Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.6 Texcoco ago./sep. 2018
https://doi.org/10.29312/remexca.v9i6.788
Articles
Evaluation of three protocols for the rapid extraction of total tissue RNA from Prosopis juliflora (SW)
1Institute of Agricultural Sciences-Autonomous University of Baja California. Highway to Delta s/n, ejido Nuevo León, Mexicali, Baja California, Mexico. CP. 21705.
2Department of Resources of the Sea-CINVESTAV-Merida Unit. Old road to Progreso km 6, Cordemex, Loma Bonita Xcumpich, Mérida, Yucatán, Mexico. CP. 97310. (ozapata@cinvestav.mx).
3Technological Institute of Merida. Av. Tecnológico km 4.5, Mérida, Yucatán, México. CP. 97118. (jrubiopina@gmail.com).
4Tecomán Experimental Field-INIFAP. Highway Colima-Manzanillo km 35, Tecomán, Colima, Mexico. (bermudez.manuel@inifap.gob.mx).
The extraction of high quality genomic RNA for PCR amplification, from Prosopis spp., is complicated due to the presence of a high percentage of secondary metabolites that bind or co-precipitate nucleic acids. In the present study, we report a modified protocol of sodium dodecyl sulfate/phenol that includes the elimination of liquid nitrogen in the maceration process, β-mercaptoethanol in the extraction of buffer and step of precipitation of ethanol. The absorbance ratios A260/A280 of the isolated RNA were around 2 to 1.9, suggesting that the DNA fraction was pure and can be used for a subsequent PCR analysis. The RNA isolated by this protocol is of sufficient quality for molecular applications. This technique could be applied to other organisms that have similar substances that make it difficult to extract RNA. Finally, this proposal represents a fast, cheap and effective alternative for the isolation of genomic RNA from fresh leaves of Prosopis spp., even in low-tech laboratories.
Keywords: extraction; mezquites; PCR; RNA
La extracción de ARN genómico de alta calidad para la amplificación por PCR, a partir de Prosopis spp., es complicada debido a la presencia de un alto porcentaje de metabolitos secundarios que se unen o coprecipitan con los ácidos nucleicos. En el presente estudio reportamos un protocolo modificado de sodio dodecil sulfato/fenol que incluye la eliminación de nitrógeno líquido en el proceso de maceración, β-mercaptoetanol en la extracción de tampón y paso de precipitación de etanol. Las proporciones de absorbancia A260/A280 del ARN aislado estaban en torno a 2 a 1.9, lo que sugiere que la fracción de ADN era pura y se puede usar para un análisis de PCR posterior. El ARN aislado por este protocolo es de calidad suficiente para aplicaciones moleculares; Esta técnica podría aplicarse a otros organismos que tienen sustancias similares que dificultan la extracción del ARN. Por último, esta propuesta representa una alternativa rápida, barata y eficaz para el aislamiento del ARN genómico de las hojas frescas de Prosopis spp., incluso en los laboratorios de baja tecnología.
Palabras claves: ARN; extracción; mezquites; PCR
Introduction
The use of molecular techniques for studies related to the expression of differential genes requires the synthesis of complementary DNA of good quality (Aslam et al., 2017). In this way, the availability of a good quality RNA is the first requirement for the understanding of research in plants. Different methods have been described for the isolation of RNA using various detergents such as cetyltrimethylammonium bromide (CTAB) because it is economical, SDS, phenol, chloroform, lithium chloride and different centrifugation gradients (Bermúdez-Guzmán et al., 2016; Silva et al., 2016). For its part, the preservation of the integrity of RNA during extraction is paramount.
The precise quantification of mRNA used for the gene expression profile depends on the integrity of the extracted RNA. Therefore, the use of poor quality RNA during the quantification of mRNA levels may compromise the accuracy of gene expression results (Bleitler et al., 2016).
The extraction of tissue RNA from mesquite plants (Prosopis spp.), is difficult because it is a plant rich in secondary metabolites, polysaccharides and polyphenols (Ghangal et al., 2009; Chang et al., 2016). The presence of compounds can affect the quality and quantity of the isolated nucleic acids (Abdel-Latif and Osman, 2017). In turn, it is hindered due to differences in the content of metabolites present in different tissues (roots, stems and leaves) and stages of plant development (Aslam et al., 2016). In addition, there are phenolic compounds that can be easily oxidized, which enables the formation of quinones due to their similar chemical properties, affecting the quality of the sample and the yield of RNA (Ghangal et al., 2009; da Luz et al., 2016).
The advances in the cultivation of the passion fruit were obtained by genetic improvement. Then, the best method for extracting RNA in P. edulis should be determined so that analyzes such as the construction of complementary DNA library (cDNA), subtractive suppressive hybridization (SSH), RT-PCR, amplified fragment length polymorphism with cDNA (AFLP cDNA) and Northern blot are not limited by low quality samples
Prosopis juliflora (SW), commonly called ‘sweet mesquite’, is a tree belonging to the family Fabaceae and is a plant tolerant to drought, salinity, and heavy metals. It is widely distributed in arid and semi-arid regions. In Mexico, it is the most important species because of its size and uses, it occupies almost all Mexican territory, it grows in extreme climates with temperatures above 48 °C and in a variety of soils (Rodríguez-Sauceda et al., 2014).
Materials and methods
Material collection
The present study was carried out in the Institute of Agricultural Sciences (ICA-UABC) of the Autonomous University of Baja California. The germplasm of P. juliflora was provided by the bank specialized in the safeguard of forest seeds, trees and shrubs, native or endemic of commercial interest, operated by the National Forestry Commission in Baja California National Forestry Commission (CONAFOR). The seeds were transported to the Biotechnology laboratory for disinfection with a 1% NaOCl (Clorox) solution for 3 min. Finally, four successive washes were made with sterile distilled water (two minutes per wash) to eliminate excess disinfectant. Subsequently, seeding and harvesting of tissues (stem, leaf, root and embryo) of the mesquite plants of approximately two months of age was carried out to carry out the corresponding tests.
Solutions and buffers required
Three different extraction protocols were evaluated, each with slight modifications:
Extraction buffer 1: 0.25M sodium chloride, 0.05M Tris-HCl (pH 7.5), 20 mM EDTA, sodium dodecyl sulfate (SDS 1%) (w/v) and polyvinylpyrrolidone (PVP 4%) (w/v) (Llanes et al., 2016).
Extraction buffer 2: Buffer Phenol-Chloroform (Ghawana et al., 2011) with slight modifications.
Extraction buffer 3: Trizol® reagent; solution of phenol saturated with buffer 0.1 M citrate, pH 8), DNase I, ethanol, chloroform, isoamyl alcohol and isopropanol of analytical purity and following the considerations of the manufactured.
Chloroform-isoamyl alcohol (24:1)
Phenol-chloroform isoamyl alcohol (1:1)
3M sodium acetate
Lithium chloride
Ethanol 70%
Absolute ethanol
Distilled water free of RNases or DEPC
Total RNA isolation protocol
For the three extraction methods, samples of different tissues (stem, leaf, root and embryo) were collected and treated with distilled water for further analysis. Approximately 100 mg of the fresh tissues were sprayed with liquid nitrogen, the material was collected in 1.5 mL eppendorf tubes and then 750 μL of extraction buffer and 750 μL of chloroform-isoamyl alcohol (24:1) were added and the samples were vortexed.
The samples were then centrifuged at 12 800 rpm for 2 min at room temperature 25 °C. The supernatant was recovered and transferred to new tubes adding an equal volume of phenol-chloroform isoamyl alcohol (1:1), then centrifuged at 12 800 rpm for 2 min at room temperature 25 °C (repeat this step until a clear phase is observed), transfer the supernatant to new tubes and add 10% of the recovered volume of 3M sodium acetate and 2.5 volumes of absolute ethanol, mix and incubate at -20 °C for 1 h. Subsequently, a 70% ethanol wash was performed, the samples were centrifuged at 12 800 rpm for 10 min at 4 °C, the last sample was washed with absolute ethanol, decanted and allowed to dry at room temperature. Finally, the tablet obtained was resuspended in 30 μL of distilled water free of RNases for later use (Kam-Lock et al., 2007).
Estimation of RNA quality and quantity
The purity and concentration of the RNA samples obtained from the tissues of P. juliflora (SW) were evaluated spectrophotometrically by means of NanoDrop 2000 (Thermo Scientific) at different wavelengths, obtaining the radii of OD A260/280 and A260/230 nm for polyphenol contamination measure-carbohydrates and proteins, respectively. The RNA integrity was assessed by means of 1% agarose gel electrophoresis, a total of 3 μl was loaded per sample and the gels were stained with ethidium bromide and visualized in a UV light photodocument MultiDoc-It Digital Imaging System UVP (Rubio-Piña and Vázquez-Flota, 2008). Subsequently, a treatment with DNases was carried out using RQ1 RNase-Fee DNase (Promega) and following the commercial protocol.
Synthesis of complementary DNA and RT-PCR
The extracts obtained by the three extraction protocols were evaluated by RT-PCR. The synthesis of cDNA was synthesized from 3 μg of the total RNA obtained using ImProm-IITM Reverse Transcriptase (Promega) and oligo (dT) and applying the instructions of the manufacturer. The synthesis of single chain cDNA was later used for PCR to estimate the levels of expression of the universal gene of actin in plants using specific primers: sense (5’CACIACTACTGCTAAACGGGAAA3’), antisense (5’ACATCTGCTGGAAGGTGCTG3’) which were designs using software Premier Premier v5 (PrimerBiosoft) and based on conserved regions of previously reported sequences for other species.
The PCR program implemented consisted of denaturing the DNA at 94 °C for 3 min, then 35 cycles for 30 s at 94 °C to denature the DNA, 30 s at 60 °C for the alignment of the primers and extension for 30 s 72 °C, finally the program concludes with 10 min of final extension at 72 °C. The obtained amplified products were separated in a 1% agarose gel, stained with ethidium bromide (10 mg mL-1) and visualized with ultraviolet light in a photodocument.
Statistical analysis
For the analysis of the samples, a completely randomized variance analysis model (Anova) was designed with its three replications and Tukey’s mean separation test (p< 0.05) was applied to determine the differences between absorbances obtained for each of the treatments. In the same way, the Duncan a test (p< 0.01) was used to evaluate the differences between treatments. The analysis of the data was carried out using the SAS statistical package. The integrity and functionality of the DNA were evaluated visually with agarose gels.
Results and discussion
Currently there are published a large number of methods for the isolation of RNA from plants, in which some of the difficulties that exist during the extraction procedure have been mentioned. Generally, high-quality RNA from plant tissues can be obtained once the activities of the RNases are inhibited (Wang et al., 2008). In the present study, we compared the results of three different protocols with the results of other protocols that were designed to extract RNA from plant tissues with high content of polysaccharides and polyphenols.
Obtaining high quality RNA is the basis of many investigations of plant molecular biology; RT-PCR, cDNA synthesis and subsequent genetic analysis, require RNA with high purity and integrity (Liu et al., 2018). In Figure 1, the representation of the characteristic bands of the 28S and 18S subunits of the ribosomal RNA can be observed, thereby confirming the purification of the RNA. In turn, the quality of the total RNA was detected by the polymerase chain reaction. On the other hand, in this study, the use of polyvinylpyrrolidone (PVP) in the extraction buffer resulted in an improvement in the yield and quality of extracted RNA compared with the application of other methods for P. juliflora tissues (SW). Wang et al. (2008), mention that PVP efficiently absorbs polyphenols and removes secondary metabolites of nucleic acids in plants, as well as most macromolecular substances.
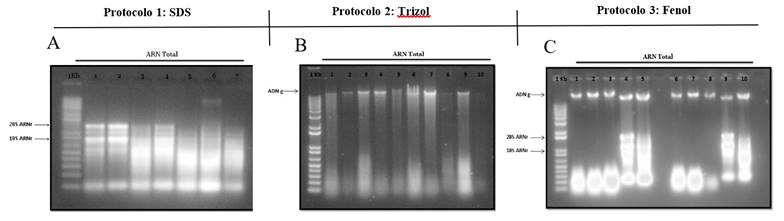
Figure 1 The 1% agarose gel electrophoresis of the total RNA extracted from the tissues (leaf, stem, root and embryo) of mesquite P. juliflora (SW) of the three protocols evaluated. The two distinct bands, 18S and 28S of ribosomal RNA show that the RNA is intact (A, C) and (B) only samples of total genomic DNA were obtained.
The phenolic compounds present in the tissue samples are rapidly oxidized to form quinones. These quinones can easily bind with nucleic acids and act as a barrier to the isolation of good quality RNA (George, 2018).
The successful isolation and obtaining of an intact total RNA from tissues rich in polysaccharides and polyphenols is important to prevent the binding of contaminants to nucleic acids (Malnoy et al., 2001). In turn, it has been reported that DNA contamination may not be completely avoided, however, the digestion of RNA with DNases free of RNases is essential; that is, digestion with DNases is a common step in the analysis of RNA protocols (Ghawana et al., 2011).
The Trizol-based method has been widely used for the isolation of RNA from various tissues and species. First, we tried to use the Trizol reagent to isolate the RNA from the tissues of P. juliflora. However, the Trizol buffer could not efficiently remove the high content of metabolites causing the aqueous phase to turn red after purification with chloroform. However, protocol 2 was found to be favorable for the extraction of DNA from mesquite plant tissues.
Studies have shown that there are different factors that affect the amplification profiles obtained by molecular techniques based on PCR, some of the main factors that affect these amplification profiles are the quality, purity and quantity of the RNA extracted, which mainly has to do with the co-isolation of polysaccharides, phenols and secondary metabolites during RNA extraction (Solano-Florez et al., 2009). In this study, the quality of total RNA was detected by the polymerase chain reaction. The RNA samples were reverse transcribed into cDNA and the result was amplified using PCR (Liu et al., 2018). In this way, it is demonstrated that the total RNA extracted from P. juliflora through protocol 1 and 3 meet the requirements for future molecular research.
On the other hand, agarose gel electrophoresis shows that the extraction protocol implemented produced high quality RNA shown by the defined cytosolic and plasmid ribosomal RNA bands (Box et al., 2011). The electrophoresis observed in Figure 2 indicates that the pair of primers used to amplify the actin gene fragment exhibits high specificity. The amplification curves showed that the mRNA sequences were virtually intact in the RNA samples and that the actin gene was present in all tissues in moderate abundance; in this way, it is demonstrated that the total RNA produced is of good quality for the RT-PCR analysis of constitutive genes such as actin with an approximate size of 300 bp (Kam-Lock et al., 2007).
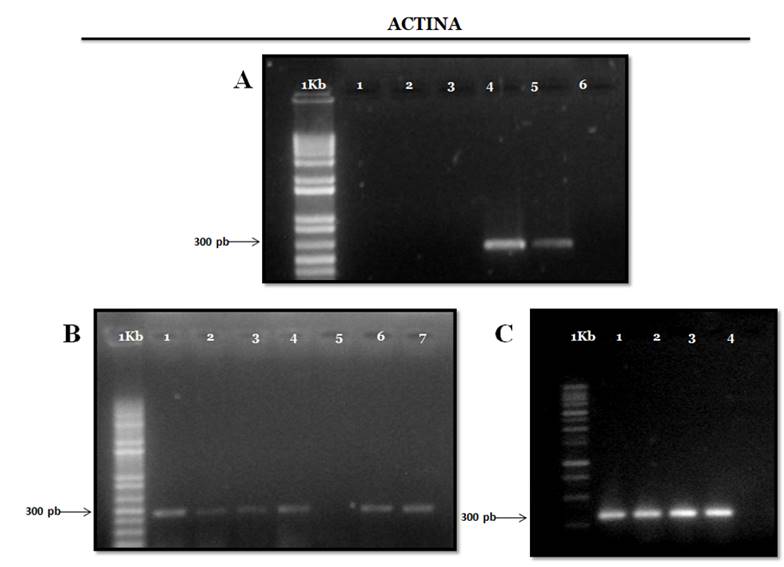
Figure 2 Electrophoresis in 1% agarose gel of the RT-PCR products of the actin gene with extracts of all tissues evaluated with the three extraction protocols and with plant-specific primers (expected size 300pb). For the three gels MP corresponds to the molecular weight marker GeneRuler 1Kb Plus DNA Ladder (ferment).
The large number of original and modified methods is well known, which mostly report modest yields and partially intact RNA, which cannot be generalized or applied to different species, especially for RNA extraction, where the content of this nucleic acid is relative to the moment and state of the cells to be evaluated. In this study, the absorbance radii of all the analyzed samples were greater than 1.8, indicating a weak contamination produced by polysaccharides and polyphenols (Table 1), these results are similar to those reported by Malnoy et al. (2001) in species of the Rosaceae family.
Table 1 Spectrophotometric readings used to evaluate the quantity and quality of total tissue RNA of P. juliflora.
| Tissue | Protocols | Quality radios OD | |
|---|---|---|---|
| A260/A280 | A260/A230 | ||
| Leaf | 1 | 2.11 | 2.19 |
| 2.2 | 2.07 | ||
| 2.26 | 2.11 | ||
| 2 | 1.85 | 1.76 | |
| 1.95 | 1.52 | ||
| 1.94 | 1.47 | ||
| 3 | 1.97 | 1.33 | |
| 1.77 | 1.59 | ||
| 1.72 | 1.17 | ||
| Stem | 1 | 2.02 | 2.12 |
| 2 | 2.12 | ||
| 1.97 | 2 | ||
| 2 | 1.92 | 1.25 | |
| 1.91 | 1.41 | ||
| 1.95 | 2.07 | ||
| 3 | 2.02 | 1.99 | |
| 1.94 | 1.2 | ||
| 1.92 | 1.25 | ||
| Root | 1 | 2.11 | 2.32 |
| 2.05 | 2.1 | ||
| 2.01 | 1.9 | ||
| 2 | 2.12 | 2.09 | |
| 2.13 | 2.08 | ||
| 2.06 | 1.83 | ||
| 3 | 1.96 | 2.09 | |
| 2 | 1.9 | ||
| 2 | 1.9 | ||
| Embryo | 1 | 2.13 | 2.23 |
| 2.11 | 2.17 | ||
| 2 | 1.93 | 1.4 | |
| 1.91 | 1.19 | ||
| 3 | 2.04 | 1.94 | |
| 2.02 | 1.99 | ||
1= SDS protocol; 2= phenol protocol; 3= trizol protocol (ITG).
Reliable and proven methodologies, sometimes they cannot be implemented in all laboratories, due to the cost of certain reagents or the difficulty of their acquisition in the laboratory. Although the results of the evaluated protocols differ, samples were obtained that fulfilled the characteristics of yield and quality, which can be used in the application of more advanced molecular techniques.
Conclusions
In conclusion, a rapid and efficient method for extracting RNA from tissues of plants with a high content of polysaccharides and polyphenols has been developed, as is the case of P. juliflora (SW). The RNA obtained by this method can be used for the detection of specific RNAs. Also, this method can also be applicable for the extraction of other tissues in different plants with characteristics similar to P. juliflora (SW).
In turn, with this document, we offer the possibility of an efficient, fast, safe and economic protocol for the simultaneous extraction of DNA and RNA, thus allowing the extraction of high quality genetic material, as well as the simultaneous extraction of nucleic acids, can be an advantage in the case of material of limited origin or to minimize the variation of the sample when the DNA has to be compared with the RNA of the same source or biological condition.
Literatura citada
Abdel, A. A. and Osman, G. 2017. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plant Methods. 13(1):1-9. [ Links ]
Aslam, S.; Tahir, A.; Aslam, M. F.; Alam, M. W.; Shedayi, A. A. and Sadia, S. 2017. Recent advances in molecular techniques for the identification of phytopathogenic fungi - a mini review. J. Plant Interactions. 12(1):493-504. [ Links ]
Bermúdez, G. M. de J.; Guzmán, G. S.; Orozco, S. M.; Velázquez, M. J. J.; Buenrostro, N. M. T. y Michel, L. C. Y. 2016. Optimización de un protocolo para aislamiento de DNA de hojas de Saccharum officinarum. Rev. Mex. Cienc. Agríc. 7(4):897-910. [ Links ]
Bleitler, J. C.; Campa, C.; Georget, F.; Bertrand, B. and Etienne, H. 2016. A single-step method for RNA isolation from tropical crops in the field. Sci Rep. 6(38):1-6. [ Links ]
Box, M. S.; Coustham, V.; Dean, C. and Mylne, J. S. 2011. Protocol: a simple phenol-based method for 96-well extraction of high quality RNA from Arabidopsis. Plant Methods . 7(7):1-10. [ Links ]
Chang, E.; Zhao, Y.; Wei, Q.; Shi, S. and Jiang, Z. 2016. Isolation of high-quality RNA from Platycladus orientalis and other Cupressaceae plants. Electronic J. Biotechnol. 23(1):21-27. [ Links ]
da Luz, A. C.; Rodrigues, P. I. and Pimentel, B. M. C. 2016. Comparison of RNA extraction methods for Passiflora edulis sims leaves. Rev. Bras. Frutic. 38(1):226-232. [ Links ]
George, A. 2018. Simple and efficient method for functional RNA extraction from tropical medicinal plants rich in secondary metabolites. Tropical Plant Res. 5(1):08-13. [ Links ]
Ghangal, R.; Raghuvanshi, S. and Chand, S. P. 2009. Isolation of good quality RNA from a medicinal plant seabuckthorn, rich in secondary metabolites. Plant Physiol Bio. 47(1):11-12. [ Links ]
Ghawana, S.; Asosii, P.; Kumar, H.; Kumar, A.; Singh, H.; Bhardwaj, P.; Rani, A.; Ravi, S.; Raizada, S.; Singh, J. and Kumar, S. 2011. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Research Notes. 4(85):1-5. [ Links ]
Kam, L. C.; Chai, L. H.; Parameswari, N. and Suhami, N. 2007. A simple and rapid method for RNA isolation from plant tissues with high phenolic compounds and polysaccharides. Protocol Exchange. [ Links ]
Liu, L.; Han, R.; Yu, N.; Zhang, W.; Xing, L. and Xie, D. 2018. A method for extracting highquality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS ONE. 13(5):1-9. [ Links ]
Llanes, A.; Restrepo, C. M. and Rajeev, S. 2016. Whole genome sequencing allows better understanding of the evolutionary history of Leptospira interrogans Serovar Hardjo. PLoS ONE . 11(7):1-12. [ Links ]
Malnoy, M.; Reynoird, J. P.; Mourgues, F.; Chevreau, E. and Simoneau, P. 2001. A method for isolating total RNA from pear leaves. Plant Mol. Biol. Reporter. 19(1):69-69. [ Links ]
Nwokeoji, A. O.; Kilby, P. M.; Portwood, D. E. and Dickman, M. J. 2016. RNASwift: a rapid, versatile RNA extraction method free from phenol and chloroform. Analy. Biochem. 512(1):36-46. [ Links ]
Rodríguez, S. E. N.; Rojo, M. G. E.; Ramírez, V. B.; Martínez, R. R.; Cong, H. M.; Medina, T. S. M.; y Piña, R. H. H. 2014. Análisis técnico del árbol del mezquite (Prosopis laevigata Humb. & Bonpl. ex Willd.) en México. Ra Ximhai. 10(3):173-193. [ Links ]
Rubio, P. J. and Vázquez, F. F. 2008. Isolation of functional total RNA from Argemone mexicana tissues. Electronic J. Biotechnol . 11(4):1-5. [ Links ]
Sahebi, M.; Hanafi, M.; Abdullah, S.; Nejat, N.; Rafii, M. Y. and Azizi, P. 2013. Extraction of RNA from mangrove plants to identify different genes involved in its adaptability to the variety of stresses. Pakistan J. Agric. Sci. 50(4):529-536. [ Links ]
Silva, D. V.; Branco, S. M.; Holanda, J. I. A.; Royaert, S.; Motamayor, J. C.; Marelli, J. P. and Corrêa, R. X. 2016. Comparative evaluation of total RNA extraction methods in Theobroma cacao using shoot apical meristems. Genet. Mol. Res. 15(1):1-8. [ Links ]
Solano, F. G.; Márquez, C. M. y Schuler, I. 2009. Optimización de la extracción de ADN de Passiflora ligularis para el análisis por medio de marcadores moleculares. Universitas Scientiarum. 14(1):16-22. [ Links ]
Wang, X.; Tian, W. and Li, Y. 2008. Development of an efficient protocol of RNA isolation from recalcitrant tree tissues. Mol Biotechnol. 38(1):57-64. [ Links ]
Received: May 2018; Accepted: August 2018











 texto en
texto en 


