Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 spe 20 Texcoco Abr./Mai. 2018
https://doi.org/10.29312/remexca.v0i20.988
Articles
Zinc content and yield of biofortified cowpea beans
1Division of Agricultural Sciences Academic-Autonomous University Juarez of Tabasco. Highway Villahermosa-Teapa km 25, R/A La Huasteca 2nd. Section, Villahermosa Centro, Tabasco, Mexico. CP. 86280. Tel. 52 (993) 3581500, ext. 6607. (cesar.marquez@ujat.mx, rodolfo.osorioo@gmail.com, javier.huijara@ujat.mx).
2Center of Research in Food and Development AC-Unit Delicias. Delicias, Chihuahua. Mexico. (were@ciad.mx; johnvih@gmail.com).
Zinc (Zn) is an essential trace element for plants, animals and humans; consequently, their deficiency affects their growth and development. It is estimated that between 15 and 30% of the human population in the world exhibit Zn deficiencies. The objective was to study the effect of biofortification with Zn2+ on the mineral content and yield of cowpea beans. The experiment was developed under a completely random design in two production cycles. The Zn was applied as zinc sulphate (T1: 0 µM L-1, T2: 25 µM L-1, T3: 50 µM L-1 and T4: 100 µM L-1) and as zinc chelate (T5: 0 µM L-1, T6: 25 µM L-1, T7: 50 µM L-1 and T8: 100 µM L-1). The applications of 25 μM L-1 of ZnSO4 and 50 μM L-1 of Zn-EDTA for both production cycles were the most effective in increasing the content of this element in the bean seed cowpea, determining 1.14 and 0.93 times more zinc respectively, compared to the control. The yield, in cycle 1, was decreased by 53.8 and 20.3% by applying 50 μM L-1 of ZnSO4 and 25 μM L-1 of Zn-EDTA, respectively. The addition of 50 μM L-1 of ZnSO4 and 25 μM L-1 of Zn-EDTA, in cycle 2, increased the yield by 16.7 and 37.3%, respectively, compared to the control. The best treatments to biofortify cowpea beans were 25 μM L-1 of ZnSO4 and 50 μM L-1 of Zn-EDTA for both production cycles.
Keywords: Vigna unguiculata subsp. unguiculata; functional food; iron; zinc
El zinc (Zn) es un elemento traza esencial para las plantas, los animales y los seres humanos; por consiguiente, su deficiencia afecta el crecimiento y el desarrollo de los mismos. Se estima que entre 15 y 30% de la población humana en el mundo exhiben deficiencias de Zn. El objetivo fue estudiar el efecto de la biofortificación con Zn2+ sobre el contenido mineral y rendimiento del frijol caupí. El experimento se desarrolló bajo un diseño completamente al azar en dos ciclos de producción. El Zn se aplicó como sulfato de zinc (T1: 0 µM L-1, T2: 25 µM L-1, T3: 50 µM L-1 y T4: 100 µM L-1) y como quelato de zinc (T5: 0 µM L-1, T6: 25 µM L-1, T7: 50 µM L-1 y T8: 100 µM L-1). Las aplicaciones de 25 µM L-1 de ZnSO4 y 50 µM L-1 de Zn-EDTA para ambos ciclos de producción fueron las más efectivas en aumentar el contenido de este elemento en la semilla de frijol caupí, determinándose 1.14 y 0.93 veces más zinc, respectivamente, en comparación con el testigo. El rendimiento, en el ciclo 1, se decrementó 53.8 y 20.3% al aplicar 50 µM L-1 de ZnSO4 y 25 µM L-1 de Zn-EDTA, respectivamente. La adición de 50 µM L-1 de ZnSO4 y 25 µM L-1 de Zn-EDTA, en el ciclo 2, incrementó el rendimiento en 16.7 y 37.3%, respectivamente, en comparación con el testigo. Los mejores tratamientos para biofortificar frijol caupí fueron 25 µM L-1 de ZnSO4 y 50 µM L-1 de Zn-EDTA para ambos ciclos de producción.
Palabras clave: Vigna unguiculata subsp. unguiculata; alimento funcional; hierro; zinc
Introduction
The cowpea bean Vigna unguiculata (L.) Walp. Subsp. unguiculata is a legume that occurs in tropical and subtropical regions, humans can consume the leaves, roots, grains and immature pods (Lim, 2012) also, is a source of protein, dietary fiber, carbohydrates, vitamins, essential nutrients and phytochemicals in the human diet (Awika and Duodu, 2017). The zinc (Zn) content in the seed is often low (7.3 mg kg-1, Espinosa-Moreno et al., 2013), especially when it is produced in soils with microelement deficiencies (Alloway, 2008). However, values of 43 mg kg-1 to 65 mg kg-1 of Zn have been reported in cowpea beans biofortified with iron (Márquez-Quiroz et al., 2015). On the other hand, per capita consumption worldwide is 3.89 kg year-1, while in Mexico it is 1.89 kg year-1 (FAOSTAT, 2017).
The Zn is an essential component of various dehydrogenases, proteases and peptidases (Fageria and Baligar, 2005). In this sense, the deficiency of this microelement constitutes a public health problem (Pereira et al., 2014). To correct it, strategies have been implemented to increase Zn content in legumes (Praharaj et al., 2016). In this sense, the biofortification of the crop with Zn fertilizers has increased the content of this element in legumes by 74.6%, it has increased the antioxidant capacity of the grain 60% (Sida-Arreola et al., 2017), and has reduced the content of antinutrients (Sharma et al., 2017); likewise, it has been observed that when increasing the dose of Zn2+, the P content tends to decrease (Cakmak et al., 2010). In the seeds of various crops, most of the Zn is associated with proteins, peptides (Persson et al., 2016), enzymes (Broadley et al., 2007) and phytic acid (Broadley et al., 2012).
Several studies have shown that biofortification with zinc fertilizers increased the content of the microelement in potato Solanum tuberosum (L.) (White et al., 2017), rice Oryza sativa (L.) (Tuyogon et al., 2016), wheat Triticum aestivum (L.) (Cakmak, 2008; Zhao et al., 2014), maize Zea mays (L.) (Potarzycki et al., 2015), onions Allium cepa (L.) (Almendros et al., 2015; Manna and Maity, 2016), common bean Phaseolus vulgaris (L.) (Ram et al., 2016) and safflower Carthamus tinctorius (L.) (Movahhedy-Dehnavy et al., 2009). In general, there is little literature on the biofortification of legumes of the genus Vigna and the results obtained from other works are mainly focused on the production of biofortified cereals, so the objective of this research was to study the effect of biofortification with Zn2+ on the mineral content and yield of cowpea beans.
Materials and methods
Production cycles
The work was carried out in the Academic Division of Agricultural Sciences of the Universidad Juarez Autonoma of Tabasco, geolocated at 17° 47’ north latitude, 92° 57’ west longitude and 29 meters above sea level. The variety of cowpea bean “De Castilla” was used, of indeterminate growth habit.
Production cycle autumn-winter 2013 and spring-summer 2014
The seeds were planted in black polyethylene pots of 25 cm x 30 cm, using inert substrate tepetzil. During the autumn-winter cycle the average temperature was 30 °C, with relative humidity of 86 to 94%, while the spring-summer cycle registered an average temperature of 37 °C, with relative humidity of 80 to 94%.
In both production cycles, the pots were established in a 200 m2 tropical Megavent protected system, with lateral cover of anti-aphid mesh and Grown Cover mesh in the ground. The plants were arranged in a double row, with a separation of 30 cm between plants and 90 cm between rows, for a planting density of 44 444 plants per hectare. The plants were guided vertically with raffia wire, and phytosanitary management was carried out with applications of Karate® (lambda cyalotrine) and Sulfacob 25® (copper sulfate pentahydrate).
Fertilization and treatments
To the plant of each pot was applied irrigation with nutrient solution (Hoagland and Arnon, 1950), which contained 14 mM of NO3 -, 1 mM of H2PO4 -, 2 mM of SO4 2-, 6 mM of K+, 4 mM of Ca2+ and 2 mM of Mg2+. The microelements of the nutrient solution were supplied with the TradeCorp AZ®. product. The nutrient solution was adapted according to the stages of development of the crop at 50 and 100%, at 10-30, 31-100 days after sowing (DDS), respectively, the pH of the solution was maintained between 5.5 and 6, using sulfuric acid. To the ten DDS, 0.25 L of solution was applied per pot day-1, at 31 DDS it was increased to 0.5 L day-1 and 1 L day-1 was applied after 61 DDS.
The treatments consisted in the addition of Zn + 2 in the form of zinc sulphate (ZnSO4·7H2O reactive grade, 21% Zn, 0 μM L-1, 25 μM L-1, 50 μM L-1 and 100 μM L-1) and as zinc chelate (Zn-EDTA, TradeCorp Zn®, 14% Zn, 0 μM L-1, 25 μM L-1, 50 μM L-1 and 100 μM L-1) (Table 1). Both compounds were dissolved in distilled water, and applied from day 31 to day 100 DS, every third day. The total of treatments was eight for each production cycle, with four repetitions. A completely random design was used.
Variables evaluated
The variables evaluated were: mineral content and yield of cowpea beans obtained. The harvest of the pods began at 70 DDS and ended at 100 DDS. The harvested pods were placed in labeled brown paper bags, to be later weighed. On the other hand, the nitrogen content (N) in the seed was determined with the Dumas method (1831), the total crude protein was calculated by multiplying the N content by the factor 6.25, the phosphorus (P), potassium (K), iron (Fe) and zinc (Zn) of the seed were determined by optical emission spectrometry with inductively coupled plasma (ICP-OES ICAP® 7200 Duo, Thermo Fisher Scientific), according to the procedure 984.27 (Horwitz, 2002) and previous wet digestion.
Statistical analysis
The data obtained were subjected to an analysis of variance. For the difference between means of the treatments, the orthogonal contrasts test (p≤ 0.05) was used (SAS Institute, 2013).
Results and discussion
Mineral content of biofortified beans
The results of the mineral content of biofortified cowpea beans with different doses of zinc fertilizers in the two production cycles are shown in Figure 1.
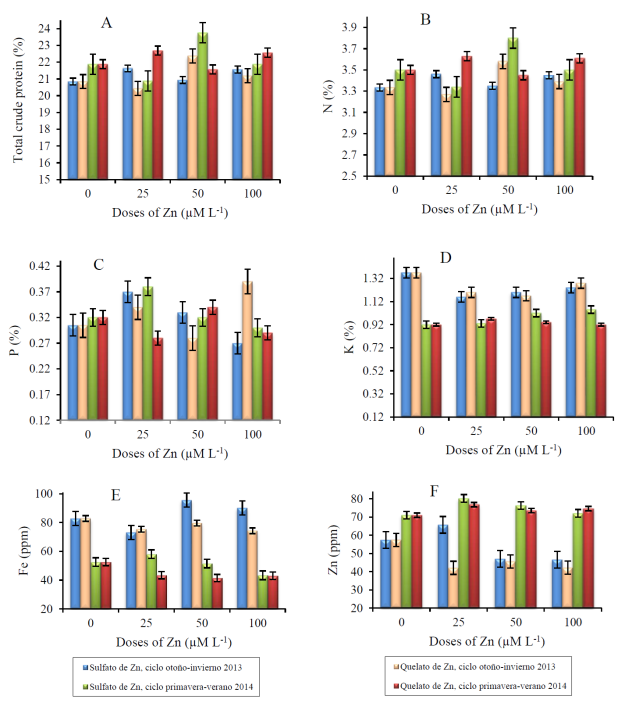
Figure 1 Content of total crude protein (A), nitrogen (B) phosphorus (C), potassium (D), iron (E) and zinc (F) of biofortified cowpea beans in two production cycles. Mean values ± standard error.
In cycle 1, the addition of 25 and 100 μM L-1 of ZnSO4, and 50 μM L-1 of Zn-EDTA increased the total crude protein content and N in 3.7, 3.4 and 7.3%, respectively, compared to the addition of 0 μM L-1 of the chemical compound (Figure 1A and Figure 1B). On the other hand, when adding 25 μM L-1 of Zn-EDTA, the content of total crude protein and N decreased by 1.9%, respectively, compared to the addition of 0 μM L-1 of the chemical compound. On the other hand, the addition of 25 and 50 μM L-1 of ZnSO4, and 25 and 100 μM L-1 of Zn-EDTA decreased the P content by 21.3, 8.2, 11.5, and 27.9%, respectively, compared to the addition of 0 μM L-1 of the chemical compound (Figure 1C). The content of K was decreased by 15.3, 12.4, 9.5, 12.4, 14.6 and 6.6% when adding 25, 50 and 100 μM L-1 of ZnSO4, and 25, 50 and 100 μM L-1 of Zn-EDTA, respectively (Figure 1D).
At the same time, the application of 25 μM L-1 of ZnSO4, and 25, 50 and 100 μM L-1 of Zn-EDTA decreased the Fe content by 11.8, 8.9, 3.9 and 10.4%, respectively, compared with the application of 0 μM L-1 of the chemical compound (Figure 1E). Similarly, the addition of 50 and 100 μM L-1 of ZnSO4 and 25, 50 and 100 μM L-1 of Zn-EDTA and a decrease in the content of Zn in 18.2, 18.9, 26.7, 20.6 and 26.5%, respectively, compared to the application of 0 μM L-1 of the chemical compound (Figure 1F).
In cycle 2, the addition of 25 μM L-1 of ZnSO4, and 50 μM L-1 of Zn-EDTA decreased the total crude protein content and N in 4.6 and 1.4%, respectively, compared to the addition of 0 μM L-1 of the chemical compound (Figure 1A and Figure 1B). In contrast, when adding 50 μM L-1 of ZnSO4, and 25 and 100 μM L-1 of Zn-EDTA, the total crude protein content and N decreased by 8.6, 3.7 and 3.1%, respectively, compared to the addition of 0 μM L-1 of the chemical compound. On the other hand, the addition of 25 μM L-1 of ZnSO4 and 50 μM L-1 of Zn-EDTA increased the P content by 18.88 and 6.3%, respectively, compared to the addition of 0 μM L-1 of the compound chemical (Figure 1C). However, adding 100 μM L-1 of ZnSO4 and 25 and 100 μM L-1 of Zn-EDTA decreased the P content by 6.3, 12.5 and 9.4%, respectively, compared to the application of 0 μM L-1 of the chemical compound.
The content of K was increased by 1.1, 10.9, 14.1, 5.4 and 2.2% by adding 25, 50 and 100 μM L-1 of ZnSO4, and 25 and 50 M L-1 of Zn-EDTA, respectively (Figure 1D). At the same time, the application of 50 and 100 μM L-1 of ZnSO4 and 25, 50 and 100 μM L-1 of Zn-EDTA lowered the Fe content in 1.9, 17.5, 17.3, 21.1 and 18.1%, respectively, in comparison with the application of 0 μM L-1 of the chemical compound (Figure 1E). However, the addition of 25, 50 and 100 μM L-1 of ZnSO4 and 25, 50 and 100 μM L-1 of Zn-EDTA increased the Zn content by 13, 7.5, 1.4, 8.2, 3.5 and 5.1%, respectively, compared to the application of 0 μM L-1 of the chemical compound (Figure 1F).
The value obtained in the content of total crude protein and N are similar to those reported in the literature, which range between 16 and 30% and 2.5 and 4.8%, respectively (Carvalho et al., 2012). In the present study, the concentration of macroelements in the seed contrast with the results reported by Espinosa-Moreno et al. (2013), who reported the total crude protein content, N, P and K in the seeds of 21.9, 3.5, 0.35 and 1.52%, respectively. On the other hand, the content of Fe and Zn showed values that are frequently reported in the literature (Carvalho et al., 2012; Kalidass and Mohan, 2012). Consequently, the content of Zn in the biofortified cowpea bean seed is not considered toxic because it does not exceed values of 150 ppm Zn (Mengel et al., 2001).
In addition to the above, the increase in the dose of Zn2+ will not always allow a greater accumulation in the seed, since it depends on the mobilization of the microelement as a free ion or chelated from the stems to the seed (Olsen et al., 2016), which suggests that the retranslocation, via phloem, of the microelements deposited in the stems have an important role in the accumulation of Fe and Zn in the seed (Cakmak et al., 2010). In this sense, the cowpea plants of the present experiment could exhibit different capacities to absorb and mobilize the nutriment, due to a lower or higher rate of transpiration during the production cycles (White, 2012).
Similarly, it has been reported that the application of high doses of Zn2+ interferes with the absorption and translocation of P, calcium and Fe, besides causing cytological disorders in the plant (Cakmak, 2000; Khudsar et al., 2008). Likewise, a decrease in the content of Fe in the wheat grain (Shekari et al., 2015) and root protein in the common bean has been reported when applying high doses of Zn2+ due to a decrease in the enzymatic activity of nitrate reductase (Chaoui et al., 1997).
Performance
In cycle 1, the weight of 100 seeds ranged between 12. 1 and 20.5 g, which is consistent with the data reported in the literature (Giami, 2005). In the present study, the application of ZnSO4 had a negative effect on the number of pods per plant, number of seeds per plant and seed yield per plant, with decreases of up to 37.4, 54.9 and 53.8%, respectively, compared to the addition of 0 μM L-1 of ZnSO4. On the other hand, not all the responses of the plants were negative, adding 100 μM L-1 of Zn-EDTA increased the number of pods per plant, the number of seeds per plant and the yield per plant in 14.0, 20.3 and 17.2 %, respectively, with respect to the application of 0 μM L-1 of Zn-EDTA.
In contrast, plants grown with 50 μM L-1 of Zn-EDTA showed an increase of 2.6 and 1.6% in the number of seeds per plant and yield per plant, compared to the application of 0 μM L-1 of the chemical compound (Cuadro2). As part of the comparison, by means of orthogonal contrasts, among the fertilizers of Zn, it was determined that the use of Zn-EDTA presented significant differences in the comparisons of T3 versus T7, and T4 versus T8.
Table 2 Performance components of cowpea bean biofortified with zinc, autumn-winter 2013 agricultural cycle.
| Treatment | Doses (µM L-1) | Weight of 100 seeds (g) | Num. pod per plant | Num. seeds per plant | Seed yield (g plant-1) |
| T1) ZnSO4 | 0 | 13.2 | 9 | 144 | 16 |
| T2) ZnSO4 | 25 | 20.5 | 7 | 89 | 9.8 |
| T3) ZnSO4 | 50 | 16.2 | 6 | 65 | 7.4 |
| T4) ZnSO4 | 100 | 12.3 | 8 | 144 | 10.8 |
| T5) Zn-EDTA | 0 | 12.4 | 9 | 118 | 12.8 |
| T6) Zn-EDTA | 25 | 12.1 | 7 | 91 | 10.2 |
| T7) Zn-EDTA | 50 | 13.1 | 9 | 121 | 13 |
| T8) Zn-EDTA | 100 | 13.8 | 10 | 142 | 15 |
| Orthogonal contrasts (values of p) | |||||
| T1 vs T2 + T3 + T4 | 0.3815 | 0.9607 | 0.9607 | 0.9216 | |
| T2 vs T3 + T4 | 0.0743 | 0.0162 | 0.0001 | 0.0001 | |
| T2 vs T6 | 0.0001 | 0.9165 | 0.0425 | 0.6309 | |
| T3 vs T7 | 0.0016 | 0.0027 | 0.0001 | 0.0001 | |
| T4 vs T8 | 0.0848 | 0.0677 | 0.1057 | 0.0001 | |
| T5 vs T6 + T7 + T8 | 0.0002 | 0.0076 | 0.0001 | 0.0001 | |
| T6 vs T7 + T8 | 0.0001 | 0.6273 | 0.0001 | 0.3369 | |
In cycle 2, biofortification with ZnSO4 and Zn-EDTA had a positive effect on the number of pods per plant, number of seeds per plant and yield per plant. In this sense, the addition of 25, 50 and 100 μM L-1 of ZnSO4 caused an increase in the number of pods of 25, 5 and 21.6%, respectively, in the number of seeds per plant of 31.3, 35.3 and 58.4%, respectively, and in the yield of 7.2, 16.7 and 33.3%, respectively, compared to the addition of 0 mM L-1 of the chemical compound (Table 3). Similarly, the number of pods of 28.3, 35.9 and 32.9% was increased by adding 25, 50 and 100 μM L-1 of Zn-EDTA, respectively, compared to the addition of 0 mM L-1 of the chemical compound.
Table 3 Performance components of cowpea beans biofortified with zinc, spring-summer 2014 agricultural cycle.
| Treatment | Doses (µM L-1) | Weight of 100 seeds (g) | Num. pod per plant | Num. seeds per plant | Seed yield (g plant-1) |
| T1) ZnSO4 | 0 | 13.4 | 6 | 62 | 9.6 |
| T2) ZnSO4 | 25 | 11.7 | 8 | 81 | 10.3 |
| T3) ZnSO4 | 50 | 13.5 | 6 | 84 | 11.2 |
| T4) ZnSO4 | 100 | 13.1 | 7 | 98 | 12.8 |
| T5) Zn-EDTA | 0 | 13.4 | 5 | 54 | 8.3 |
| T6) Zn-EDTA | 25 | 13 | 7 | 88 | 11.4 |
| T7) Zn-EDTA | 50 | 13.4 | 7 | 88 | 11.9 |
| T8) Zn-EDTA | 100 | 12 | 7 | 82 | 9.9 |
| Orthogonal contrasts (values of p) | |||||
| T1 vs T2 + T3 + T4 | 0.3467 | 0.0346 | 0.0159 | 0.1602 | |
| T2 vs T3 + T4 | 0.6538 | 0.8408 | 0.7866 | 0.8332 | |
| T2 vs T6 | 0.0976 | 0.4897 | 0.6243 | 0.6543 | |
| T3 vs T7 | 0.8967 | 0.4897 | 0.7717 | 0.7472 | |
| T4 vs T8 | 0.166 | 0.4897 | 0.3018 | 0.2326 | |
| T5 vs T6 + T7 + T8 | 0.3132 | 0.1025 | 0.048 | 0.3545 | |
| T6 vs T7 + T8 | 0.0242 | 0.5489 | 0.4658 | 0.4134 | |
Moreover, the number of seeds per plant and yield per plant increased by 65.1, 63.9 and 53.1% and 37.4, 43.4 and 19.3%, respectively, when adding 25, 50 and 100 μM L-1 of Zn-EDTA, in comparison with the addition of 0 mM L-1 of the chemical compound. As part of the comparison, by means of orthogonal contrasts, among Zn fertilizers, it was determined that the use of ZnSO4 and Zn-EDTA did not present significant differences in the comparisons of T2 versus T6, T3 versus T7, and T4 versus T8.
The yield obtained in the present study is greater than the 7 g of seed per plant reported by Apaez-Barrios et al. (2011), but less than 13 g of seed per plant obtained by the producers of cowpea beans of the state (SIAP, 2013). In this regard, Cakmak et al. (2010), reported that the addition of ZnSO4 via fertigation increased significantly the yield and Zn content in the wheat grain, however they recommend that foliar application be combined with the application via fertigation to increase these variables by 200%. In this regard, Prasad et al. (2014), report an increase of 69% in the Zn content of the rice when making foliar applications instead of the application to the soil. Likewise, Das and Green (2016), reported that the application of Zn2+ improved the performance and nutraceutical quality of the potato.
Conclusions
The applications of 25 μM L-1 of ZnSO4 and 50 μM L-1 of Zn-EDTA for both production cycles were the most effective in increasing the content of this element in the bean seed cowpea, determining 1.14 and 0.93 times more zinc respectively, compared to the control. The yield, in cycle 1, was reduced 53.8 and 20.3% by applying 50 μM L-1 of ZnSO4 and 25 μM L-1 of Zn-EDTA, respectively. The addition of 50 μM L-1 of ZnSO4 and 25 μM L-1 of Zn-EDTA, in cycle 2, increased the yield by 16.7 and 37.3%, respectively, compared to the control. Considering the set of responses, the best treatments to biofortify cowpea beans with Zn2+ were 25 μM L-1 of ZnSO4 and 50 μM L-1 of Zn-EDTA for both production cycles.
Literatura citada
Almendros, P.; Obrador, A.; Gonzalez, D. and Alvarez, J. M. 2015. Biofortification of zinc in onions (Allium cepa L.) and soil Zn status by the application of different organic Zn complexes. Sci. Hortic. 186:254-265. [ Links ]
Alloway, B. J. 2008. Zinc in soils and crop nutrition. International Zinc Association Brussels, Belgium. 135 p. [ Links ]
Apáez, B. P.; Escalante, E. J. A. S. and Rodríguez, G. M. T. 2011. Growth and yield of cowpea bean in relation with trellises type and climate. Trop. Subtrop. Agroecosys. 13:307-315. [ Links ]
Awika, J. M. and Duodu, K. G. 2017. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Functional Foods. 10.1016/j.jff.2016.1012.1002. [ Links ]
Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z. and Zhao, F. 2012. Function of nutrients: Micronutrients. In: Marschner, P. (Ed.). Marschner's mineral nutrition of higher plants (third edition). Academic Press, San Diego, USA. Chapter 7. 191-248 pp. [ Links ]
Broadley, M. R.; White, P. J.; Hammond, J. P.; Zelko, I. and Lux, A. 2007. Zinc in plants. New Phytologist. 173:677-702. [ Links ]
Cakmak, I. 2000. Possible roles of zinc in protecting plant cell from damage by reactive oxygen species. New Phytologist. Tansley review No. 111. 146:185-205. [ Links ]
Cakmak, I. 2008. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant and Soil. 302:1-17. [ Links ]
Cakmak, I.; Pfeiffer, W. H. and McClafferty, B. 2010. Biofortification of durum wheat with zinc and iron. Cereal Chem. J. 87:10-20. [ Links ]
Carvalho, A. F. U.; de Sousa, N. M.; Farias, D. F.; da Rocha-Bezerra, L. C. B.; da Silva, R. M.P.; Viana, M. P.; Gouveia, S. T.; Sampaio, S. S.; de Sousa, M. B.; de Lima, G. P. G.; de Morais, S. M.; Barros, C. C. and Filho, F. R. F. 2012. Nutritional ranking of 30 Brazilian genotypes of cowpeas including determination of antioxidant capacity and vitamins. J. Food Composition and Anallysis. 26:81-88. [ Links ]
Chaoui, A.; Mazhoudi, S.; Ghorbal, M. H. and El Ferjani, E. 1997. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 127:139-147. [ Links ]
Das, S. and Green, A. 2016. Zinc in crops and human health. In: Singh, U.; Praharaj, S. C.; Singh, S. S. and Singh, P. N. (Eds.). Biofortification of food crops. Springer India, New Delhi. 31-40 pp. [ Links ]
Dumas, J. B. A. 1831. Procédés de l'analyse organique. Annales de Chimie et de Physique. 47:198-212. [ Links ]
Espinosa, M. J.; Centurión, H. D.; Solano, M. L. y Lagunes, G. L. M. 2013. Las leguminosas de grano. In: Velázquez, M, J. R.; López-Hernández, E. y García-Alamilla, P. (Eds.). Desarrollo científico y tecnológico de los recursos alimentarios en el estado de Tabasco. Universidad Juárez Autónoma de Tabasco. Tabasco, México. 183-190 p. [ Links ]
Fageria, N. K. and Baligar, V. C. 2005. Nutrient availability. In: Hillel, D.(Ed.). Encyclopedia of soils in the environment. Elsevier, Oxford. 63-71 pp. [ Links ]
FAOSTAT. 2017. Suministro alimentario. Cultivos equivalentes primario, año 2013. http://www.fao.org/faostat/es/#data/CC [ Links ]
Giami, S. Y. 2005. Compositional and nutritional properties of selected newly developed lines of cowpea (Vigna unguiculata L.Walp). J. Food Composition and Analysis. 18:665-673. [ Links ]
Hoagland, D. R. and Arnon, D. I. 1950. The water-culture method for growing plants without soil. California agricultural experiment station, circular 347. College of Agriculture, University of California in Berkeley, USA. 32 p. [ Links ]
Horwitz, W. 2002. Official methods of analysis of AOAC international. 17th edition. Gaithersburg, MD. USA. [ Links ]
Kalidass, C. and Mohan, V. R. 2012. Nutritional composition and antinutritional factors of little-known species of vigna. Trop. Subtrop. Agroecosys. 15:525-538. [ Links ]
Khudsar, T.; Arshi, A.; Siddiqi, T. O.; Mahmooduzzafar. and Iqbal, M. 2008. Zinc-induced changes in growth characters, foliar properties, and Zn-accumulation capacity of pigeon pea at different stages of plant growth. J. Plant Nutr. 31:281-306. [ Links ]
Lim, T. K. 2012. Vigna unguiculata cv-gr. Unguiculata. Edible medicinal and non-medicinal plants: fruits. Springer Netherlands, Dordrecht. 2:976-988 pp. [ Links ]
Manna, D. and Maity, T. K. 2016. Growth, yield and bulb quality of onion (Allium cepa L.) in response to foliar application of boron and zinc. J. Plant Nutr. 39:438-441. [ Links ]
Márquez, Q. C.; de-la-Cruz, L. C.; Osorio, O. R.; Sánchez, C. E. 2015. Biofortification of cowpea beans with iron: Iron’s influence on mineral content and yiel. J. Soil Sci. Plant Nutr. 15(4):839-847. [ Links ]
Mengel, K.; Kirkby, E. A.; Kosegarten, H. and Appel, T. 2001. Principles of plant nutrition. Springer Netherlands. 849 pp. [ Links ]
Movahhedy, D. M.; Modarres, S. S. A. M. and Mokhtassi, B. A. 2009. Foliar application of zinc and manganese improves seed yield and quality of safflower (Carthamus tinctorius L.) grown under water deficit stress. Industrial Crops and Products. 30:82-92. [ Links ]
Olsen, L. I.; Hansen, T. H.; Larue, C.; Østerberg, J. T.; Hoffmann, R.D.; Liesche, J.; Krämer, U.; Surblé, S.; Cadarsi, S.; Samson, V. A.; Grolimund, D.; Husted, S. and Palmgren, M. 2016. Mother-plant-mediated pumping of zinc into the developing seed. Nature Plants. 2(5) 16036. [ Links ]
Pereira, E. J.; Carvalho, L. M. J.; Dellamora, O. G. M.; Cardoso, F. S. N.; Carvalho, J. L. V.; Viana, D. S.; Freitas, S. C. and Rocha, M. M. 2014. Effects of cooking methods on the iron and zinc contents in cowpea (Vigna unguiculata) to combat nutritional deficiencies in Brazil. Food Nutr. Res. 58:20694. [ Links ]
Persson, D. P.; de Bang, T. C.; Pedas, P. R.; Kutman, U. B.; Cakmak, I.; Andersen, B.; Finnie, C.; Schjoerring, J. K. and Husted, S. 2016. Molecular speciation and tissue compartmentation of zinc in durum wheat grains with contrasting nutritional status. New Phytologist. 211:1255-1265. [ Links ]
Potarzycki, J.; Przygocka, C. K.; Grzebisz, W. and Szczepaniak, W. 2015. Effect of zinc application timing on yield formation by two types of maize cultivars. Plant, Soil Environ. 61:468-474. [ Links ]
Praharaj, C. S.; Singh, U.; Singh, S. S. and Kumar, N. 2016. Improving protein density in food legumes through agronomic interventions. In: Singh, U.; Praharaj, S. C.; Singh, S. S. and Singh, P. N. (Eds.) Biofortification of food crops. Springer. New Delhi. India. 199-215 p. [ Links ]
Prasad, R.; Shivay, Y. S. and Kumar, D. 2014. Chapter Two-Agronomic biofortification of cereal grains with iron and zinc. In: Donald, L. S. (Ed.). Adv. Agron. Academic Press. 55-91 pp. [ Links ]
Ram, H.; Rashid, A.; Zhang, W.; Duarte, A. P.; Phattarakul, N.; Simunji, S.; Kalayci, M.; Freitas, R.; Rerkasem, B.; Bal, R. S.; Mahmood, K.; Savasli, E.; Lungu, O.; Wang, Z. H.; Barros, V. L. N. P.; Malik, S. S.; Arisoy, R. Z.; Guo, J. X.; Sohu, V. S.; Zou, C. Q. and Cakmak, I. 2016. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant and Soil. 403:389-401. [ Links ]
SAS Institute I. 2013. Base SAS(R) 9.4. Procedures guide: Statistical procedues. Second edition. Cary, NC, USA. SAS Institute Inc. 212 pp. [ Links ]
Sharma, P.; Aggarwal, P. and Kaur, A. 2017. Biofortification: a new approach to eradicate hidden hunger. Food Reviews International. 33:1-21. [ Links ]
Shekari, F.; Javanmard, A. and Abbasi, A. 2015. Zinc biofortification, preference or essential? International J. Agric. Crop Sci. 8:320-327. [ Links ]
SIAP. 2013. Servicio de Información Agroalimentaria y Pesquera. Anuario estadístico de la producción agrícola. Cierre de la producción agricola por estado de frijol pelón. [http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-estado/. [ Links ]
Sida, A. J. P.; Sánchez, E.; Ojeda, B. D. L.; Ávila, Q. G. D.; Flores, C. M. A.; Márquez, Q. C.; Preciado, R. P. 2017. Can biofortification of zinc improve the antioxidant capacity and nutritional quality of beans? Emirates J. Food Agric. 29(3):237-241. [ Links ]
Tuyogon, D. S. J.; Impa, S. M.; Castillo, O. B.; Larazo, W. and Johnson-Beebout, S. E. 2016. Enriching rice grain zinc through zinc fertilization and water management. Soil Sci. Soc. Am. J. 80:121-134. [ Links ]
White, P. J. 2012. Long-distance transport in the xylem and phloem In: Marschner, P. (Ed.). Marschner’s mineral nutrition of higher plants (Third edition). Academic Press, San Diego. USA. Chapter 3. 49-70 pp. [ Links ]
White, P. J.; Thompson, J. A.; Wright, G. and Rasmussen, S. K. 2017. Biofortifying Scottish potatoes with zinc. Plant and Soil. 411:151-165. [ Links ]
Zhao, A-q.; Tian, X-h.; Cao, Y-x.; Lu, X-c. and Liu, T. 2014. Comparison of soil and foliar zinc application for enhancing grain zinc content of wheat when grown on potentially zinc-deficient calcareous soils. J. Sci. Food Agric. 94:2016-2022. [ Links ]
Received: January 2018; Accepted: March 2018











 texto em
texto em 


