Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 spe 20 Texcoco Abr./Mai. 2018
https://doi.org/10.29312/remexca.v0i20.986
Articles
Biofortification of cowpea beans (Vigna unguiculata L. Walp) with zinc: effect on yield and mineral content
1Division of Agricultural Sciences Academic- Autonomous University Juarez of Tabasco. Road Villahermosa-Teapa km 25, R/a La Huasteca 2nd. section, Villahermosa, Tabasco, Mexico. CP. 86298.
2Center of Research in Food and Development AC-Unit Delicias. Delicias, Chihuahua. Mexico.
Zinc is an essential element in biological systems, which is required in small quantities. The objective was to know the effect of the foliar application of the doses of 0, 7, 14 and 28 mM L-1 of chelate (Zn-EDTA) and zinc sulfate (ZnSO4) in the yield and mineral content of cowpea (Vigna unguiculata L.). In plants were counted the days to flowering, weight of grain, number of pods and number of grains per plant, in addition to the weight of 100 grains, while in the grains the content of total crude protein, nitrogen, phosphorus, potassium, calcium, magnesium, iron, zinc, boron, manganese, copper and molybdenum was determined. With the doses of 7, 14 and 28 mM L-1 of Zn-EDTA the yield of grain per plant with respect to the control was increased by 53.29, 41.58 and 38.42%, respectively, while with the same doses of ZnSO4 the increase it was 27.80, 32.09 and 16.83%, respectively. The highest contents of nitrogen, crude protein, phosphorus, potassium, calcium, iron, manganese and copper were obtained with the doses of 7 and 14 mM L-1 of Zn-EDTA. While the highest levels of zinc, boron, molybdenum and magnesium were found with the doses of ZnSO4, it was observed that the zinc content increases proportionally with the increase in the dose. The addition of different doses of Zn-EDTA and ZnSO4 for the biofortification of cowpea beans has an effect on grain yield, crude protein content and mineral content.
Keywords: dosage; legumes; protein; chelates; sulfates
El zinc es un elemento esencial en los sistemas biológicos, que se requiere en pequeñas cantidades. El objetivo, fue conocer el efecto de la aplicación foliar de las dosis de 0, 7, 14 y 28 mM L-1 de quelato (Zn-EDTA) y sulfato de zinc (ZnSO4) en el rendimiento y contenido mineral de frijol caupí (Vigna unguiculata L.). En planta se contabilizaron los días a floración, peso de grano, número de vainas y número de granos por planta, además del peso de 100 granos, mientras que en los granos se determinó el contenido de proteína cruda total, nitrógeno, fósforo, potasio, calcio, magnesio, hierro, zinc, boro, manganeso, cobre y molibdeno. Con las dosis de 7, 14 y 28 mM L-1 de Zn-EDTA se incrementó el rendimiento de grano por planta con respecto al testigo en un 53.29, 41.58 y 38.42%, respectivamente, mientras que con las mismas dosis de ZnSO4 el incremento fue de 27.8, 32.09 y 16.83%, respectivamente. Los mayores contenidos de nitrógeno, proteína cruda, fósforo, potasio, calcio, hierro, manganeso y cobre se tuvieron con las dosis de 7 y 14 mM L-1 de Zn-EDTA. Mientras que los mayores contenidos de zinc, boro, molibdeno y magnesio se tuvieron con las dosis de ZnSO4, observándose que el contenido de zinc incrementa de forma proporcional con el incremento de la dosis. La adición de diferentes dosis de Zn-EDTA y ZnSO4 para la biofortificación del frijol caupí, tiene efecto en el rendimiento de grano, contenido de proteína cruda y contenido mineral.
Palabras clave: dosis; leguminosas; proteína; quelatos; sulfatos
Introduction
The cowpea bean (Vigna unguiculata L. Walp) is an important source of food in the tropics and subtropics, provides adequate contents of protein, dietary fiber, carbohydrates, vitamins and phytochemicals (Devi et al., 2015). Dry beans, green pods, growing tips are consumed from cowpea beans and the whole plant is used as fodder (Márquez-Quiroz et al., 2015). For what is an important source of food for the rural population of the states of Campeche, Chiapas, Guerrero, Jalisco, Oaxaca, Tabasco, Veracruz, Yucatan and Tamaulipas (Lagunes-Espinoza et al., 2008; Apaez-Barrios et al., 2011).
Zinc is an essential micronutrient in biological systems, which is required in small quantities. It is involved in the formation and activation of enzymes that impact on the growth, development and production of plants (Poblaciones and Rengel, 2016). Its deficiency impacts on growth, pollen viability, flowering and grain production (Pandey et al., 2006). In humans, its deficiency is associated with problems of growth and learning capacity in children, and increases the risk of infections, cancer and DNA damage (Ahmed et al., 2014). It is present in around one third of the world population, which represents the fifth risk factor for diseases in developing countries (Shahzad et al., 2014).
Biofortification is the agronomic process by which the content of elements and microelements in the edible parts of the cultivation plants is increased, which are applied in a foliar or edaphic form (Guillén-Molina et al., 2016). In this regard, Prasad et al. (2015) mention that the success of biofortification depends on the mobility of the element in the soil and the plant, zinc, is one of the most suitable microelements for biofortification, presenting its greatest absorption when applied as a foliar ZnSO4. While Cakmak et al. (2010) report that the most effective method to increase the zinc content in the wheat grain is the edaphic and foliar application at the same time.
In cabbage (Brassica oleracea cv. Bronco) it is reported that the application of doses between 80 and 100 μM of Zn increase the concentration of zinc in the edible part of the plant (Barrameda-Medina et al., 2017). While Sida-Arreola et al. (2015b) report that with 50 μM dose of zinc sulfate has the highest growth and yield of Phaseolus vulgaris.
Regarding cowpea beans, it is reported that the zinc content in the grain is low, especially when planted in soils with deficiencies of elements (Morales-Morales et al., 2016). While Guillén-Molina et al. (2016) report that the zinc content in the cowpea bean grain is increased when it is biofortified with Zn chelate in an edaphic way. Therefore, the objective of this work was to know the effect of foliar application of different doses of zinc chelate (Zn-EDTA) and zinc sulfate (ZnSO4) on the yield and mineral content of cowpea beans (V. unguiculata L.).
Materials and methods
Description of the study area
The work was carried out in the nurseries and greenhouses area of the Academic Division of Agricultural Sciences of the Universidad Juárez Autónoma of Tabasco, which is located in the municipality of Centro, Tabasco, Mexico at 17° 46’ 56” north longitude and 92° 57’ 28” west latitude, at an altitude of 21 m. We used a tropical Megavent type protected structure of 200 m2, with lateral cover of anti-aphid mesh and Grown Cover mesh to prevent the growth of weeds.
Establishment of the crop
The sowing of cowpea beans (V. unguiculata L.) cv. De Castilla, was held on September 23, 2016. To have uniformity in germination, the seeds were submerged in water for one hour, then planted in black polyethylene bags 30 cm wide by 35 cm high, which They filled with porous white stone of volcanic origin (tepetzil). The planting density was 44 444 plants per hectare.
Fertilization and treatments
Fertilization was carried out with the nutrient solution of Hoagland and Arnon (1950) containing 14 mM of NO3 -, 1 mM of H2PO4 -, 4 mM of SO4 2-, 6 mM of K+, 8 mM of Ca2 + and 4 mM of Mg2+. To supply the microelements, we used the TradeCorp AZ® product that contains iron-EDTA 7.5% p/p, manganese-EDTA 3.4% p/p, zinc-EDTA 0.70% p/p, boron 0.68% p/p, copper-EDTA 0.28% p/p and molybdenum 0.26% p/p. The pH was adjusted to 6 and the electrical conductivity to 2 dS m-1. The nutritive solution was applied manually, providing 250 ml of the nutritive solution at 50% the first 20 days after sowing (dds), and increasing to 1 000 ml of the 100% nutrient solution from day 21 to 90 dds
To wash the excess salts, an irrigation of 1 500 ml of water was applied every eight days. The irrigation water used was classified as C1S1 according to the U.S. Laboratory Salinity (Wilcox, 1955), which indicates that it has low salinity and sodium content, with C.E: 1.3 dS m-1, pH: 7, cations (mM L-1): Ca2+= 4.6; Mg2+= 13; K+= 0.2; Na+= 3 and anions (Mm-1): HCO3-=4.6; Cl-= 4 and SO4 2-= 0.
The treatments evaluated were the doses of 0, 7, 14 and 28 mM of zinc chelate (EDTA 14% p/p (Na2Zn-EDTA) and zinc sulphate, hydrated (ZnSO4.7H2O), which resulted in eight treatments, which were evaluated in a completely randomized design with five replications. The treatments were sprinkled to the foliage of the plants with a 50 mL sprinkler, the first application was made at 21 dds, applying 25 mL of the dose, while in the applications performed at 36, 51, 66 and 81 dds were applied 50 mL per plant.
Management and phytosanitary control
The plants were guided and separated vertically from each other with raffia thread tutors. For control of atracnosis (Colletotrichum lindemuthianum) that occurred at 50 dds, Sulfacob 25® was applied at a dose of 3 kg ha-1.
Agronomic variables evaluated
The grain yield per plant was evaluated at 90 dds, the grain weight of all the pods harvested per plant was added, days to flowering, the number of days elapsed from sowing to the appearance of the first flowers; number of pods per plant, the number of mature pods harvested at 90 dds, number of grains per plant, the sum of all the grains harvested per plant and weight of 100 grains, taking 100 grains at random and then weighing them in a granataria balance (Ohaus Scout® Pro) model H-2710 with an accuracy of ±0.1 g.
Mineral content analysis
The determination of the mineral content was made in the dry grains of the third and fourth pods of the harvested plants in each treatment, which were ground in a Krups Model GX4100 mill. The determination of the total nitrogen (N) content was made by the Micro-Kjeldahl method; to determine the content of phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), zinc (Zn), boron (B), manganese (Mn), copper (Cu) and molybdenum (Mo), the 1 g of sample was mineralized by triacida digestion of each treatment. The P, was determined by the colorimetric method of ammonium metavanadate, and the K, Ca, Mg, Fe, Zn, Bo, Mn, Cu and Mo were quantified by atomic absorption spectrophotometry in a Thermo ScientificTM spectrophotometer (iCE TM 3000 series AAS).
Statistical analysis
Statistical analyzes were performed under a completely randomized design. With the obtained means, the orthogonal contrasts test was carried out, all the analyzes were performed with the statistical program SAS 9.4 for Windows (SAS, 2013).
Results and discussion
Flowering in biofortified plants with different Zn-EDTA doses occurred between 46.0 and 51.0 dds, while plants biofortified with doses of ZnSO4 flowered between 46.3 and 51.7 dds, while analysis of orthogonal contrasts detected statistical differences (p≤ 0.01) between the dose of 7 mM L-1 of ZnSO4 and the doses with 14 and 28 mM L-1 (Table 1). Because flowering at all doses (treatments) occurred after 45 days, it is considered late (Manggoel and Uguru 2012). The different doses of Zn-EDTA and ZnSO4, decreased the days to flowering, with a higher precocity of the plants treated with 7 mM L-1 of Zn-EDTA or ZnSO4. For grain weight per plant values were found between 22.20 and 34.03 g per plant for Zn-EDTA doses, while ZnSO4 doses had grain weights between 20.97 and 24.50 g, with statistical differences (p≤ 0.01) in the contrast of the control treatment (0 mM L-1) and the doses of 7, 14 and 28 mM L-1 of ZnSO4.
Table 1 Days to flowering, yield and yield components in cowpea beans (V. unguiculata L.) biofortified with different doses of chelate and zinc sulphate.
| Treatments | Days to flowering | Grain weight per plant (g) | Num. of pods per plant | Num. of grains per plant | Weight of 100 grains (g) |
| 1) 0 mM L-1 Zn-EDTA | 51 ±1 | 22.2 ±3.57 | 14.33 ±2.08 | 163.67 ±13.58 | 12.92 ±1.56 |
| 2) 7 mM L-1 Zn-EDTA | 46 ±1 | 34.03 ±4.46 | 25.67 ±6.43 | 170.33 ±11.85 | 16.57±0.12 |
| 3) 14 mM L-1 Zn-EDTA | 46.3 ±1.2 | 31.43 ±6.24 | 24.67 ±8.08 | 168.33 ±7.64 | 13.82 ±1.55 |
| 4) 28 mM L-1 Zn-EDTA | 49.7 ±1 | 30.73 ±0.06 | 22.67 ±2.52 | 181.33 ±1.53 | 15.4 ±0.25 |
| 5) 0 mM L-1 ZnSO4 | 51.7 ±1.5 | 20.97 ±2.11 | 15.33 ±3.51 | 164.67 ±4.73 | 12.5 ±1.6 |
| 6) 7 mM L-1 ZnSO4 | 46.3 ±0.6 | 26.8 ±1.23 | 18.67 ±2.08 | 177.67 ±9.24 | 14.39 ±0.5 |
| 7) 14 mM L-1 ZnSO4 | 46 ±1.7 | 27.7 ±3.84 | 19.67 ±2.31 | 172.67 ±8.08 | 12.9 ±2.63 |
| 8) 28 mM L-1 ZnSO4 | 48.3 ±2.9 | 24.5 ±1.74 | 16.33 ±1.41 | 146.33 ±7.37 | 13.1 ±0.68 |
| Orthogonal contrasts | |||||
| 1 vs 2+3+4 | 0.804 | 0.051 | 0.044* | 0.529 | 0.001** |
| 2 vs 3+4 | 0.86 | 0.317 | 0.139 | 0.111 | 0.076 |
| 3 vs 4 | 0.761 | 0.246 | 0.073 | 0.387 | 0.051 |
| 5 vs 6+7+8 | 0.159 | 0.008** | 0.005** | <.001** | 0.003** |
| 6 vs 7+8 | 0.009** | 0.36 | 0.655 | 0.003** | 0.926 |
| 7 vs 8 | 0.607 | 0.618 | 0.968 | 0.355 | 0.362 |
*= (p≤ 0.05), **= (p≤ 0.01).
In general, these yields are greater than the 7 g reported by Apaez-Barrios et al. (2011) and the 13 g of grain per plant obtained by local producers of cowpea beans (SIAP, 2013); but they are equal to the average grain weights reported by Márquez-Quiroz et al. (2015) for biofortified cowpea beans. The highest performance of the treatments with the doses of 7, 14 and 28 mM L-1 of Zn-EDTA or ZnSO4, coincides with that reported by Quddus et al. (2011) who indicate that the yield increases with increasing zinc dose.
The number of pods per plant ranged between 14.33 and 25.67 for the different doses of Zn-EDTA, and from 15.33 to 19.67 for the doses of ZnSO4. These values are higher than the 8.75 pods obtained by Guillén-Molina et al. (2016) for cowpea beans biofortified with zinc. The differences may be due to the time of year in which the experiments were planted, since the present work was planted in the autumn winter cycle, while the work of Guillén-Molina et al. (2016) was sown in the spring-summer cycle, with average temperatures higher than those presented in the present work. The effect of temperature on the flowering of cowpea beans is known to be sensitive to high temperatures in the reproductive stage, which causes flower abortion and decreased yield (Hanumantha et al., 2016).
The number of grains per plant ranged between 163.67 and 181.33 grains for the doses of Zn-EDTA, and from 164.67 to 177.33 grains for the doses of ZnSO4. For the weight of 100 grains values between 12.92 and 16.67 g were found for the different doses of Zn-EDTA, while the doses of ZnSO4 had weights of 100 grains between 12.50 and 14.39 g. Observing statistical differences (p≤ 0.01) between the control doses and the different doses of Zn-EDTA and ZnSO4 (table 1). On average, the doses of 7, 14 and 28 mM L-1 of Zn-EDTA had a 28.25, 6.97 and 19.20% greater weight of 100 grains than the control (0 mM L-1), while the doses of 7, 14 and 28 mM L-1 of ZnSO4 had weight increases of 100 grains of 15.12, 3.20 and 4.80% with respect to the control.
In the treatments with Zn-EDTA and ZnSO4, the 7 mM L-1 dose with the highest average weight of 100 grains stands out. In general, the weights of 100 grains of all the treatments are higher than the 12.0 and 12.4 g reported by Lagunes-Espinoza et al. (2008) and Apaez-Barrios et al. (2011), but according to Márquez-Quiroz et al. (2015) only the doses with 7 and 28 mM L-1 of Zn-EDTA had the higher weights of 100 grains of biofortified cowpea beans.
The doses of 7, 14 and 28 mM L-1 of Zn-EDTA and ZnSO4 decreased the days to flowering, and increased the weight of grain, number of pods per plant, number of grains per plant and the weight of 100 grains, which indicates that the different doses Zn-EDTA and ZnSO4 increase the production of biomass and grain in the plants of cowpea beans, which coincides with Ratto and Miguez (2005) and Quddus et al. (2011) who indicate that total dry matter and yield increase in plants biofortified with zinc, while Sida-Arreola et al. (2015a) and Ibrahim and Ramadan (2015) indicate that the application of the adequate dose of Zn increases biomass and yield.
The total nitrogen (N) content ranged between 4.14 and 4.38% for the doses of Zn-EDTA, while for the doses of ZnSO4 it was 4.15 to 4.32% (Figure 1B). These values are higher than those reported by Márquez-Quiroz et al. (2015) and Guillén-Molina et al. (2016). According to the factor of 5.45 to convert the nitrogen content to crude protein of V. unguiculata L. (Muranaka et al., 2016), the protein content ranged between 22.56 and 23.87% for the different doses of Zn-EDTA and from 22.62 to 23.54% for the doses of ZnSO4 (Figure 1A ); presenting the highest total crude protein contents at 14 mM L-1 doses of ZnSO4 (23.89%) and Zn-EDTA (23.54%). These crude protein contents are higher than the 20.3% reported by Guillén-Molina et al. (2016) and 22% of protein reported by Márquez-Quiroz et al. (2015) for biofortified cowpea beans.
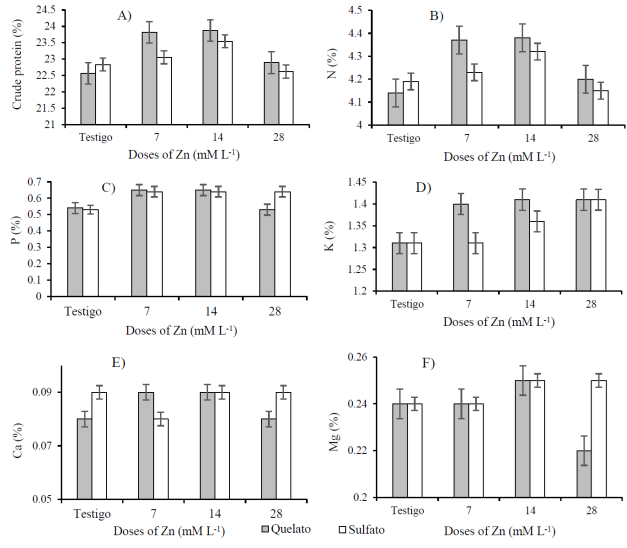
Figure 1 Content of total crude protein (A), nitrogen (B), phosphorus (C), potassium (D), calcium (E) and magnesium (F) in the bean seed of cowpea (V. unguiculata L.) biofortified with different doses of chelate and zinc sulfate.
But they are low with respect to the maximum value of the total crude protein range of 21 to 30.70% reported for V. unguiculata (Timko and Singh, 2008). The highest contents of total crude protein were obtained with the doses of 7 and 14 mM L-1 of Zn-EDTA (23.82 and 23.87%) and ZnSO4 (23.05 and 23.54%), decreasing the content of crude protein in 4.28% in the dose of 28 mM L-1 of Zn-EDTA and 4.06% in the dose of 28 mM L-1 of ZnSO4. What can be due to higher doses of Zn cause oxidative stress to the plant, which increases the production of active oxygen species, which damages the cellular components and the production of proteins (Muhammad et al., 2016).
It can also be due to the problem of toxicity that occurred with the application of 28 mM L-1 doses of Zn-EDTA and ZnSO4 at 21 dds, manifesting with problems of leaf necrosis and reduced plant growth. With the doses of 14 mM L-1 of Zn-EDTA and of ZnSO4, the highest content of nitrogen and total crude protein (Figure 1A) and the lowest grain yields per plant were observed (Table 1), which may be due to the negative correlation between nitrogen content and grain yield (Olunike, 2014).
When comparing the phosphorus content (P) of the control with the doses of 7, 14 and 28 mM L-1 of Zn-EDTA, increments of 18.46, 18.46 and 1.85% are observed, respectively; while the doses of 7, 14 and 28 mM L-1 of ZnSO4 had increases of 20.75, 20.75 and 18.86%, respectively (Figure 1C). There was a slight decrease in phosphorus with the doses of 28 mM L-1 of Zn-EDTA and ZnSO4, which indicates that with the zinc doses used, the maximum limit was not reached to observe the pronounced decrease in phosphorus reported by Cakmak et al. (2010).
For the content of potassium (K) it was found that, with the doses of 7, 14 and 28 mM L-1 of Zn-EDTA was increased by 6.87, 7.63 and 7.63%, respectively, the potassium content; while the doses of 7, 14 and 28 mM L-1 of ZnSO4 the potassium content increased by 0, 3.82 and 7.63%, respectively (Figure 1D).
The contents of potassium found in all doses are within the contents reported for cowpea beans (Frota et al., 2008; Márquez-Quiroz et al., 2015). With regard to the content of calcium (Ca), values between 0.08 and 0.9% were found in the different doses of Zn-EDTA and ZnSO4 (Figure 1E), with increases in the calcium content with the doses of 7 and 14 mM L-1 of Zn-EDTA of 12.5%, while with ZnSO4 the decrease in calcium content was observed with the dose of 28 mM L-1. For the content of magnesium (Mg) values between 0.22 and 0.25% were found with the doses of Zn-EDTA and 0.24 and 0.25% with the doses of ZnSO4 (Figure 1F), observing that with the dose of 14 mM L-1 of Zn-EDTA and ZnSO4 have the highest Mg contents, while with the dose of 28 mM L-1 of ZnSO4 there is a decrease of 8.33% with respect to the control.
The increase in mineral contents with the doses of 7 and 14 mM L-1 of Zn-EDTA and ZnSO4, indicate the influence of biofortification with zinc on the mineral content of cowpea beans (Guillén-Molina et al., 2016). For the content of crude protein and macroelements, it was found that the highest contents were obtained with doses of 7 and 14 mM L-1 of Zn-EDTA and of ZnSO4, but with a tendency to present the highest values at doses of 7 and 14 mM L-1 of Zn-EDTA, which may be due to the greater assimilation of the chelated fertilizers and that some elements are characterized by being inside the plant in a chelated form (Sánchez, 1984).
For the content of iron (Fe) it was observed that with the doses of 7 and 14 mM L-1 of Zn-EDTA there were increases of 11.14 and 9.19% of the iron, respectively; while with doses of ZnSO4, only with 7 mM L-1 there was an increase of 0.71% of iron. Meanwhile, with the doses of 28 Mm L-1 of Zn-EDTA and ZnSO4, the iron content decreases 4.37 and 14.61% (Figure 2A). Similar effects have been observed in V. unguiculata, with reports indicating that doses greater than 15 mM L-1 of ZnSO4 decreases iron content by 31.10% (Morales-Morales et al., 2016). In general, iron content is within the range of 48 to 79 ppm reported for V. unguiculata (Timko and Singh, 2008). For the zinc content there were increases of 5.81, 8.98 and 2.11% with the doses of 7, 14 and 28 mM L-1 of Zn-EDTA, while the doses of ZnSO4 had increases of 11.28, 19.99 and 23.61%, respectively (Figure 2B).
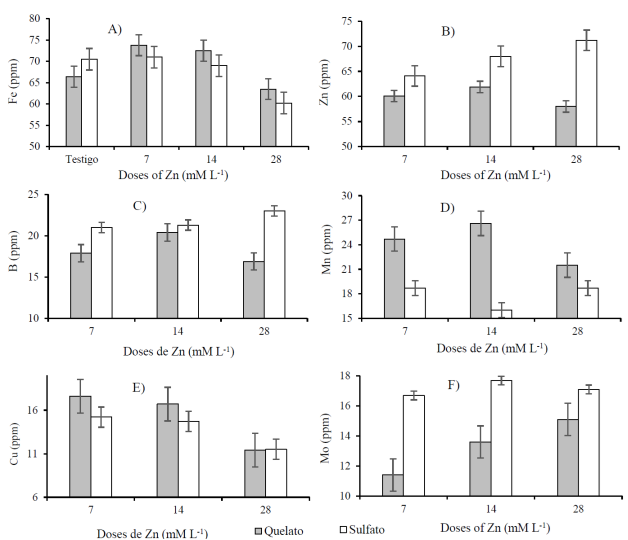
Figure 2 Content of iron (A), zinc (B), boron (C), manganese (D), copper (E) and molybdenum (F) in seeds of cowpea beans (V. unguiculata L.) biofortified with different doses of chelate and zinc sulfate.
The higher zinc contents found in the present work with respect to what was reported by Timko and Singh (2008) and Guillen-Molina et al. (2016), it can be because the foliar application of zinc is more effective than the edaphic application, to increase its content in the grain (Yue-Qiang et al., 2012). The higher zinc content in the biofortified grains with the ZnSO4 doses, may be due to the biofortification with ZnSO4 in foliar form is more effective to increase the zinc content in the grain, than that made with Zn-EDTA (Prasad et al., 2015). The increase in Zn and the decrease in Fe, with the increase in the dose of Zn-EDTA and ZnSO4, is due to the antagonistic effect between Zn and Fe (Fasaei and Ronaghi, 2015).
Regarding Shekari et al. (2015) report that the increase in the dose of Zn, decreased the Fe content in the wheat grain. With the doses of 7, 14 and 28 mM L-1 of ZnSO4 and with 14 mM L-1 of Zn-EDTA there were 64.10, 68.00, 71.20 and 61.90 ppm, values that are higher than the 61 ppm established as critical level of zinc in plants, to have a zinc sufficiency status in human nutrition (Huett et al., 1997).
The boron content was 16.90 to 21.10 ppm for the doses of Zn-EDTA, while for the ZnSO4 doses the boron content was 20.60 to 23 ppm (Figure 2C). For the different doses of Zn-EDTA it is observed that the control (0 mM L-1) had the highest boron content, while the doses of 7, 14 and 28 mM L-1 decrease 15.17, 3.32 and 19.91% on the boron content. Whereas the doses of 7, 14 and 28 mM L-1 of ZnSO4 increased the boron content by 1.94, 3.99 and 11.65%. For the manganese content values between 20.30 and 26.70 ppm were found for the doses of Zn-EDTA, while in the doses of ZnSO4 the content was 18.70 to 19.40 (Figure 2D).
The copper content ranged between 10.90 and 17.60 ppm for the different doses of Zn-EDTA, and from 9.84 to 15.20 ppm for the doses of ZnSO4 (Figure 2E), with increases of 61.47, 53.21 and 4.59% in the doses of 7, 14 and 28 mM L-1 of Zn-EDTA, while, in the doses of 7, 14 and 28 mM L-1 of ZnSO4 the increase was of 54.47, 49.39 and 16.87%. In general, it is observed that the copper content increases with the doses of 7 and 14 mM L-1 of Zn-EDTA and of ZnSO4, but decreases with the doses of 28 mM L-1. For the molybdenum content values between 10 and 15.10 ppm were found in the doses of Zn-EDTA, while the ZnSO4 doses had contents of 13.40 to 17.70 ppm (Figure 2F).
Conclusions
The biofortification of cowpea beans with different doses of Zn-EDTA and ZnSO4 have effects on grain yield, total crude protein content and mineral content. The doses of 7, 14 and 28 mM L-1 of Zn-EDTA had the greatest increases in grain yield per plant with respect to the control. The doses of Zn-EDTA and ZnSO4, decreased the days to flowering, and increased the number of pods, number of grains and the weight of 100 grains with respect to the control. The highest contents of nitrogen, crude protein, phosphorus, potassium, calcium, iron, manganese and copper were obtained with the doses of 7 and 14 mM L-1 of Zn-EDTA.
The zinc content increased proportionally with the doses of 7, 14 and 28 mM L-1 of ZnSO4 in 11.28, 19.99 and 23.61% presenting the doses of 7, 14 and 28 mM L-1 of ZnSO4 and the dose of 14 mM L-1 of Zn-EDTA values higher than 61 ppm, which is considered as critical value to have a zinc sufficiency status in the plants cultivated for human consumption.
Literatura citada
Ahmed, A.; Randhawa, M. A. and Sajid, M. W. 2014. Bioavailability of calcium., iron, and zinc in whole wheat flour. In: Wheat and rice in disease prevention and health benefits, risks and mechanisms of whole grains in health promotion. Watson, R. R.; Preedy, V. and Zibadi, S. (Eds). 1st Edition. Academic Press.USA. 67-80 pp. [ Links ]
Apáez, B. P.; Escalante, E. J. A. y Rodríguez, G. M. T. 2011. Crecimiento y rendimiento del fijol chino en función del tipo de espaldera y clima. Trop. Subtrop. Agroecosys. 13(3):307-315. [ Links ]
Barrameda, M. Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D. A. and Ruiz, J. M. 2017. Zinc biofortification improves phytochemicals and amino-acidic profile in Brassica oleracea cv. Bronco. Plant Sci. 258:45-51. [ Links ]
Cakmak, I.; Pfeiffer, W. H. and McClafferty, B. 2010. Biofortification of durum wheat with zinc and iron. Cereal Chem. 87(1):10-20. [ Links ]
Devi, C. B.; Kushwaha, A. and Kumar, A. 2015. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). Ind. J. Food Sci. Technol. 52(10):6821-6827. [ Links ]
Fasaei; R. G. and Ronaghi, A. 2015. The influence of iron chelate and zinc sulfate on the growth and nutrient composition of chickpea grown on a calcareous soil. Ir. Agric. Res. 34(2):35-40. [ Links ]
Frota, K. M. G.; Soares, R. A. M. e Arêas, J. A. G. 2008. Composição química do feijão caupi (Vigna unguiculata L. Walp), cultivar BRS-Milênio. Ciênc. Tecnol. Alimentos 28(2):470-476. [ Links ]
Guillen, M. M.; Márquez, Q. C.; De La Cruz, L. E.; Velázquez, M. J. R.; Soto, P. J. M.; García, C. M. y Orozco, V. J. A. 2016. Biofortificación de frijol caupí (Vigna unguiculata L. Walp) con hierro y zinc. Rev. Mex. Cienc. Agríc. 17:3427-3438. [ Links ]
Hanumantha, R. B.; Nair, R. M and Nayyar, H. 2016. Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Frontiers in Plant Science. 957. [ Links ]
Hoagland, D. R. and Arnon, D. I. 1950. The water-culture method for growing plants without soil. College of Agriculture, University of California, Berkeley, Calif. 32 p. [ Links ]
Huett, D. O.; Maier, N. A.; Sparrow, L. A. and Piggot, T. J. 1997. Vegetable crops. In:Reuter, D. J. and Robinson, J. B. (Eds.). Plant analysis: an interpretation manual. 2nd ed. CSIRO. Collingwood, Victoria, Australia. CSIRO. 383-464 pp. [ Links ]
Ibrahim, E. A. and Ramadan, W. A. 2015. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hortic. 184:101-105. [ Links ]
Lagunes, E. L. C.; Gallardo, L. F.; Becerril, H. H. y Bolaños, A. E. 2008. Diversidad cultivada y sistema de manejo de Phaseolus vulgaris y Vigna unguiculata en la región de la Chontalpa, Tabasco. Rev. Chapingo Ser. Hortic. 14(1):13-21. [ Links ]
Manggoel, W. and Uguru, M.I. 2012. Evidence of maternal effect on the inheritance of florewing time in cowpea (Vignia unguiculata (L.) Walp). Inter. J. Plant Breed. Gen. 6(1):1-16 [ Links ]
Márquez, Q. C. ; De La Cruz, L. E. ; Osorio, O. R. and Sánchez, C. E. 2015. Biofortification of cowpea beans with iron: iron´s influence on mineral content and yield. J. Soil Sci. Plant Nutr. 15(4):839-847. [ Links ]
Morales, M. A. E.; De La Cruz, L. E. ; Osorio, O. R.; Sánchez, C. E.; Montemayor, T. A. y Márquez, Q. C. 2016. Contenido mineral y rendimiento de germinados de frijol caupí biofortificados. Rev. Mex. Cienc. Agríc . 17:3415-3425. [ Links ]
Muhammad, I; Rehim, A.; Sarwar, N.; and Hussain, S. 2016. Zinc bioavailability in maize grains in response of phosphorous- zinc interaction. J. Plant Nutr. Soil Sci. 179(1):60-66. [ Links ]
Muranaka, S.; Shono, M.; Myoda, T.; Takeuchi, J.; Franco, J.; Nakazawa, Y.; Boukar, O. and Takagi, H. 2016. Genetic diversity of physical, nutritional, and functional properties of cowpea grain and relationships among the traits. Plant Gen. Res. 14(1):67-76. [ Links ]
Olunike, A. A. 2014. Utilization of legumes in the tropics. J. Biol. Agric. Healthcare 4(12):77-84 [ Links ]
Pandey, N.; Pathak, G. C. and Sharma, C. P. 2006. Zinc is critically required for pollen function and fertilization in lentil. J. Trace Element and Medical Biology. 20(2):80-96. [ Links ]
Poblaciones, M. J.; and Rengel, Z. 2016. Soil and foliar zinc biofortification in field pea (Pisum sativum L.): Grain accumulation and bioavailability in raw and cooked grains. Food Chem. 212:427-433. [ Links ]
Prasad, B. V. G.; Smaranika, M.; Rahaman, S. and Bareily P. 2015. Bio-fortification in horticultural crops. J. Agric. Eng. Food Technol. 2(2):95-99. [ Links ]
Quddus, M. A.; Rashid, M. H.; Hossain, M. A. and Naser, H. M. 2011. Effect of zinc and boron on yield and yield contributing characters of mungbean in low ganges rivel floodplain soil at Madaripur, Bangladesh. Bangladesh J. Agric. Res. 36(1):75-85. [ Links ]
Ratto, S. E. y Miguez, F. H. 2005. Zin en el cultivo del maíz, deficiencia de oportunidad. Comunicaciones Agronómicas. 63(10):8-15. [ Links ]
Sánchez, de la P. L. 1984. La alimentación mineral de las plantas. Instituto de Recursos Naturales y Agrobiología. 1a. Ed. Temas de divulgación. Salamanca, España. 36 p. [ Links ]
SAS. 2013. Base SAS® 9.4 Procedures guide statistical procedures. Second Edition. Cary, NC: SAS Institute Inc. 550 p. [ Links ]
Shahzad, Z.; Rouached, H. and Rakha, A. 2014. Combating mineral malnutrition through iron and zinc biofortification of cereals. Comprehensive Reviewsin Food Science and Food Safety. 13:329-346. [ Links ]
Shekari, F.; Javanmard, A. and Abbasi, A. 2015. Zinc biofortification, preference or essential? Inter. J. Agric. Crop Sci. 8(3):320-327. [ Links ]
SIAP. 2013. Servicio de Información Agroalimentaria y Pesquera. Anuario estadístico de la producción agrícola. Cierre de la producción agricola por estado de frijol pelón. http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-estado/. [ Links ]
Sida, A. J. P.; Sánchez, C. E.; Muñoz, M. E. y Flores, C. M. A. 2015a. Parámetros nitrogenados, biomasa y rendimiento en frijol biofortificado con hierro y zinc. In: Loredo, O. C.; Lara, M. J. L.; Beltrán, L. S. y Valdez, C. R. D. (Comp.). Memorias del XL Congreso Nacional de la Ciencia del Suelo. Sociedad Mexicana de la Ciencia del Suelo. 383-387 pp. [ Links ]
Sida, A. J. P. ; Sánchez, E.; Dávila, Q. G. D.; Zamudio, F. P. B. and Acosta, M. C. H. 2015b. Can improve iron biofortification antioxidant responde, yield and nutritional in gree bean? Agric. Sci. 6(11):1324-1332. [ Links ]
Timko, M. P. and Singh, B. B. 2008. Cowpea, a multifunctional legume. In: Moore, P. H. and Ming, R. (Eds). Genomics of tropical crop plants. Springer. Volume 1. USA. 227-258 pp. [ Links ]
Wilcox, L. V. 1955. Classification and use of irrigation waters. United States Salinity Laboratory. United States of Department of Agriculture. Circular No. 969. Whasington. 19 p. [ Links ]
Yue, Q. Z.; Yi, X. S.; You, L. Y.; Rezaul, K. M.; Yan, F. X.; Peng, Y.; Qing, F. M.; Zhen, L. C.; Cakmak, I.; Fu, S. Z. and Chun, Q. Z. 2012. Zinc biofortification of wheat through fertilizer applications in different locations of China. Field Crops Res. 125:1-7. [ Links ]
Received: February 2018; Accepted: March 2018











 texto em
texto em 


