Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.4 Texcoco mar./jun. 2018
https://doi.org/10.29312/remexca.v9i4.1392
Articles
P-Ca, AG4/7 and 6-BAP in the physiology and nutrition of tomato in the greenhouse
1Autonomous Agrarian University Antonio Narro. Calzada Antonio Narro 1923, Buenavista, Saltillo, Coahuila. México. CP. 25315. (lfabdiel@gmail.com; edmundo49mx@yahoo.com.mx; zara.mg@hotmail.com; azermenog@hotmail.com.
The tomato is a crop of worldwide importance for its contribution in labor, economy and food quality. These characteristics require a continuous search for technologies that contribute to improving fruit yield and quality. In this study, the effect of bioregulators on foliar parameters, nutrient content, yield and fruit quality was evaluated in hybrid saladette tomato “Raptor-F1” at the UAAAN, Saltillo, Coahuila, during April-august 2015, under greenhouse conditions. When the plants showed floral primordia, a first foliar application was made with manual spray at the dew point of the treatments: control (water), P-Ca (50 mg L-1), AG4/7 (50 mg L-1), AG4/7 (100 mg L-1), 6-BAP (50 mg L-1), 6-BAP (100 mg L-1), AG4/7 (50 mg L-1)+6-BAP (50 mg L-1) and AG4/7 (100 mg L-1)+6-BAP (100 mg L-1) and 15 days later, a second application of the same doses was performed. A completely randomized statistical design with ten repetitions per treatment was established. The results were analyzed using the DMS test (p≤ 0.05). P-Ca and 6-BAP at 50 mg L-1 did not modify the foliar parameters and yield per plant; however, they increased the level of potassium in leaves and nitrogen and calcium in fruits. 6-BAP at 50 mg L-1 increased the fresh and dry matter, while when combined with gibberellin AG4/7 at 100 mg L-1 increased the vitamin C and lycopene content in fruits. It is concluded that the individual or combined concentrations of P-Ca, AG4/7 and 6-BAP, favor the quality of hybrid tomato “Raptor-F1” saladette in the greenhouse.
Keywords: cytokinins; chlorophyll; prohexadione-calcium; gibberellins; lycopene; minerals
El tomate es un cultivo de importancia mundial por su aportación en mano de obra, economía y calidad alimenticia. Estas características obligan a una continua búsqueda de tecnologías que contribuyan a mejorar el rendimiento y calidad del fruto. En este estudio se evaluó el efecto de biorreguladores en parámetros foliares, contenido de nutrientes, rendimiento y calidad del fruto en tomate saladette híbrido “Raptor-F1” en la UAAAN, Saltillo, Coahuila, durante abril-agosto de 2015, bajo condiciones de invernadero. Cuando las plantas mostraron primordios florales se realizó una primera aplicación foliar con atomizador manual a punto de rocío de los tratamientos: control (agua), P-Ca (50 mg L-1), AG4/7 (50 mg L-1), AG4/7 (100 mg L-1), 6-BAP (50 mg L-1), 6-BAP (100 mg L-1), AG4/7 (50 mg L-1)+6-BAP (50 mg L-1) y AG4/7 (100 mg L-1)+6-BAP (100 mg L-1) y 15 días después, se realizó una segunda aplicación de las mismas dosis. Se estableció un diseño estadístico completamente al azar con diez repeticiones por tratamiento. Los resultados fueron analizados usando la prueba DMS (p≤ 0.05). P-Ca y 6-BAP a 50 mg L-1 no modificaron los parámetros foliares y rendimiento por planta; sin embargo, aumentaron el nivel de potasio en hojas y de nitrógeno y calcio en frutos. 6-BAP a 50 mg L-1 incrementó la materia fresca y seca, mientras que al combinarse con las giberelinas AG4/7 a 100 mg L-1 aumentaron el contenido de vitamina C y licopeno en frutos. Se concluye que las concentraciones individuales o combinadas de P-Ca, AG4/7 y 6-BAP, favorecen la calidad del tomate saladette híbrido “Raptor-F1” en invernadero.
Palabras clave: citocininas; clorofila; prohexadiona-calcio; giberelinas; licopeno; minerales
Introduction
The tomato (Solanum lycopersicum L.), is currently one of the most important vegetables in many countries in the world because of the large number of by-products obtained from it and the foreign exchange it contributes to its economy. In Mexico, approximately 80 000 hectares of greenhouse are destined for cultivation (Betancourt and Pierre, 2013), which is why it is necessary to develop or adapt technologies that allow improving their yield and fruit quality without causing adverse effects to the environment.
The use of bioregulators in agriculture has intensified in recent years with the aim of improving production and fruit quality through the various actions offered by this technology. In several investigations, it has been discovered that the bioactivity of bioregulators is directly related to a better link of the bioregulator to the reception site in the cell and a greater reactive capacity at the point of induction when stimulating or inhibiting a physiological process. This characteristic places them as an excellent technology to increase quality and production in terms of early or late ripening of fruits, higher antioxidant content, longer shelf life, translocation of nutrients and even in the fight against pests and diseases (Nickell, 1988).
Prohexadione calcium (P-Ca) is a growth retardant, which acts through the inhibition of the synthesis of biologically active gibberellins (A1, A4, and A7) which reduces vegetative growth (Ramírez et al., 2016a). It has been reported in pepper and cherry peppers that P-Ca increases the content of cytokinins in the apical meristem, which is related to flower formation and fruit set (Ramírez et al., 2010). Similar effects have been reported in temperate climate fruit species such as pears, peaches and apples (Ramírez et al., 2003; Costa et al., 2004a; Costa et al., 2004b); however, little is known about the P-Ca effect, gibberellins AG4/7 and cytokinin 6-benzyl amino purine (6BAP) in tomato culture.
Therefore, in this study it was proposed to know the effects produced by the prohexadione of calcium gibberellins AG4/7 and 6-benzylaminopurine in tomato (Solanum lycopersicum L.) under greenhouse conditions.
Materials and methods
The study was conducted at the Autonomous Agrarian University Antonio Narro, Saltillo, Coahuila, Mexico, in a greenhouse with a metal upper structure covered with white plastic (720 caliber) on the roof and polycarbonate side plates. As vegetable material, two-month old tomato plantlets of hybrid indeterminate growth “Raptor-F1” were used, which were transplanted on april 15, 2015 to black plastic bags with a capacity of 12 liters using as substrate: soil, tezontle and perlite (2:1:2 v/v). The bags were distributed at a distance of 50 cm between plants and 75 cm between rows, the climatic conditions inside the greenhouse were maintained at 25 °C and 65% relative humidity during the experiment. A high-flow drip irrigation system was used in each pot, with three daily irrigations at different times per day (9:00, 13:00 and 18:00 h), where 900 ml of water was applied in each pot in each irrigation carried out, this amount being the expense per pot having been previously calibrated. The crop was managed on a stalk with the traditional cultural tasks of the horticulture department at the UAAAN.
The treatments with bioregulators were the following: control (water), P -Ca (50 mg L-1), AG4/7 (50 mg L-1), AG4/7 (100 mg L-1), 6-BAP (50 mg L-1), 6-BAP (100 mg L-1), AG4/7 (50 mg L-1) + 6-BAP (50 mg L-1) and AG4/7 (100 mg L-1) + 6-BAP (100 mg L-1). When the plants showed the first floral primordia on May 18, 2015, the first foliar application was applied to dew point using an atomizer and 15 days later the second application was made with the same treatments. The experiment was established in a completely randomized design with 10 repetitions per treatment.
The variables evaluated were: leaf temperature, chlorophyll content, foliar transpiration, photosynthesis, intrinsic efficiency, yield, vitamin C and lycopene content in fruits, content of minerals in leaves and fruits (N, P, K, Ca, and Mg) and fresh and total dry biomass. The results data were evaluated with the analysis of variance (Anova) and a DMS means test (p≤ 0.05) by using the statistical program R version 2.14.2 for Windows 8.1.
Foliar parameters
Temperature (°C) and chlorophyll (SPAD Units) in leaves were measured simultaneously with a Thermometer IR digital thermometer brand Radioshack and a Chlorophyll Meter SPAD 502 brand Konica Minolta respectively. The youngest and best developed leaf in each plant was taken as reference. Three measurements were made after each application every 5 days. Leaf transpiration (mmol H2O m-2 s-1) and photosynthesis (µmol CO2 m-2 s-1) were obtained with a LI-COR Model LI-6400 TX Portable Meter, in sheets described above.
These measurements were made twice, 5 days after the first application and 37 days after the second spray. Finally, the intrinsic efficiency of the water use ((µmolCO2) (mmolH2O)-1) was obtained with the ratio of photosynthesis and leaf transpiration.
Yield
The total yield per plant resulted from the sum of the weight of fruits harvested in 10 cuts, using a Scout® Pro scale with a capacity of 1 000 g.
Antioxidants
The vitamin C content in fruits was determined using the method reported by Padayatt et al. (2001). The 10 g of pericarp of the fruit were macerated with 10 ml of 2% hydrochloric acid (v/v), then the mixture was homogenized in 40 ml of distilled water, filtered through gauze and collected in an Erlenmeyer flask. The 10 ml of the solution were taken and titrated with 2,6-dichlorophenolindophenol (1x10-3 N), until the solution turned pink. The vitamin C content was determined using the following formula:
The lycopene content was obtained from 3 g of fresh pericarp weight of the fruit. The samples were placed in a frozen mortar containing 3 ml of phosphate buffer (pH 7) and ground, from the obtained mixture 2 ml were taken and placed in centrifuge tubes, 4 ml of the hexane-acetone mixture was added (3: 2), the mixture was stirred to separate and dissolve the pigments from the membranes (Davis et al., 2003), centrifuged at 3 000 rpm for 10 min for phase separation, the colored phase was extracted and quantified at a wavelength of 450 nm on a Varian brand HPLC kit, model 500-MS. To quantify the lycopene content in the samples, a standard lycopene calibration curve (Sigma, Co) was constructed with a range of 0-40 mg mL-1 previously dissolved in the aforementioned solution. The samples were compared to the calibration curve and the lycopene content was determined using a linear regression equation.
Mineral content
For the analysis of minerals (N, P, K, Mg and Ca) in fruits and leaves, three plants were randomly selected for each treatment. The mineral content in leaves was determined by carrying out three samplings in an interval of 20 days starting from the beginning of flowering. The determination of minerals in fruits was carried out in the first cut when 3 samples were taken at random from the 10 repetitions per treatment. The process of mineral analysis corresponding to Ca, Mg, K and P was carried out in two stages, extraction and quantification. For the extraction, the samples were dried in a Felisa Model FE-291 stove at 70 °C for 72 h, they were ground and one gram of the sample was subjected to the calcination process at 600 °C for two hours, the ashes obtained they were recovered with hydrochloric acid 1:1 and gauging at 100 m with distilled water. In the second phase the concentration of the minerals Ca, Mg, and K (mg kg-1) were read in an atomic absorption spectrophotometer Varian Spectrc AA5, while the concentration of phosphorus (mg kg-1). It was carried out by obtaining the absorbance by photocolorimetry with a wavelength of 560 nm. Nitrogen (%) was determined using the modified procedure of micro-Kjeldahl digestion (Jones, 1991).
Total biomass
To determine the biomass (g), a destructive sampling of three randomly selected plants was carried out in each treatment. Samples were weighed on a Scout® Pro scale with a scale of 0 - 1 000 g to obtain fresh weight, then the plants were placed in brown paper boses and placed inside a Felisa FE 291 drying ovens at 70 °C four days and then the dry weight was obtained.
Results and discussion
Foliar parameters
The effect obtained when applying bioregulators on the temperature of the leaf is shown in Figure 1. The individual treatments with gibberellin A4/7 when compared with the control, significantly increased leaf temperature (DMS p≤ 0.05), whereas Applications with P-Ca and 6-BAP at 50 mg L-1 showed the lowest temperature in the tissue. Ferreyra et al. (2002) have related the temperature of the plant with the evolution of the hydric state of the plant; effect that they relate to the variations of water supply to tissues. Goldhamer et al. (1999) and Matthews et al. (1987) indicate that the temperature of the plant is related to the water stress of the atmosphere adjacent to it.
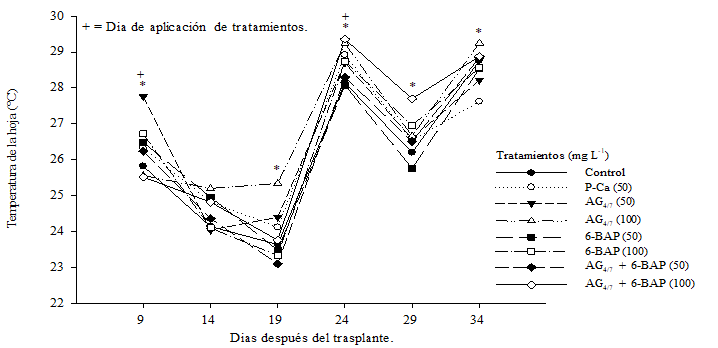
Figure 1 Effect of bioregulators on leaf temperature of tomato plants hybrid “Raptor-F1” saladette. Each point represents the average of ten repetitions. *= statistical differences. DMS (p≤ 0.05).
The gibberellins are hormones that increase cellular elongation, causing a greater flow of water to the tissue, a condition that would cause a greater fluctuation of the temperature of the leaf and manifest a faster water deficit (Ramírez et al., 2016a) as it can appreciate in Table 1 where the water efficiency ((µmolCO2) (mmolH2O)-1) shown by the plants under treatment were lower than the control in the first sampling. The P-Ca is a plant retardant that causes in plants a partial closure of stomata and therefore a reduction in water loss through the leaf (Rademacher, 2004) which causes this hormone to slightly increase the temperature of the plant. leaf having an intrinsic efficiency of water use ((µmolCO2) (mmolH2O)-1) (Table 1) lower than the control.
Table 1 Effect of bioregulators on photosynthesis, leaf transpiration and water efficiency in tomato plants hybrid “Raptor-F1” saladette.
| Treatments (mg L-1) | Photosynthesis (µmol CO2 m-2s-1) |
Leaf perspiration (mmolH2Om-2S-1) |
Intrinsic efficiency (µmolCO2) (mmolH2O)-1 |
|||||
| 5 DD1A | 37 DD2A | 5 DD1A | 37 DD2A | 5 DD1A | 37 DD2A | |||
| Control | 24.1006 a | 11.0341 ab | 9.4949 c | 5.3013 a | 2.5279 a | 2.184 b | ||
| P-Ca(50) | 23.9093 a | 8.22962 b | 10.1735 abc | 3.9729 ab | 2.3569 ab | 2.5308 b | ||
| AG4/7(50) | 21.6628 ab | 10.3904 ab | 10.0333 abc | 2.274 b | 2.1703 bc | 5.2571 a | ||
| AG4/7(100) | 20.006 ab | 11.5683 ab | 10.1711 abc | 4.0577 ab | 1.9596 cd | 2.94 b | ||
| 6-BAP(50) | 21.9897 ab | 10.8346 ab | 10.7223 ab | 4.9765 a | 2.0441 c | 2.1835 b | ||
| 6-BAP(100) | 16.7876 b | 12.5993 a | 9.589 bc | 4.0472 ab | 1.7597 d | 3.149 b | ||
| AG4/7+6-BAP(50) | 23.7023 a | 10.9025 ab | 11.1604 a | 3.7085 ab | 2.135 bc | 3.4587 b | ||
| AG4/7+6-BAP(100) | 19.3155 ab | 10.1388 ab | 9.9116 a | 3.8445 ab | 1.9542 cd | 3.1022 b | ||
| CV (%) | 14.89 | 21.68 | 9.71 | 38.64 | 11.5 | 48.67 | ||
| SE | * | * | * | * | * | * | ||
Values with the same letter in each column are statistically equal according to the DMS test; *= significant differences at a p≤ 0.05; SE= statistical significance; CV= coefficient of variation; DD1A= days after the first application. DD2A= days after the second application. Each value represents the average of six plants.
The chlorophyll content in the leaves of all treatments with bioregulators was lower than the control (Figure 2). The levels of chlorophyll in the leaves treated with P-Ca were close to the control. Bekheta et al. (2009) showed in bean plants that P-Ca at 10, 20 and 30 mg L-1 increased the pigment content. Fletcher and Hofestra (1985) proposed that the optimal application of bioregulators cause improvements in various plant plants such as increased levels of chlorophyll and a wide range of chloroplasts. The authors carried out their studies with plants in the open field. The present study was carried out in a greenhouse and with different concentrations of bioregulators. The lower amount of chlorophyll observed among them could reflect the concept of supra-optimal concentration reported in other crops (Rademacher, 2000).
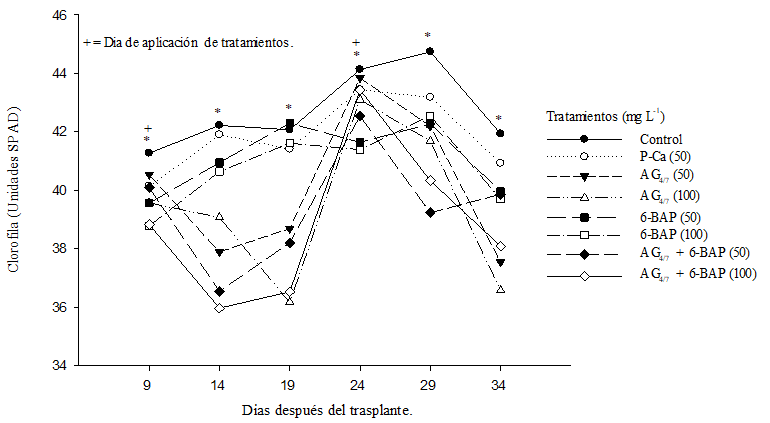
Figure 2 Effect of bioregulators on the chlorophyll content of hybrid saladette tomato plants “Raptor-F1”. Each point represents the average of ten repetitions. *= statistical differences (DMS p≤ 0.05).
It is important to point out that chlorophyll is an exponential growth factor in the plant that often adopts a curve that grows at the beginning of the crop but that usually decreases at the end of the production cycle (Zermeño et al., 2015). This natural behavior was modified by applying AG4/7 at 50 and 100 mg L-1 and with the combination of AG4/7 and 6-BAP at 50 and 100 mg L-1. It can be observed that days after the applications, the greenness of the leaves (represented in SPAD units) is reduced immediately and managed to recover 15 days after the application (Figure 2). P-Ca follows a natural pattern of chlorophyll production, which could be explained by its ability to inhibit the formation of biologically active gibberellins (Ramírez et al., 2003; Ramírez et al., 2016a). Plants sprinkled with 6-BAP at concentrations of 50 and 100 mg L-1 showed values lower than P-Ca; however, the behavior of the chlorophyll curve was not affected, this agrees with that reported by Ros et al. (2004) who observed these same trends when making 6-BAP applications in a cotton crop.
In the Table 1 shows the effects of bioregulators on photosynthesis (µmol CO2 m-2 s-1), leaf transpiration (mmol H2O m-2 s-1) and the intrinsic efficiency of water use ((µmolCO2) (mmolH2O)-1) in sheets. It was observed, in the majority of the treatments with bioregulators that they did not modify these physiological processes adversely and therefore they can be considered very positive for future studies that allow to broaden the knowledge about photosynthesis and stomatal conductance in tomato under greenhouse conditions.
Total biomass
Figure 3 shows the total fresh and dry biomass of the treatments. P-Ca and 6-BAP at 50 mg L-1 were similar (p≤ 0.05) to the control in the variable Total Fresh Weight (PFT). In previous P-Ca investigations in doses higher than 100 mg L-1 increased the number of leaves, stem diameter and number of internodes as well as the fresh and dry biomass of the plant (Ramírez et al., 2016a). In Golden Delicious apple tree this relationship was also observed (Ramírez et al., 2003). This demonstrates the fact that P-Ca is a biologically active gibberellin synthesis blocker (Rademacher, 2004) that acts as an apical growth retardant and stimulates the synthesis of cytokinins causing the vegetative increase of other organs of the plant (Costa, et. al., 2004a). This experience would lead to consider in the present study the non-effect due to low dose of those bioregulators used in tomato.
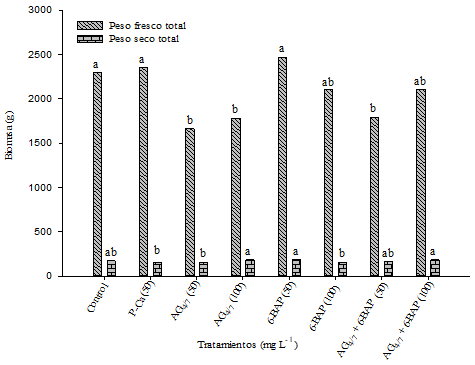
Figure 3 Influence of bioregulators on fresh and total dry biomass in tomato plants hybrid “Raptor-F1” saladette. Each bar represents the average of ten repetitions. Bars with the same letter are statistically equal (DMS p≤ 0.05).
In total dry weight (PST) (Figure 3) we observed a statistically significant difference (p≤ 0.05) among the treatments where AG4/7 + 6-BAP showed to be effective when increasing the dry matter of the plants. These effects have been observed in cocoa by Cardenas-Hernandez et al. (2010) who evaluated the effect of gibberellic acid and 6-benzylaminopurine on the development of buds in grafts of that species. At a general level, these results support the postulate that gibberellins and 6-BAP promote cell elongation, increasing the growth of the plant (El Fouly et al., 1988; Wilson-García et al., 2007).
The application of any bioregulator did not alter the content of N, Mg and Ca in leaves (Table 2). According to Gutiérrez (1997) the flow of minerals in plants is varied since it depends on the availability and demand between organs, as well as the phenological stage of the same; however, these elements are required for the metabolism of the plant since they fulfill structural functions in organic molecules, in the energy and ion reserve and redox reactions (Aven et al., 1992). Based on these experiences, the effects on the physiology of the tomato observed in this study (Table 2) are interesting since they do not observe detriment in nutrient levels.
Table 2 Effect of bioregulators on the mineral content in leaves of tomato plants hybrid “Raptor-F1” saladette.
| Treatments (mg L-1) | N (%) | P (mg kg-1) | K (mg kg-1) | Mg (mg kg-1) | Ca (mg kg-1) |
| Control | 3.052 a | 2562.7451 a | 24466.66 ab | 4383.33 a | 36933.33 a |
| P-Ca(50) | 3.2351 a | 2669.4117 a | 21800 b | 4370 a | 39800 a |
| AG4/7(50) | 3.3266 a | 2563.5294 a | 21233.33 b | 4316.66 a | 38000 a |
| AG4/7(100) | 3.2656 a | 2447.4509 ab | 22400 b | 4366.66 a | 36066.66 a |
| 6-BAP(50) | 3.0825 a | 2561.1764 a | 25100 ab | 4340 a | 35733.33 a |
| 6-BAP(100) | 3.0825 a | 2463.9215 ab | 23600 ab | 4363.33 a | 36566.66 a |
| AG4/7+6-BAP(50) | 3.113 a | 2502.3529 ab | 27800 a | 4420 a | 35266.66 a |
| AG4/7+6-BAP(100) | 3.1435 a | 1960.3921 a | 26800 a | 4406.66 a | 34500 a |
| CV | 8.5004 | 20.7393 | 15.4744 | 2.2418 | 14.1219 |
| SE | ns | * | * | ns | ns |
Values with the same letter in each column are statistically equal according to the DMS test; *= significant differences at a p≤ 0.05; NS= non-significant differences at a p≤ 0.05; SE= statistical significance; CV= coefficient of variation. Each value represents the average of six plants.
In most treatments, the phosphorus content was similar to the control; whereas, the potassium content was higher in the AG4/7 + 6-BAP treatments at any dose (Table 2). The efficiency of bioregulators in plant nutrition has been related to the translocation of nutrients to certain tissues (Medjdoub et al., 2002; Rademacher, 2004) where a stimulus or inhibition of their appearance or development can be caused (Ramírez et al., 2010).
The Table 3 shows the nitrogen levels accumulated in the fruit. The treatments AG4/7 at 50mg L-1 and AG4/7+ 6-BAP at 100 mg L-1 presented a greater amount of this element, surpassing the control in 2.1 and 8% respectively. It has been shown that nitrogen is directly related to the protein content and is also a promoter of the synthesis and production of sugar in fruits (Kjellbom and Larsson, 1984). In a study conducted by Ramírez et al. (2016a) it was shown that AG4/7 at 100 mg L-1 increased the content of brix degrees and firmness and prolonged the shelf life in the fruits. The above is relevant since the higher sugar content in the fruit there is the possibility of a shorter shelf life (Siller-Cepeda et al., 2004).
Table 3 Effect of bioregulators on the mineral content of tomato fruits hybrid “Raptor-F1” saladette.
| Treatments (mg L-1) | N (%) | P (mg kg-1) | K (mg kg-1) | Mg (mg kg-1) | Ca (mg kg-1) |
| Control | 2.7468 ab | 2169.0196 abc | 41233.33 a | 1966.66 a | 2200 abc |
| P-Ca (50) | 2.6857 ab | 2273.3333 ab | 38133.33 a | 1860 ab | 2300 ab |
| AG4/7 (50) | 2.8078 a | 2053.7254 bc | 35166.66 a | 1686.66 b | 1933.33 bc |
| AG4/7 (100) | 2.5636 ab | 2374.5098 a | 37733.33 a | 1770 ab | 2233.33 ab |
| 6-BAP (50) | 2.5942 ab | 1986.2745 c | 36000 a | 1720 ab | 2366.66 a |
| 6-BAP (100) | 2.3805 b | 2380 a | 38300 a | 1856.66 ab | 2100 abc |
| AG4/7+6-BAP (50) | 2.7468 ab | 2237.2549 ab | 39800 a | 1803.33 ab | 1833.33 c |
| AG4/7+6-BAP (100) | 2.9604 a | 2283.5294 ab | 38600 a | 1790 ab | 2066.66 abc |
| CV (%) | 12.8088 | 9.4362 | 14.1087 | 9.6691 | 15.7478 |
| SE | * | * | NS | * | * |
Values with the same letter in each column are statistically equal according to the DMS test; *= significant differences at a p≤ 0.05; NS= non-significant differences at a p≤ 0.05; SE= statistical significance; CV= coefficient of variation. Each value represents the average of 6 plants.
The content of phosphorus in fruits varied between treatments (Table 3). The bioregulators AG4/7 and 6-BAP at 100 mg L-1 showed increases of 9.4 and 9.7% respectively in relation to the control. Gutiérrez (1997) showed that phosphorus is translocated in greater quantity to the fruits when they begin their development. The potassium content was not affected by the bioregulators and they maintained levels similar to the control.
Ramírez et al. (2010) showed that P-Ca does not affect K levels in peel tomato fruits. Barrera et al. (2008) reported that the potassium content in the plant ranges between 2.7% and 4.5%, influences photosynthesis, transport of carbohydrates and plays an important role as an antagonistic element of nitrogen in fruits, besides regulating the entry and metabolism of nutrients. In human medicine, potassium occupies the third place among the minerals that act most in our body and plays an important role in most vital functions (Hopkinson et al., 1998); however, Hobson and Davies (1980) indicate that a high concentration of potassium in fruit is associated with a low quality of it.
A slight and lower content of magnesium in fruits resulted in most of the treatments with bioregulators (Table 3). It is possible that this variation is caused by the concentration of hormones used and by the physiological state of the moment in the development-maturation of the fruit. Gutiérrez (1997) mentions that the flow of Mg in plants is varied and depends on the availability and demand between organs of the plant and the stage of development of the same. On the other hand, Betancourt and Pierre (2013) point out that the order of Mg extraction in tomato is of leaf > stem > fruit > root, making it possible to demonstrate that the amount of Mg that accumulates in fruits depends on the amount stored in stems and leaves.
This is reflected when comparing the Mg found in leaves (Table 2) where the plants sprinkled with the bioregulators were similar to the control, condition that could cause an unbalanced shipment of Mg towards the fruits. The previous thing, would reflect the greater amount of Mg observed in leaves when being compared with fruits. These data coincide with those published by Fayad et al. (2002) who reported a greater Mg accumulation in the vegetative part of the tomato in relation to the fruit.
It can be observed that the calcium content was higher in the treatment 6-BAP at 50 mg L-1 (Table 3) while P-Ca at 50 mg L-1 also showed a greater tendency to control. Betancourt and Pierre (2013) observed that the level of Ca accumulated in tomato fruits was gradual starting from leaves > stems and that generally the accumulation in fruits is usually very low, this presumably because the Ca has little mobility in the phloem, transported in the plant basically through the xylem (Malone et al., 2002). It is important to consider then that the high level of Ca with 6-BAP and P-Ca at 50 mg L-1 highlights the potential of these bioregulars as good alternatives to increase the quality of the tomato fruit.
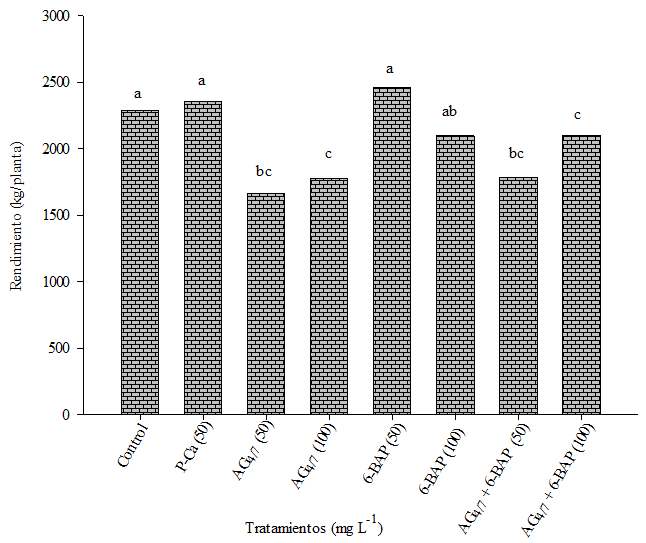
Performance
Figure 4 shows that the yield in the 6-BAP and P-Ca treatments in 50 mg L-1 was similar to the control. This behavior could be due to the concentration effect. Previous investigations (Ramírez et al., 2010) report that P-Ca at 200 mg L-1 increased yield 83% in rind tomato. This influence that P-Ca has had on increasing yield was also obtained in pear (Costa et al., 2004a) and apple tree (Greene, 1996; Unrath, 1999; Basak and Rademacher, 2000). The rest of the treatments had a tendency to have a lower yield than the control. This effect may reflect greater stimulus to vegetative development which competed with floral formation and therefore less yield (Rademacher, 2004).
Antioxidants
The effect of the treatments on the lycopene and vitamin C content in tomato fruits is shown in Table 4. The combination of AG4/7 + 6-BAP at 100 mg L-1 caused significant increases in both antioxidants; these, surpassed the control in 55.4% and 47.8% in lycopene and vitamin C respectively. The individual concentrations of AG4/7 showed trends in higher content of the two antioxidants; while P-Ca also reflected that effect for vitamin C.
Table 4 Effect of bioregulators on lycopene and vitamin C content in tomato fruits hybrid “Raptor-F1” saladette.
| Treatments (mg L-1) | Lycopene (mg L-1) | Vitamin C (mg 100 g-1) |
| Control | 3.2343 abc | 14.485 b |
| P-Ca(50) | 3.834 abc | 15.625 ab |
| AG4/7(50) | 3.9233 ab | 19.8 ab |
| AG4/7(100) | 2.618 bc | 20.26 ab |
| 6-BAP(50) | 2.6376 bc | 14.465 b |
| 6-BAP(100) | 1.7523 c | 14.555 b |
| AG4/7+6-BAP(50) | 4.118 ab | 20.61 ab |
| AG4/7+6-BAP(100) | 5.0263 a | 21.41 a |
| CV (%) | 66.4166 | 29.8391 |
| SE | * | * |
Values with the same letter in each column are statistically equal according to the DMS test; *= significant differences at a p≤ 0.05; CV= coefficient of variation; SE= statistical significance, each value represents the average of 10 plants.
Previous research shows that P-Ca increases the content of vitamin C and lycopene in red tomatoes (Ramírez et al., 2003) and habanero peppers (Ramírez et al., 2016b). These findings allowed P-Ca to be classified as a retardant that increases the antioxidant content, which affects good health in humans since it strengthens the immune system that protects against diseases such as diabetes, cancer and blood pressure (Ramírez et al., 2010). Little information exists on the stimulation of gibberellins and cytokinins in the synthesis of lycopene and vitamin C in tomato (Ramírez et al., 2010). Therefore, more research is needed on this subject.
The higher content of lycopene and vitamin C observed in several treatments with bioregulators gives an added value to the fruit. This characteristic allows a higher price in the international market than that of a tomato with normal levels of these antioxidants (Ramírez et al., 2016a), which compensates for a yield equal to or perhaps less than that of the control.
Conclusions
In greenhouse tomato “Raptor-F1” cultivated in greenhouse, the P-Ca and 6-BAP biorregulators at 50 mg L-1 do not alter the foliar physiology and yield, increase the level of potassium in leaves and nitrogen and calcium in fruits. 6-BAP at 50 mg L-1 increases the fresh and dry matter of the plant; when combined with gibberellins A4/7 at 100 mg L-1 increase the content of vitamin C and lycopene in fruits.
Literatura citada
Aven, P.; Evert, F. y Eichhorn, S. E. 1992. Biología de plantas. Vol. 2. Traducido al español por Santamaría, S.; Lloret, F.; Mas, M. y Cardona, M. Edit. Reverté. Barcelona, España. 773 p. [ Links ]
Barrera, L.; Basilo, P.; Durango, P. y Ramos, A. 2008. Efecto de las épocas de lluvia y sequia sobre la absorción de potasio y fósforo en las plantaciones de plátano. Acta Agron. 57(1):55-59. [ Links ]
Basak, A. and Rademacher, W. 2000. Growth regulation of pome and stone fruits trees by use of Prohexadione-Ca. Acta Hortic. 514:41-50. [ Links ]
Bekheta, M.; Abdelhamid, M. and El-Morsi, A. 2009. Physiological response of vicia faba to prohexadione-Calcium under saline conditions. Planta Daninha. 27(49):769-779. [ Links ]
Betancourt, P. y Pierre, F. 2013. Extracción de macronutrimentos por el cultivo de tomate (Solanum lycopersicum Mill var. Alba) en casas de cultivo en Quibor. Estado de Lara. Bioagro. 25(3):181-188. [ Links ]
Cárdenas-Hernández, J.; Álvarez-Herrera, J.; Barragan, E. y Rivera, C. 2010. Efecto del ácido giberelico y la 6-bencilaminopurina sobre el desarrollo de yemas en injertos de cacao (Theobrama cacao L.). Agron. Colomb. 28(1):19-27. [ Links ]
Costa, G.; Sabatini, E.; SpinellI, F.; Andreotti, C.; Spada, G. and Mazini, F. 2004a. Prohexadione-Ca control vegetative growth and cropping performance in pear. Acta Hortic. 653:43-48. [ Links ]
Costa, G.; Sabatini, E.; Spinelli, F.; Andreotti, C.; Bomben, C. and Vizzotto, G. 2004b. Two years of application of prohexdione-Ca on apple: effect on vegetative and cropping performance, fruit quality, return bloom and residual effect. Acta Hortic . 653:35-40. [ Links ]
Davis, A.; Fish, W. and Perkins‐Veazie, P. 2003. A rapid hexane-free for analysing lycopene content in watermelon. J. Food Sci. 68(1):328-332. [ Links ]
El Fouly, M.; Sakr, R.; Fouad, M.;Zaher, A. y Fawzi, A. 1988. Efecto de GA, CCC y B-9 en los personajes morfofisiológicos y el rendimiento de frijoles (Phaseolus vulgaris L.). J. Crop Sci. Agron. 160(1):94-101. [ Links ]
Fayad, J.; Fontes, P.; Cardoso, A.; Finger, F. e Ferrreira, F. 2002. Absorcao de nutrientes pelo tomateiro cultivado sob condicoes de campo e de ambiente protegido. Hortic. Bras. 20(1):90-94. [ Links ]
Ferreyra, E.; Selles, V.; Peralta, A.; Burgos, L. y Valenzuela, J. 2002. Efectos de la restricción del riego en distintos periodos de desarrollo de la vid CV. Cabernet sauvignon sobre producción y calidad del vino. Agric. Téc. 62(3):406-417. [ Links ]
Fletcher, R. and Hofstra, G. 1985. Triadimefon a plant multi-protectant. Plant Cell Phisiol. 26(4):775-780. [ Links ]
Goldhamer, A.; E. Fereres , and Cohen, M. 1999. Sensitivity of continuous and discrete plant and soil water status monitoring in peach trees subjected to deficit irrigation. J. Am. Soc. Hortic. Sci. 124:437-444. [ Links ]
Greene, W. 1996. The use of BAS 125W to control growth of apple trees. Proceedings PGRSA. 24(2):283-286. [ Links ]
Gutiérrez, M. 1997. Nutrición mineral de las plantas: avances y aplicaciones. Agron. Costarric. 21(1):127-137. [ Links ]
Hobson, G. and Davies, J. 1980. The biochemistry of fruits and their products. Vol. 2. Academic Press Inc. Ed. A.C. Hulme. London and New York. 788 p. [ Links ]
Hopkinson, D.; Bhabra, M. and Hooper, T. 1998. Pulmonary graft preservation: a worldwide survey of current clinical practice. J. Heart Lung Transplant. 17(22):525-531. [ Links ]
Jones, J. 1991. Kjeldahl method for nitrogen determination. MicroMacro Publ., Athens, GA. 79 p. [ Links ]
Kjellbom, P. and Larsson, C. 1984. Prepation and polypeptide composition of chlorophyll-free plasma membranes from leaves of light-griwn spinach and barley. Physiol. Plant. 62(4):501-509. [ Links ]
Malone, M.; White, P. and Morales, M. 2002. Mobilization of calcium in glasshouse tomato plants by localized scorching. J. Exp. Bot. 53(336):83-88. [ Links ]
Matthews, M.; Anderson, M. and Shultz, H. 1987. Phenologic and growth responses to early and late season water deficits in Cabernet franc. Vitis. 26:147-160. [ Links ]
Medjdoub, R.; Bordonaba, M.; Pilar, A.; Val, J. y Blanco, A. 2002. Efecto del prohexadione-ca sobre el crecimiento y la nutrición del manzano. In: Actas del IX Simposio Ibérico sobre Nutrición Mineral de las Plantas. 10-13 septiembre, Zaragoza-España. 38:11-12. [ Links ]
Nickell, L. G. 1988. Plant growth regulator use in cane and sugar production. Update. Sugar J. 50:7-11. [ Links ]
Padayatt, J.; Daruwala, R.; Wang, Y.; Eck, P.; Song, J.; Koch, W. S. and Levine, M. 2001. Vitamin C: from molecular actions to optimum intake. In: Handbook of antioxidants. Cadenzas, E. and Packer, L. (Eds) 2nd edition. CRC press. Washington DC, USA. 117-145. [ Links ]
Rademacher, W. 2000. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Ann. Rev. PlantPhysiol. Plant . Mol. Biol. 51:501-531. [ Links ]
Rademacher, W. 2004. Chemical regulation of shoot growth in fruit trees. Acta Hortic . 653:9-15. [ Links ]
Ramírez, H.; Gómez-Castañeda, J.; Benavides-Mendoza, A.; Robledo-Torres, V.; Encina-Rodríguez, L. y Coello-Coutiño, C. 2003. Influencia de Prohexadiona-Ca sobre crecimiento vegetativo-producción y calidad de fruto en manzano (Malus domestica Borkh). Rev. Chapingo Ser. Hortic. 9(2):279-289. [ Links ]
Ramírez, H.; Rivera-Cruz, C.; Benavides-Mendoza, A.; Robledo-Torres, V. y Reyna-Sustaita, G. 2010. Prohexadiona-Ca, una alternativa en la producción de tomate de cáscara (Physalis ixocarpa Brot.). Rev. Chapingo Ser. Hortic . 16(2):139-146. [ Links ]
Ramírez, H.; Zavala- Ramírez, M.; Sánchez-López, A.; Aguilar-Zarate, P.; Cristóbal-Aguilar, N.; Rodríguez -García, R.; Jasso-Cantú, D. Zermeño-González, A.; Villareal-Quintanilla, J. and López-Fabián, A. 2016a. Tomato responses to bioregulators grown under greenhouse conditions. Inter. J. Plant Soil Sci. 10(6):1-13. [ Links ]
Ramírez, H.; Mendoza-Castellanos, J.; Vázquez-Badillo, M. y Zermeño-González, A. 2016b. La prohexadiona de calcio (P-CA): una alternativa hormonal viable en chile habanero. Rev. Mex. Cienc. Agríc. 7(3):631-641. [ Links ]
Ros, A.; Gómez, P.; José, A. y Báidez, A. 2004. Incremento de la tolerancia frente a Fusarium oxysporum de plantas de algodón (var. Delta opalo) por tratamientos con 6-benzilaminopurina. In: metabolismo y modo de acción de fitohormonas. Ediciones Universidad de Salamanca. 231-236 pp. [ Links ]
Siller-Cepeda, J.; Muy-Rangel, D.; Baez-Sañudo, M.; García-Estrada, R. y Araiza-Lizarde E.. 2004. Calidad en frutos de carambola (Avrrhoa carambola L.) cosechada en cuatro estados de madurez. Rev. Chapingo Ser. Hortic . 10(1):23-29. [ Links ]
Unrath, C. 1999. Prohexadione-Ca: a promising chemical for controlling vegetative growth of apples. HortSci. 34(7):1191-1200. [ Links ]
Wilson-García, C.; Zavaleta-Mancera, A.; López-Delgado, H. y Hernández-Garay A. 2007. La citocinina BAP retrasa senescencia, aumenta antioxidantes, proteína y crecimiento del pasto ocillo (Dactylis glomerata L.). Agrociencia. 42(7):799-806. [ Links ]
Zermeño, A.; López, B.; Melendrez, A.; Ramírez, H.; Cárdenas, J. y Munguía, J. 2015. Extracto de alga marina y su relación con fotosíntesis y rendimiento de una plantación de vid. Rev. Mex. Cienc. Agríc . Núm. Esp. 12:2437-2446. [ Links ]
Received: March 2018; Accepted: May 2018











 texto en
texto en