Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.5 Texcoco jun./ago. 2018
https://doi.org/10.29312/remexca.v9i5.242
Articles
Incompatibility of the capulín (Prunus serotina ssp. capuli (Cav.) McVaugh) as rootstock of the sweet cherry tree (Prunus avium L.)
1Institute of Horticulture-UACH. Mexico-Texcoco Highway km 38.5, Chapingo, Mexico. CP 56230.
2ITVM. Road Morelia-Salamanca km 6.5, Morelia, Michoacán. CP. 58100.
3CRUCO-Chapingo Autonomous University. Perif. Ind. 1000, Morelia, Michoacán. CP. 58170.
4Postgraduate College-Campus Montecillo. Mexico-Texcoco Highway km 36.5, Montecillo, Mexico. CP. 56230.
The capulín (Prunus serotina ssp. capuli (Cav.) McVough 1951) is a subspecies of P. serotina Ehnr. of Mesoamerican origin used mainly as fruit. Although from the 80’s there were reports of a collection of capulín from central Mexico, called San Martín, which showed compatibility with the cherry tree (Prunus avium L.) possibly because it was a diploid capulín, this possible quality had not been the subject of a study that the cherry tree is little cultivated in the country. This situation is changing due to the release of new cherry tree varieties with low winter cold requirements. It returned to the idea of testing the materials of capulines that had been described as possible patterns of the cherry tree given the quality of the first one to adapt to the soils in Mexico and the susceptibility of the traditional rootstocks of the cherry tree to cancer (Dibotryon sp.) or blight of fire (Phytophtora sp.) mainly. In this investigation, remarkable compatibility of the Chapingo capulín with the cherry tree was not found and by looking for the causes analyzing both species by means of flow cytometry we found that the San Martin capulín is tetraploid and not diploid as it was supposed. The above is added to the possible production of phenolic compounds that is common in Prunus, producing a translocalized incompatibility that we observe in most of the grafted plants.
Keywords: Prunus; cytometry; Mexico; propagation
El capulín (Prunus serotina ssp. capuli (Cav.) McVough 1951) es una subespecie de P. serotina Ehnr. de origen mesoaméricano utilizada como frutal principalmente. Aunque desde los 80’s se tenían reportes de una colecta de capulín del centro de México, llamada San Martín, que presentaba compatibilidad con el cerezo (Prunus avium L.) posiblemente porque fuera un capulín diploide, esta posible cualidad no había sido objeto de estudio dado que el cerezo es poco cultivado en el país. Esta situación está cambiando por la liberación de nuevas variedades de cerezo de bajo requerimiento de frío invernal. Retomamos la idea de probar a los materiales de capulines que habían sido descritos como posibles patrones del cerezo dada la cualidad del primero de adaptarse a los suelos en México y a la susceptibilidad de los portainjertos tradicionales del cerezo al cáncer (Dibotryon sp.) o tizón de fuego (Phytophtora sp.) principalmente. En esta investigación, no se localizó compatibilidad notable del capulín Chapingo con el cerezo y al buscar las causas analizando a ambas especies por medio de citometría de flujo encontramos que el capulín San Martín es tetraploide y no diploide como se suponía. Lo anterior, se agrega a la posible producción de compuestos fenólicos que es común en Prunus produciendo entonces una incompatibilidad traslocalizada que observamos en la mayoría de las plantas injertadas.
Palabras clave: Prunus; citometría; México; propagación
Introduction
The capulín (Prunus serotina ssp. capuli (Cav.) VcVaugh) has been used in the country with multiple purposes, among which are: consumption of fresh fruit, roasted seeds and forestry (Rzendowski and Calderon, 2005; Fresnedo et al., 2011). McVaugh (1951), classified the capulín as one of the five subspecies of Prunus serotina Ehnr. based on their morphological characters and distribution zones. Avendaño-Gómez (2015); Rzendowski and Calderón (2005); Rohrer (2014) affirm that the capulines are the only subspecies really domesticated and that their range of distribution was extended by human influence to what the American territory comprises from the south of Canada, to the south of Bolivia between 1 200 and 3 200 meters above sea level. According to Halarewicz et al. (2017), this species is now a powerful invader in Europe. Fresnedo et al. (2011) studied the morphology of the capulines of the center-west of Mexico and found a structuring of the variation where the capulines of the center of the country seem to be domesticated as suggested by Avendaño-Gómez (2015).
Muratalla (1984) reported new perspectives of use of the capulín when introducing some varieties of sweet cherry by grafting them in collections of capulín coming from Puebla, Mexico. Of his essays, only one collection presented compatibility with the Cristobalina cherry cv. and named it the San Martin capulín. From this experience also note that the type of slit graft gave the best results, either using the capulín as a cherry tree pattern or using the capulín as an intergraft. When looking for a use of capulines in Central America, Navarro et al. (1996) in Honduras tested different types of grafting and times in capulines and distinguished that the simple English graft in October produced a success of more than 60% of grafted plants.
As noted by Olmstead et al. (2006); Gainza et al. (2015), modern production of cherry trees needs high density plantings, use of rootstocks that control vigor and increase precocity with low management costs and economically viable for producers. In the search for a rootstock for the new varieties of cherry tree with low cold requirement, the objective of our study was to test the San Martin capulín as a cherry tree rootstock given its adaptation to the soils in Mexico and the susceptibility of the traditional rootstocks of the cherry to cancer (Dibotryon sp.) or fire blight (Phytophtora sp.) mainly. The above in the interest of exploring the cultivation of cherry trees in the country.
Materials and methods
The research was carried out in the facilities of the Center Regional University Center West of the Autonomous University Chapingo (CRUCO) in Morelia, Michoacan. The CRUCO is located at latitude 19° 52’ and longitude 101° 02’ with a height of 1 920 masl.
Vegetal material
Seeds were multiplied by individuals from a collection identified by Muratalla (1984) as San Martin de (Prunus serotina ssp. capuli). The 160 capulín trees were established in the CRUCO field at a distance of 1 m between trees and 2 m between rows. In the Table 1 shows the characteristics of the San Martin type capulín.
Table 1 Origin, environment and taxonomic identity reported for the San Martín de Prunus serotina ssp. collection. capuli that served as a rootstock.
| Key code |
Population and State |
Ambient | Taxonomic identity reported |
|
|---|---|---|---|---|
| Geographical location | Conditions | |||
| Mex | Chapingo, Mexico |
19° 29’ 58’’ N 98° 52’ 44’’ W 2270 masl |
C(w0) wb(i)g Collection in experimental fruit orchard Encino |
Prunus serotina ssp. capuli (Cav.) McVaugh |
The trees of capulín that were grafted with cherry tree presented a year of age from its germination, plants were selected that presented homogenous characteristics as for height and diameter presenting approximately 2.5 cm of diameter and 120 cm of height, the plants were free of diseases. The simple slit grafting method recommended by Muratalla (1984) was used and Stella, Van, Lapin, Brooks and Bourlat varieties were grafted in an equal number of repetitions. Finally, the graft was tied with a green plastic tape 1 cm wide and 20 cm long, leaving the yolk free for free growth Figure 1.
Variables evaluated
Next, the variables that were evaluated are recorded in Table 2.
Table 2 Variables evaluated in the Prunus serotina capuli/Prunus avium grafts.
| Organ | Morphological descriptor | Abbreviation | Unit of measurement |
|---|---|---|---|
| Complete tree | Height of the plant | ADP | (m) |
| Stem and branches | Stem diameter pattern Stem diameter graft 1 Stem diameter graft 2 Number of branches |
DDTP DDTI1 DDTI2 NDR |
(cm) (cm) (cm) Number |
| Leaves | Number of sheets Width of leaves Length of leaves | NDH ADH LDH |
Number (cm) (cm) |
| Percent engraftment | PDP | Number | |
Estimation of nuclear DNA content by flow cytometry
Isolation of cell nuclei
The protocol for the isolation of cell nuclei recommended by Arumuganathan and Earle (1991) with modifications served to analyze the young leaves of the plants. Then the summary of the protocol: place in a Petri dish a small amount of tissue from the study plant and the reference standard used (approximately 30 mg of each tissue). Place both tissues one on top of the other to standardize a possible effect of cytosolic compounds. Add 1 mL of the buffer (LB01, tris MgCl2, etc) to the Petri dish.
Immediately cut both tissues with a knife or scalpel (use in each sample analyzed a new blade) in very thin slices in the chosen buffer and incubate on ice for at least 15 minutes. Mix the homogenate by pipetting up and down several times (avoid the formation of air bubbles). Filter the suspension through a 42 μg nylon mesh in a microcentrifuge tube. Add the fluorochrome (in the case of DAPI a concentration of 4 μg mL-1 is normally used, while in the case of IP and EB it is 50 μg mL-1 together with 50 μg mL-1 of RNase), and shake gently. Incubate the sample in ice and darkness before analysis (15 min to 1 h), occasionally shaking the tubes.
Flow cytometry analysis
The samples were analyzed in the laboratory in an Attune® acoustic flow cytometer configuration of argon blue/violet laser at an intensity of 50 mW at 450 nm/20 mW at 488 nm respectively (Life Techologies, San Diego CA, USA). For this, propidium iodide fluorochrome (PI Sigma - Aldrich) to stain the isolated plant nuclei and was used as a tissue reference species of Hordeum vulgare “Sultan” (5.19 pg of DNA) from the germplasm bank of the International Maize and Wheat Center (CIMMYT)-Mexico. The measurements were made to each individual to make the representation of the taxa evaluated. In each performed analysis, at least 10 000 events and a sample volume of 200-300 μL were measured. The coefficient of variation in each analysis was less than 5. The genome size of each sample was estimated according to the formula:
DNA 2C (pg)= value G1 shows X= the standard genome size (5.19 pg)/G1 value of the standard.
Results and discussion
The capulín P. serotina ssp. capuli and the cherry tree cultivars Stella, Van, Lapin, Brooks and Bourlat did not develop a graft union that could be considered compatible. There was no notable difference between the cherry tree cultivars, the individuals with pruning, two were of the Stella variety, two of Brooks and two of the Lapin variety. In Figure 2, the percent yield obtained in general for the grafts of cherry tree cultivars on capulín is verified. The arrest was 3.75% of all grafted individuals.
Individual development record
In the Figure 3 shows the development of grafted individuals taking into account the means of the eight variables measured.
The incompatibility between the capulín and the cherry tree was revealed by the number of individuals with arrest (6 individuals representing 3.75% of the total grafted individuals). This did not allow the application of inferential tests and soon the development of the surviving plants is described which also allows us to take elements to understand the incompatibility of much of plants. Thus, it was important to note that plant height (AP) presented an upward development except in the second half of April where individuals showed lower growth, it was resumed from the first half of May and remained up to 5 cm for fifteen days. In the end the growth reached up to 81 cm on average.
In the same way, the stem diameter (DT) has an ascending development, but in the second half of April it decreased. The stem diameter of the graft (DTI) presented a similar pattern reaching a final development of 1.10 cm. In the variable number of leaves (NH) the individuals initially presented on average around 7 leaves and at the end of the period they had an average of 20 leaves. The width of leaf (AH) presented a development generally on average of 0.5 cm in each fortnight reaching a final growth of 5.55 cm. The leaf length (LH) presented a development in its general averages of 2 cm every fortnight. The lateral branches (RL) of the individuals could be observed from the first half of April and at the end of the registration of the individuals, on average they came to present 2 lateral branches in the second half of July.
The registration of the diameters of the individual’s stem diameter pattern (DTP) and stem diameter of the cherry tree (DTC) in the surviving individuals describes a modest but favorable progression.
In the Figure 4 shows the means of the variables evaluated of the individuals who survived. The figure refers to the fact that the variables with the highest development were plant height (AP), a development higher than 66 cm x 10^-1 is obtained in its general mean, number of leaves (NH) in which the individuals presented a maximum of 20 leaves in its record x 10^-1, leaf width (ADH) variable in which the surviving individuals came to observe an average development of 3.8 cm and finally the variable leaf length (LDH) the general average obtained by the surviving individuals present a development of 7.33 cm.
The results of this study coincide with what was pointed out by Gainza et al. (2015) who recognizes the translocalized incompatibility in Prunus as one that during the first year of grafting presents a defoliation, discoloration of the leaf and a subsequent non-development associated with a carbohydrate translocation block mainly.
In the different cultivated species of the Prunus genus, there are few high-end rootstocks, due to the incompatibility present between rootstock and graft, preventing a strong and lasting functional union. Incompatibility in the genus Prunus is very present in species such as cherry, almond, apricot, peach and plum (Gainza et al., 2015).
Usenik (2006) mentioned that recent data show that several biochemical pathways are affected during the formation of the union of a graft. In Prunus, the metabolism of phenolic compounds is common. According to Olmstead (2006); Gainza et al. (2014), mention that small amounts of phenols can be extremely sufficient to produce limiting dysfunctions at the local level at the interface between two or more cells. Meanwhile, on the other hand, studies using callus cultures of Prunus avium L. have shown that podaflavine (phenol typical of Prunus species) interferes with the permeability of tissues, resulting in damage to the membrane. In incompatible graft unions, there is mobility of phenols from the vacuole in the cytoplasm, causing stress that results in growth dysfunction, which is probably caused by the inhibition of the lignin pathway (Kueger et al., 2012). In addition, concentrations of catechins and proanthocyanidins, these flavonoids, increase under stress and grafting is no exception (Pina and Errea, 2005).
Sorce et al. (2002); Koepke and Dhingra (2013); Souza (2015) mention that in a complex disorder such as incompatibility there is a biochemical background also complex and dependent on the specific genetic interactions between the cells of the pattern and graft. The success of the union depends primarily on the compatibility of the graft union to allow a rapid development of the vascular connections between the pattern and the graft (Olmstead et al., 2006) allowing this in turn the rapid resumption pattern and graft and the vascular regeneration of the tissues of the xylem and phloem of both parts through a cellular differentiation (Gainza, 2014; Souza, 2015).
Genome sizes
Next, the histograms obtained from the estimation of the nuclear DNA content of our evaluated species are presented, allowing us to determine the genome size of P. avium L. and P. serotina ssp. capuli (Cav.) McVaugh. All cherry tree cultivars presented the same value.
In the genetic field Dickson et al. (1992) indicate variations of ploidy in the capulín ranging from diploid to hexaploid. Downey and Iezzoni (2000), mention that Prunus serotina is a tetraploid species and Pairon and Jaquemond (2005) based on an analysis with DNA markers type microsatellites determined it as allotetraploid. Various authors such as Beck et al., (2014); Guzmán et al. (2018) and Pairon and Jacquemart (2005) have revealed a narrow genetic diversity in the species. This is the first study that determines the genome size of P. serotina ssp. capuli that coincides with the value of 1C= 0.5 pg that Dickson et al. (1992) reported for the species P. serotina Ehrn. and with the value of 1C= 0.35 pg that Arumuganathan and Earle (1991) reported for P. avium L. In Figure 5, the results obtained from the flow cytometric analysis are observed where it can be seen that the sweet cherry tree (P. avium L.) has a smaller genome size San Martin capulín (P. serotina ssp. capuli) possibly this difference is related to the low engraftment of the graft.
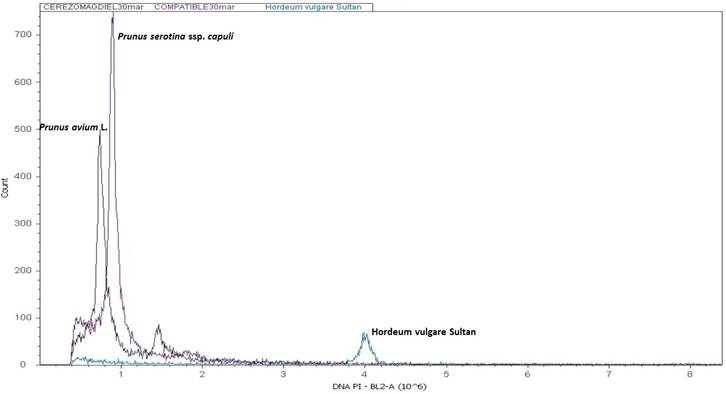
Figure 5 Histogram of the estimation of the DNA content by flow cytometry of the cherry tree (cv Stella) and the capulín type San Martín.
Considering the genetic and anatomical factors that affect the compatibility of the capulín and the cherry tree, the analysis of the size of the genome can be important to understand this relationship. Possibly one of the causes of the incompatibility of the capulín as a rootstock of the cherry tree may be associated with the genetic factors that are translated into anatomical characters as indicated by Souza et al. (2014) in a similar study in Psidium. In this study, differences in genome sizes can be linked to the difficulty of fusion and cell exchange because the tissue of each species continues to reproduce by mitosis maintaining its own genome number and genetic dosages in the metabolic pathways and this leads to non-union of tissues. Thus, the technique of flow cytometry can help in the early stages of the evaluation of the fruit utility of the other subspecies of P. serotina Ehrn.
Conclusion
The cultivars Stella, Van, Lapin, Brooks and Bourlat de Cerezo (Prunus avium L.) present an incompatibility presumably translocalised by being grafted onto the capulín (Prunus serotina ssp. capuli (Cav.) McVough) identified as type San Martín by Muratalla (1984). Possibly one of the causes of the incompatibility of the capulín as a cherry tree rootstock may be associated with the genetic factors that result in defoliation, discoloration of the leaf and a subsequent non-development associated with a possible blockage of carbohydrate translocation mainly. The determination of the genome size by flow cytometry can help in the first stages of the evaluation of the fruit utility of the other subspecies of P. serotina Ehrn.
Acknowledgments
The first author received a grant from CONACYT, for doctoral studies at UACH. The financing was provided by the project: CB 2011 169334 of CONACYT- Mexico and by DCRU of UACH. We appreciate the suggestions of the M.C. Alfonso Muratalla of the CP and the facilities of Dr. Peaubelle of the HCU Lyon-Sud, Lyon France.
REFERENCES
Arumuganathan, K. and Earle, E. D. 1991. Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol. Biol. Reporter. 9(3):229-233. [ Links ]
Avendaño-Gómez, A.; Lira-Saade, R.; Madrigal-Calle, B.; García-Moya, E.; Soto-Hernández, M. y Romo de Vivar- Romo, A. 2015. Manejo y síndromes de domesticación del capulín (Prunus serotina Ehrh ssp. capuli (Cav.) Mc Vaugh) en comunidades del estado de Tlaxcala. Agrociencia. 49(2):189-204. [ Links ]
Beck, J. B.; Ferguson, C. J.; Mayfield, M. H. and Shaw, J. 2014. Reduced genetic variation in populations of black cherry (Prunus serotina subsp. serotina: Rosaceae) at its Western Range Limit in Kansas. Northeastern Nat. 21(3):472-478. [ Links ]
Dickson, E. E.; Arumuganathan, K.; Kresovich, S. and Doyle, J. J. 1992. Nuclear DNA content variation within the Rosaceae. Am. J. Bot. 79(9):1081-1086. [ Links ]
Fresnedo, J.; Segura, S. and Muratalla-Lúa, A. 2011. Morphovariability of capulín (Prunus serotina Ehrh.) in the central-western region of Mexico from a plant genetic resources perspective. Gen. Res. Crop Ev. 58(4):481-495. [ Links ]
Gainza, F.; Opazo, I. and Muñoz, C. 2015. Graft incompatibility in plants: metabolic changes during formation and establishment of the rootstock/scion union with emphasis on Prunus species. Chilean J. Agric. Res. 75(1):28-34. [ Links ]
Guzmán, F.; Segura; S. Aradhya, M. and Potter, D. 2018. Evaluation of the genetic structure present in natural populations of four subspecies of black cherry (Prunus serotina Ehrh.) from North America using SSR markers. Sci. Hortic. 232:206-215. [ Links ]
Halarewicz, A.; Pruchniewicz, D. and Kawalko, D. 2017. Black Cherry (Prunus serotina) invasion in a scots pine forest: relationships between soil properties and vegetation. Polish J. Ecol. 65(2):295-302. [ Links ]
Kueger, S.; Steinhauser, D.; Willmitzer, L. and Giavalisco. P. 2012. High-resolution plant metabolomics: from mass, spectral features to metabolites and from whole-cell analysis to subcellular metabolite distributions. The Plant. J. 70(1):39-50 [ Links ]
Muratalla, A. 1984. Factores limitantes en la producción de capulín (Prunus serotina ssp. capuli). Colegio de Posgraduados. Montecillo, Estado de México. 18 p. [ Links ]
Olmstead, M. A.; Lang, N. S. Lang, F. G. A.; Ewers, W. and Owens, S. A. 2006. Examining the vascular pathway of sweet cherries grafted onto dwarfing rootstocks. Am. Soc. Hortic. Sci. 41(3):674-679. [ Links ]
Pairon, C. M. and Jacquemart, L. 2005. Disomic segregation of microsatellites in the tetraploid Prunus serotina Ehrh. (Rosaceae). J. Am. Soc. Hortic. Sci. 130:729-734. [ Links ]
Pairon, M.; Jacquemart, A-L. and Potter, D. 2008. Detection and characterization of genome-specific microsatellite markers in the allotetraploid Prunus serotina. J. Am. Soc. Hortic. Sci . 133(3):390-395. [ Links ]
Pina, A. and Errea, P. 2005. A review of new advances in mechanism of graft compatibility-incompatibility. Sci. Hortic. 106(1):1-11. [ Links ]
Popenoe, W. and Pachano, A. 1922. The capulín Cherry. J. Heredity. 13(2):51-62. [ Links ]
Rohrer, J. R. 2014. Prunus (Rosaceae). In flora of North America. (Ed.). Committee Flora of North America North of Mexico. New York: Oxford. 9:363-365. [ Links ]
Rzedowski, J. y Calderón, R. G. 2005. Prunus serotina Ehrh. In: flora del Bajío y regiones adyacentes. Rzedowski, J. y Calderón de, R. G. (Eds.). Instituto de Ecología AC. Centro Regional del Bajío, Pátzcuaro, Michoacán, México. 101-111. pp. [ Links ]
Sorce, C.; Massai, R.; Picciarelli, P. and Lorenzi, R. 2002. Hormonal relationships in xylem sap of grafted and ungrafted Prunus rootstocks. Sci. Hortic . 93(3-4):333-342 [ Links ]
Souza, A.; Das Graças, de L.; Vilela, R.; Pereira, de L. L.; Semen, M. S. and V. Techio, H. 2014. Chromosome number and nuclear DNA amount in Psidium spp. resistant and susceptible to Meloidogyne enterolobii and its relation with compatibility between rootstocks and commercial varieties of guava tree. Plant Syst. Evol. 301(1):231-237. [ Links ]
Usenik, V.; Krska, B.; Vican, M. and Stampar, F. 2006. Early detection of graft incompatibility in apricot (Prunus armeniaca L.) using phenol analyses. Sci. Hortic . 109(4):332-338. [ Links ]
Received: May 2018; Accepted: June 2018











 texto en
texto en 






