed that allows to establish lots of commercial production of this species. The percentage of reduced germination in the piquin pepper seed is attributed to physical or physiological latency, caused by the impermeability of its seed coat or cover, immaturity of the seed or resting of the embryo (Besnier, 1989, Ramírez-Meraz, 2001; Rodríguez Del Bosque et al., 2003). To solve this problem, different techniques are reported, including chemical and physical scarification, conditioning or seed treatment prior to sowing with gibberellic acid, potassium nitrate, hydrogen peroxide (Rodríguez Del Bosque et al., 2003; De la Rosa et al., 2012; Cano-Vázquez et al., 2015).
However, the results are very variable and low germination rates persist. On the other hand, previous studies show that the age of the seed significantly affects its germination, in addition there is no evidence that the seeds of the wild peppers are benefited and stored properly. The objective of this work was to evaluate seed germination and seedling production, depending on the time elapsed from the extraction to the sowing, the benefit to the seed and the conditioning of the seed with gibberellic acid prior to sowing.
Materials and methods
The work was carried out in the Seed Production and Testing Laboratories of the Seed Technology Training and Development Center (CCDTS) and in the Horticulture Department of the Antonio Narro Autonomous Agrarian University, in Buenavista Saltillo, Coahuila, Mexico, during the period from 2006 to 2011. The plant material consisted of seeds of different ages, 0, 2, 4 and 12 months, obtained from fresh red fruits from collections of the Linares region, Nuevo Leon, Mexico.
Extraction and benefit of the seed.
The seed was extracted by macerating the fruits with hands and latex gloves, the macerate was placed in drinking water and the seeds were allowed to settle, then dried at room temperature. Half of the seed was conditioned and the other half was only extracted, dried by sun exposure and stored in a conventional way in a Ziploc brand polyethylene bag and at the ambient temperature of the seed laboratory of the CDDTS. The conditioning consisted of drying the seed with forced air, until reaching a humidity of 9%, for which a South Dakota brand blower was used, it was homogenized by weight and size and it was stored in Uline brand plastic bags of 10 × 20 cm, hermetically sealed and at a temperature of 15 °C (Aguirre and Peske, 1998; Reveles-Hernández et al., 2013).
Germination tests
Four ages of the seed, 0, 2, 4 and 12 months after extraction were evaluated, seeds benefited and not benefited and seeds preconditioned with gibberellic acid at 5 000 mgL-1 and seeds without preconditioning (dry seeds) each treatment with three repetitions and each repetition with 50 seeds. The experimental design was factorial A × B × C, where: A= age, B= benefit and C= conditioning (Zar, 1996). The seeds were germinated in Petri dishes on Whatman No. 1 filter paper in a Lab-line branching chamber at 25 ±1 °C, with 8 hours of light and 16 hours of darkness. Germinated seeds, ungerminated seeds and abnormal plants were recorded (AOSA, 1993).
Imbibition test of the seed
This test was performed additionally to determine if the seed was impervious to water. The 50 seeds of each age were weighed with four repetitions on an Ohaus brand precision balance, model Adventurer Pro, then placed in a 13 × 100 mm test tube, 10 cc of distilled water was added with an Eppendorf brand micropipette and by difference of the volume, the water absorbed was quantified, the excess water was removed and the hydrated seed was weighed again, to determine the weight increase by the water absorption. This test was performed at 12, 24, 36, 48 and 60 h.
Viability test with tetrazolium
To determine the viability of the seed, the 2, 3, 5-Triphenyl test, Tetrazolium chloride (TZ), Sigma-Aldrich brand (ISTA, 2004) was carried out. It started with an osmoconditioning of the seed, which consists of placing the seeds in distilled water for 16 hours. Once the seed was hydrated, longitudinal cuts were made with a scalpel, to expose the cotyledons. The sections were placed in a test tube of 13 × 100 mm, adding the solution of 2, 3, 5 Tetrazolium Trifenil chloride 0.1% to cover the seed, then the test tubes were wrapped with aluminum foil to avoid light and they were placed in a Lab-Line brand incubation chamber at 30-35 °C for 90 min. Subsequently, in a Carl Zeiss brand stereoscope, seed staining was observed.
Seedling production
In this test, three different ages of the seed, 2, 4 and 12 months were evaluated (the seeds were removed recently harvested, because there was no germination in that test), seeds benefited and not benefited, seeds preconditioned with gibberellic acid at 5 000 mgL-1 and seeds without preconditioning (dry seeds); each treatment with three repetitions and each repetition with 50 seeds. The experimental design was factorial A × B × C where, A= age, B= benefit and C= conditioning (Zar, 1996). The seeds were planted in polystyrene trays of 200 cavities and as a substrate moss peat moss brand Sunshine was used. At 48 days after sowing, percentage of seedlings suitable for transplanting, height and diameter of the seedlings and number of true leaves were evaluated.
Results and discussion
Germination tests
The results show that age and benefit, are the factors that have the most effect on germination, the conditioning with gibberellic acid at 5 000 mgL-1, was also significant, but to a lesser degree. The highest germination percentages of piquín or wild pepper were obtained with the seeds of 2 and 4 months of age, which were benefited and conditioned before sowing with gibberellic acid (Table 1). The piquín or wild pepper seed that is currently used for research, backyard production or small scale, comes from collections that are made directly in the field, fruits left behind vendors or nuts that retain fans of this crop or that are purchased in stores.
Table 1 Effect of age, benefit, gibberellic acid and interactions on piquín pepper germination.
| Treatments | Mean | Standard error |
| Age 0 months | 0.5 c | 0.35 |
| Age 2 months | 74.5 ab | 5.22 |
| Age 4 months | 90.66 a | 2.26 |
| Age 12 months | 42.5 bc | 12.45 |
| With benefit | 37.25 b | 7.99 |
| Without benefit | 66.83 b | 8.06 |
| Without conditioning with gibberellic acid 5 000 mg L-1 | 49.91 b | 8.48 |
| With conditioning with gibberellic acid 5 000 mg L-1 | 54.16 b | 8.69 |
| Age 0 * without benefit | 0.33 c | 0.33 |
| Age 0 * with benefit | 0.66 c | 0.66 |
| Age 2 * without benefit | 61 b | 6.08 |
| Age 2 * with benefit | 88 ab | 3.18 |
| Age 4 * without benefit | 86.33 ab | 3.63 |
| Age 4 * with benefit | 95 a | 1.34 |
| Age 12 * without benefit | 1.33 c | 0.98 |
| Age 12 * with benefit | 83.66 ab | 1.81 |
| Age 0 * without conditioning | 0 c | 0 |
| Age 0 * with conditioning t | 1 c | 0.68 |
| Age 2 * without conditioning | 67.33 b | 8.02 |
| Age 2 * with conditioning | 81.66 ab | 5.91 |
| Age 4 * without conditioning | 89.66 ab | 2.8 |
| Age 4 * with conditioning | 91.66 a | 3.77 |
| Age 12 * without conditioning | 42.66 bc | 19.12 |
| Age 12 * with conditioning | 42.33 bc | 17.78 |
| Without benefit * without conditioning | 34.33 bc | 11.14 |
| Without benefit * with conditioning | 40.16 bc | 11.9 |
| with benefit * without conditioning | 65.5 b | 11.51 |
| with benefit * with conditioning | 68.16 b | 11.78 |
| Age 0 * without benefit * without conditioning | 0 c | 0 |
| Age 0 * without benefit * with conditioning | 0.66 c | 0.66 |
| Age 0 * with benefit * without conditioning | 0 c | 0 |
| Age 0 * with benefit * with conditioning | 1.33 c | 1.33 |
| Age 2 * without benefit * without conditioning | 50.66 b | 4.37 |
| Age 2 * without benefit * with conditioning | 71.33 ab | 7.68 |
| Age 2 * with benefit * without conditioning | 84 ab | 5.03 |
| Age 2 * with benefit * with conditioning | 92 a | 3.05 |
| Age 4 * without benefit * without conditioning | 86.66 ab | 5.45 |
| Age 4 * without benefit * with conditioning | 86 ab | 6 |
| Age 4 * with benefit * without conditioning | 92.66 a | 0.66 |
| Age 4 * with benefit * with conditioning | 97.33 a | 1.76 |
| Age 12 * without benefit * without conditioning | 0 c | 0 |
| Age 12 * without benefit * with conditioning | 2.66 c | 1.76 |
| Age 12 * with benefit * without conditioning | 85.33 ab | 2.9 |
| Age 12 * with benefit * with conditioning | 82 ab | 2.3 |
Means with different letters are statistically different (Tukey p ≤ 0.05).
The form of storage is also very varied and goes from the conservation in nuts or the extraction and conservation in containers, between the most common, polyethylene bags, paper and glass jars. The management of the seed, from the production or collection, form of extraction, selection, conditioning, drying and storage, as a whole is called profit (Aguirre and Peske, 1988). This benefit has a high impact on the germination and viability of the seed and no evidence was found to be made on piquin pepper seeds. This may be a cause of the low and variable germination rate reported from 5 to 80% (Ramírez-Meraz, 2001; Rodríguez et al., 2003; Cano-Vázquez et al., 2015).
Among other possible causes, are cited from myths such as the fact that the seed of wild pepper, require passing through the digestive system of birds to germinate (Ariza-Lizarde et al., 2011). The investigations to determine the causes of the latency, have basically started from two hypotheses; One is that the seed has physiological latency, for this purpose germination tests have been carried out with hydrogen peroxide (Flores et al., 2008), potassium nitrate and gibberellic acid (García-Federico et al., 2010; Araiza-Lizarde et al., 2011), another hypothesis is that the seed presents physical latency, attributed to the impermeability of the testa or seminal cover and to the low permeability of the endosperm (Bañuelos et al., 2008; Araiza et al., 2011), for this studies of scarification, stratification and previous hydration of the seed are reported. Therefore, in a complementary way, this study included a conditioning test with gibberellic acid and another one of imbibition that are cited as the most common tests to check whether there is physical or physiological latency.
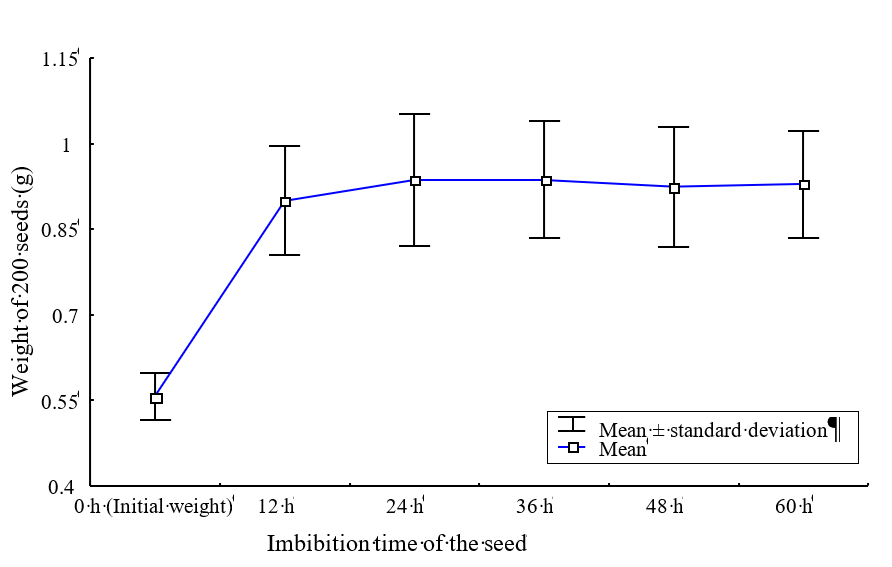
Imbibition test
The results show that the piquín pepper seed absorbed a greater volume of water during the first 12 hours, while in the subsequent 48 h the absorption was minimal. The above shows that the seed is not impervious to water. The impermeability of the testa or seminal cover is reported as the most probable cause of the physical latency of the piquín pepper seed (Ramírez-Meraz, 2001, Rodríguez-Del Bosque et al., 2004). However, the imbibition tests show that the seed is hydrated during the first 12 hours of exposure to water (Figure 1), and therefore the latency is not associated with the impermeability of seed coat or cover (García-Federico et al., 2010; Prado-Urbina et al., 2015).
This information is important, because in practice, performing osmoconditioning prior to sowing, commonly known as seed soaking, makes this activity difficult. If the seeding is manual, the seed adheres to the hands and if it is mechanical, the seeders are designed to plant dry seeds, which is the way to plant commercial pepper seeds, such as serrano peppers, jalapeños, morrones, habaneros, etc.
Tetrazolium stain test
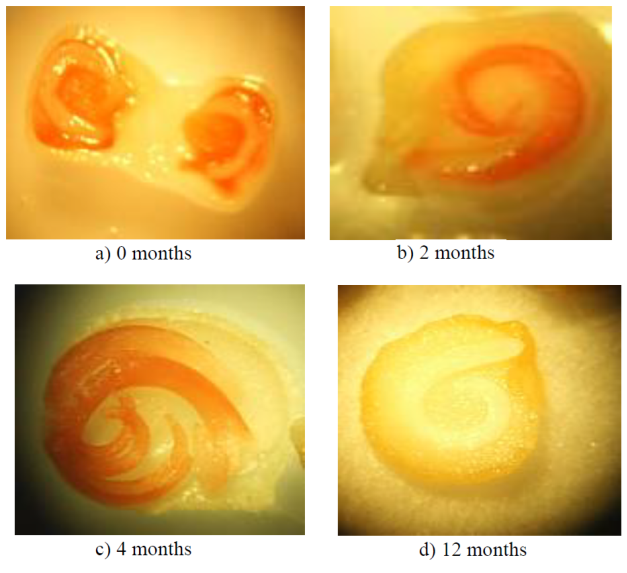
The tetrazolium staining test (2, 3, 5 triphenyl tetrazolium chloride) is a technique used to determine if the seeds have physiological activity. It is based on the reduction of tetrazolium by the hydrogen that is released by the activity of the hydrogenase enzymes, which are activated as a result of respiration, once the seeds have begun their germination process. Because of the reduction of tetrazolium, which is colorless, a red color is formed, which is indicative that the seed has begun its germination (ISTA, 2007, SNICS, 2017). The red coloration can appear in any part of the seed that has respiratory activity, so it is necessary to identify if this red color is present in the embryo, because in this way it can be more certain that the seed is able to germinate.
The results of this test are presented with photographs, where graphically it is shown that the seeds extracted from fresh red fruits (Figure 2a) do not present staining in the embryo, only in the endosperm of the seed, which indicates that there is activity Respiratory, not necessarily as part of the germination process, but rather as an activity of the reserve storage process. In Figures 2b and 2c corresponding to the seeds of 2 and 4 months of age, the red color is observed in the embryo, which indicates respiratory activity in this tissue, when correlating these results with the germination tests, it is concluded that respiratory activity is the result of the germination process of the seed. Finally, when looking at Figure 2d, which corresponds to the seeds of 12 months of age without benefit, no staining is observed, which indicates that there is no respiratory activity in any tissue; that is, dead seeds.
The seeds of 12 months and that were benefited, presented a similar staining to the seeds of 4 months of age, so that the benefit and adequate storage of the seed reduces the deterioration conserving the seed for more than a year, as it happens with seeds of commercial peppers.
Seedling production
In this test, the analysis of variance showed that the percentage of seedlings is affected by age, benefit and seed conditioning with gibberellic acid, but once the seedlings emerged no effect on their growth and development was observed and these results coincide with those obtained in the germination test.
Also, these results show that it is possible to obtain up to 96% of seedlings suitable for transplant if the seed benefits, later it is stored properly for 4 months and before planting it is conditioned with a solution of 5 000 mgL-1 of gibberellic acid before sowing (Table 2 and Figure 3). It was also observed that if the seed benefits and stores properly, it can maintain its viability for more than 1 year.
Table 2 Effect of age, benefit, gibberellic acid and interactions on piquín pepper seedling production.
| Treatments | Mean | Error estándar |
| Age 2 months | 65.33 b | 6.63 |
| Age 4 months | 71.33 ab | 6.84 |
| Age 12 months | 45.66 b | 13.51 |
| With benefit | 31.88 b | 5.62 |
| Without benefit | 89.66 b | 1.62 |
| Without conditioning with gibberellic acid 5 000 mgL-1 | 58.66 b | 7.96 |
| With conditioning with gibberellic acid 5 000 mgL-1 | 62.88 b | 8.27 |
| Age 2 * without benefit | 44.33 b | 2.84 |
| Age 2 * with benefit | 86.33 ab | 2.98 |
| Age 4 * without benefit | 50.33 b | 4.88 |
| Age 4 * with benefit | 92.33 a | 2.44 |
| Age 12 * without benefit | 1 c | 0.68 |
| Age 12 * with benefit | 90.33 a | 2.89 |
| Age 2 * without conditioning | 66 ab | 10 |
| Age 2 * with conditioning | 64.66 b | 9.65 |
| Age 4 * without conditioning | 66.33 b | 10.19 |
| Age 4 * with conditioning | 76.33 b | 9.61 |
| Age 12 * without conditioning | 43.66 bc | 19.37 |
| Age 12 * with conditioning | 47.66 bc | 20.73 |
| Without benefit * without conditioning | 29.77 b | 7.46 |
| Without benefit * with conditioning | 34 b | 8.8 |
| With benefit * without conditioning | 87.55 ab | 2.25 |
| With benefit * with conditioning | 91.77 a | 2.24 |
| Age 2 * without benefit * without conditioning | 44.66 b | 4.8 |
| Age 2 * without benefit * with conditioning | 44 b | 4.16 |
| Age 2 * with benefit * without conditioning | 87.33 ab | 4.66 |
| Age 2 * with benefit * with conditioning | 85.33 ab | 4.66 |
| Age 4 * without benefit * without conditioning | 44 b | 3.05 |
| Age 4 * without benefit * with conditioning | 56.66 b | 8.35 |
| Age 4 * with benefit * without conditioning | 88.66 b | 3.33 |
| Age 4 * with benefit * with conditioning | 96 a | 2.3 |
| Age 12 * without benefit * without conditioning | 0.66 c | 0.66 |
| Age 12 * without benefit * with conditioning | 1.33 c | 1.33 |
| Age 12 * with benefit * without conditioning | 86.66 ab | 5.2 |
| Age 12 * with benefit * with conditioning | 94 a | 1.15 |
Means with different letters are statistically different (Tukey p≤ 0.05).

Figure 2 Piquín pepper seedling with seeds of 4 months old, benefited and conditioned with 5 000 mg L-1 of gibberellic acid.
The response of the piquín pepper seed to the benefit and conditioning is very similar to that of other types of commercial pepper, given that commercial pepper seeds carry a period of rest after being extracted by the dynamics of their preparation for sale. For example, broad-leaved, free-pollinated peppers seeds or varieties, in the northern region of Guanajuato, Zacatecas, Aguascalientes, are harvested during the months of september to october, and later planted in the months of november to january. Which implies that the seeds are at rest at least 2 months before planting. In the case of hybrids, harvest, profit, packaging and distribution for sale, it is estimated that it lasts more than two months.
Conclusions
The seeds extracted from fresh red piquín pepper fruits, require two months of rest to germinate and the tetrazolium stain showed that the seeds of less than two months and more than a year without conditioning do not have respiratory activity in the embryo. The benefit to the seed maintains its viability for more than a year and the conditioning prior to sowing with gibberellic acid increases germination and seedling production.
Additionally, the piquin pepper seed does not have water impermeability in the seed coat or seed coat. Finally, the percentage of germination and seedling production is obtained with seeds benefited, with 4 months of rest or age and treated or conditioned with a solution of 5 000 mgL-1 of gibberellic acid, 12 h before planting.
Literatura citada
Aguirre, R. y Peske, S. T. 1988. Manual para el beneficio de semillas. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia. [ Links ]
Araiza, L. N.; Araiza, L. E. y Martínez, M. J. G 2011. Evaluación de la germinación y crecimiento de plántula de chiltepín (Capsicum annuum Lvar. glabriusculum) en invernadero. Rev. Colomb. Biotecnol. 13(2):170-175. [ Links ]
Association of Official Seed Analysis (AOSA) 1993. Rules for testing seeds. Journal of Seed Technology. 16(3): [ Links ]
Bañuelos, N.; Salido, P. L. y Gardea, A. 2008. Etnobotánica del chiltepín. Pequeño gran señor en la cultura de los sonorenses. Estudios Sociales Hermosillo, Son. 16:177-205. [ Links ]
Besnier, R F. 1989. Semillas biología y tecnología. Editorial Mundi-Prensa. España. 164-167 pp. [ Links ]
Bran, R. A. A.; Moya, C.; Ponce, P.; Álvarez, M. y Varela, M. 2007. Diagnóstico participativo de las condiciones socioculturales asociadas a la conservación de los chiles silvestres (Capsicum spp.), en la depresión central de Chiapas, México. Cultivos Tropicales. 28(1):69-73. [ Links ]
De la Rosa, M.; Arce, L.; Villarreal, J. A.; Ibarra, L. y Lozano, J. 2012. Germinación de semillas de chile simojovel (Capsicum annuum L.) previamente expuestas a NaCl y ácido giberélico. Pyton. 81:165-168. [ Links ]
Cano, V. A; López, P. M. C.; Zavaleta, M. H. A.; Cruz, H. N.; Ramírez R. I.; Gardea, B. A. y González, H. V. A. 2015. Variación en grados de latencia en semillas entre colectas de chile piquín (Capsicum Annuum Var. Glabriusculum). Bot. Sci. 93(1):175-184. [ Links ]
Ellis, R. H. Hong, T. D. and Roberts, E. H. 1998. Handbook of seed technology for genebanks. Compendium of specific germination information and test recommendations. Department of agriculture and horticulture, University of Reading, UK. Vol. II. 591 p. [ Links ]
García, F. A.; Montes, H. S.; Rangel, L. J. A; García, M. E. y Mendoza, E. M. 2010. Respuesta fisiológica de la semilla chile piquín (Capsicum annuum var. Glabriusculum (Dunal) Heiser & Pickersgill] al ácido giberélico e hidrotermia. Rev. Mex. Cienc. Agríc. 1:203-216. [ Links ]
ISTA. 1993. International rules for seeds testing. Rules 1993. Seed Sci. Technol. 21:27-28. [ Links ]
ISTA. 2007. International Seeds Testing Association. Chapter 6: Tetrazolium test. In: International Rules for Seed testing. Seed Science and Technology. 6-10 pp. [ Links ]
Flores, G. A.; Álvarez, M. J. G.; Rodríguez de la O. J. L. y Corona, A. A. 2008. Germinación in vitro de semillas de Nolina parviflora (H.B.K.) Hemsl. Foresta Veracruzana 10:27-33. [ Links ]
Latournerie, M. L.; Chávez, J. L.; Pérez, G. M.; Castañón, S. A.; Rodríguez, L.; Arias, M. y Ramírez, P. 2002. Valoración in situ de la diversidad morfológica de chiles (Capsicum annuum L. y Capsicum chinense Jacq.) en Yaxcabá, Yucatán. Fitotec. Mex. 25:25-33. [ Links ]
Medina, T.; Rodríguez del Bosque, L. A.; Villalón, H.; Pozo, O.; Ramírez, M.; López, R.; Lara M., Gaona, G.; Cardona, A. y Mora, A. 2002. El Chile piquín. (Capsicum annuum L. var. Aviculare) en el Noreste de México. Aspectos ecológicos y socioeconómicos. Biotam. 13:1-14. [ Links ]
Prado, U. G; Lagunes, E. L del C.; García, L. E.; Bautista, M. C.; Camacho, C. W.; Mirafuentes, G. y Aguilar, R. V. H. 2015. Germinación de semillas de chiles silvestres en respuesta a tratamientos pre-germinativos. Ecosistemas y Recursos Agropecuarios. 2(5):139-149. [ Links ]
Pedraza R. L. C y Gómez, G. A. A. 2008. Análisis exploratorio del mercado y la comercialización de chile piquín (C. annuum var. aviculare Dierb.) en México. Tecsistecatl, 1(5). [ Links ]
Pozo, C. O. M y Ramírez, M. 2003. Diversidad e importancia de los chiles silvestres. In: memorias del 1er Simposio regional sobre chile piquín. INIFAP campo experimental Río Bravo Tamaulipas, México. 17-19. [ Links ]
Ramírez, M. M. 2001. Inducción de la germinación en semilla de chile piquín. 13º Encuentro de Investigación Científica y Tecnológica del Golfo de México. 31 p. [ Links ]
Reveles, H. M.; Velásquez, V. R.; Reveles, T. L R. y Mena, C. J. 2013. Selección conservación de semilla de chile: primer paso para una buena cosecha. INIFAP-Campo Experimental Zacatecas. Folleto técnico núm. 51. [ Links ]
Rodríguez, Del B. L. A.; Ramírez, M. M y Pozo, C. O. 2004. Tecnologías de producción de chile piquín en el Noreste de México. INIFAP-CIRNE. Campo Experimental Río Bravo. Tamaulipas, México. Folleto técnico Núm. 29. 33 p. [ Links ]
Rodríguez, Del B. L. A.; Ramírez, M. M y Pozo, C. O. 2003. El cultivo del chile piquín bajo diferentes sistemas de producción en el noreste de México. In: memoria del 1er. Simposio regional sobre chile piquín: Avances de investigación en tecnología de producción y uso racional del recurso silvestre. INIFAP-CIRNE. Campo Experimental Río Bravo Tamaulipas. Publicación especial Núm. 26. México. 1-16 p. [ Links ]
SNICS. 2017. Servicio Nacional de Inspección y certificación de Semillas. Certificación de semillas. http://www.gob.mx/snics/acciones-y-programas/tramites-y-servicios-snics. [ Links ]
Watkins, T. J. and Cantliffe, D. J. 1983. Mechanical resistance of the seed coat and endosperm during germination of Capsicum annuum at low temperature. Plant Physiol. 72:146-150. [ Links ]
Zar, J. H. 1996. Biostatistical analysis. Third (Ed.). Prentice-Hall Inc. New Jersey, USA. [ Links ]
Received: December 2017; Accepted: January 2018











 texto em
texto em