Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 n.3 Texcoco Apr./May. 2018
https://doi.org/10.29312/remexca.v9i3.1217
Articles
Watermelon production with rootstocks in soils infested with the necrotic melon spot virus
1Doctorado BEMARENA-Centro Universitario de Ciencias Biológicas y Agropecuarias-Universidad de Guadalajara. Camino Ramón Padilla Sánchez 2100, Nextipac, Zapopan, Jalisco. CP. 44600. Tel. 01 (33) 37771150. (fgarcia@ucol.mx; pzarazua@cucba.udg.mx; ramonrod@cucba.udg.mx).
2Departamento de Ciencias Ambientales-Instituto Tecnológico de Colima. Avenida Tecnológico 128, Villa de Álvarez, Colima. CP. 28976. Tel. 01(312) 3126393. (victoria.huitron@itcolima.edu.mx).
Mexico is one of the main producers of watermelon worldwide. But, the presence of soil pathogens such as nematodes, Fusarium oxysporum f. sp. niveum and the melon necrotic spot virus (MNSV) represent a serious problem for producers. The use of grafted plants is a good alternative to deal with these problems, helping to increase yield and quality in watermelon. The rootstocks of wild watermelon (Citrullus lanatus var. citroides) have been shown to be effective for the control of most diseases transmitted through the soil, in addition to having high resistance to nematodes; however, there is no record of its resistance to MNSV. Two experiments were conducted to evaluate the resistance to MNSV of rootstocks for watermelon. In Experiment 1 the rootstocks of interspecific hybrids “RS-841” “Ercole” (Cucurbita maxima × Cucurbita moschata) and wild watermelon the rootstocks “Robust”, “RS0272” and “RS1833” were evaluated. In experiment 2 the rootstocks “RS-841”, “Ercole” and “Robust” were evaluated. In both cases in soils infested with MNSV. The rootstocks of wild watermelon were highly susceptible to MNSV, whereas the interspecific hybrids showed resistance to this disease. The rootstock “RS-841” was the one that produced the best quality fruits. The yield was lower with significant difference when the wild watermelon rootstocks were used.
Keywords: Olpidium bornovanus; grafts; MNSV; wild watermelons
México es uno de los principales productores de sandía a nivel mundial. Pero la presencia de patógenos del suelo como nematodos, Fusarium oxysporum f. sp. niveum y el virus de la mancha necrótica del melón (MNSV) representan un serio problema para los productores. El uso de plantas injertadas es una buena alternativa para hacer frente a estos problemas, ayudando a incrementar el rendimiento y calidad en sandía. Los portainjertos de sandía silvestre (Citrullus lanatus var. citroides) han demostrado ser eficaces para el control de la mayoría de las enfermedades que se transmiten a través del suelo, además de tener alta resistencia a nematodos; no obstante, no se tiene registro sobre su resistencia al MNSV. Se realizaron dos experimentos para evaluar la resistencia al MNSV de portainjertos para sandía. En el experimento 1 se evaluaron los portainjertos de híbridos interespecíficos “RS-841” “Ercole” (Cucurbita maxima×Cucurbita moschata) y de sandía silvestre los portainjertos “Robusta”, “RS0272” y “RS1833”. En el experimento 2 se evaluaron los portainjertos “RS-841”, “Ercole” y “Robusta”. En ambos casos en suelos infestados con MNSV. Los portainjertos de sandía silvestre fueron altamente susceptibles al MNSV, en cambio los híbridos interespecíficos mostraron resistencia a esta enfermedad. El portainjertos “RS-841” fue el que produjo los frutos de mejor calidad. El rendimiento fue inferior con diferencia significativa cuando se utilizaron los portainjertos de sandía silvestre.
Palabras claves: Olpidium bornovanus; injertos; MNSV; sandías silvestres
Introduction
Mexico occupies the tenth place as a producer of watermelon [Citrullus lanatus (Thunb.) Matsum. & Nakai] worldwide (FAOSTAT, 2016). However, the productive potential of Mexico is diminished mainly due to the presence of diseases transmitted through the soil (ETTS) such as Fusarium oxysporum f. sp. niveum, nematodes and the melon necrotic spot virus (MNSV). The latter has caused serious losses in the production of watermelon, because it causes the sudden death of the plant before or during harvest, increasing its pathogenicity when associated with the fungus vector Olpidium bornovanus (Guirado et al., 2009).
This virus attacks crops of cucumber (Cucumis sativus L.), melon (Cucumis melo L.) and watermelon, in countries such as Spain, Tunisia, Brazil, Guatemala, Honduras, Panama, and United States and in Mexico in the states of Sinaloa and Colima. (Herrera‐Vásquez et al., 2010). To increase production and make crops more profitable, in Mexico we have opted to use grafted plants for the production of crops such as melon, tomato (Solanum lycopersicum L.) and pepper (Capsicum annuum L.) (García-Rodríguez, 2010; Ricárdez-Salinas et al., 2010; Osuna-Ávila et al., 2012). In watermelon, increases in production up to 115% are reported when grafted plants are used in soils infested with MNSV (Huitrón-Ramírez et al., 2009)
Despite the known advantages over the use of grafted plants, there are also drawbacks, some combinations of varieties and rootstocks can cause changes in texture, color, fruit size, sugar content, as well as a higher incidence of physiopathies (Davis and Perkins-Veazie, 2005; Crinò et al., 2007; King et al., 2010).
Watermelons are usually grafted on interspecific hybrids of Cucurbita maxima Duchesne x Cucurbita moschata Duchesne, due to its vigorous root and its resistance to ETTS; however, several authors have reported that the watermelon fruits of plants grafted with these rootstocks tend to have a lower pH, greater firmness of pulp, variation in size and an insipid taste (Colla et al., 2006; Davis et al., 2008; Kyriacou et al., 2016). For this reason, some producers manifest a certain refusal to adopt this technique, mainly due to the quality parameters with which the fruit must comply in order to be exported to the United States of America.
In watermelon uniformity in size is sought, with little variation and firm consistency. The sugar content must be higher than 8%, and it is considered to be of very good quality when it exceeds 10% (USDA, 2006). The rootstocks of Citrullus lanatus var citroides (wild watermelon) have been shown to be resistant to nematodes (Meloidogyne spp.). And to most ETTS (Thies et al., 2010; Thies et al., 2015), without affecting the parameters of fruit quality (Huitrón-Ramírez et al., 2007); however, it is unknown if they are resistant to MNSV.
For these reasons, it is essential to have rootstocks for watermelon that maintain the standards of quality common in the market and that provide resistance to ETTS. The objective of this research was to evaluate the yield and quality of grafted watermelons on commercial rootstocks of interspecific hybrids and to compare them with wild watermelon rootstocks in naturally infested soils with MNSV associated with Olpidium bornovanus.
Materials and methods
The experiments were carried out in the ranch “The Carmelitas”, located in the state of Colima, Mexico (19.16° north latitude, 103.38° west longitude) in an open field plot. Experiment 1 was carried out in the Autumn-Winter 2011-2012 season and experiment 2 was carried out in the autumn-winter season 2012-2013. For both experiments, the maximum and minimum temperatures (Figure 1) of the region were consulted with the help of the INIFAP network of stations (INIFAP, 2013).
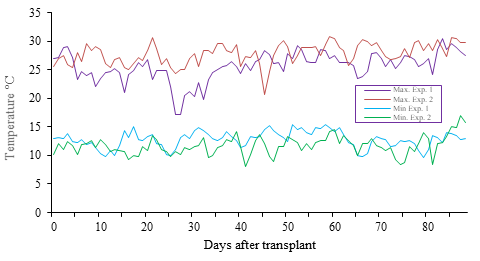
Figure 1 Maximum and minimum temperatures throughout the crop for experiment 1 (2011-2012) and experiment 2 (2012-2013).
The soil of the experimental plots was infested naturally with the melon necrotic spot virus (MNSV) associated with the vector fungus Olpidium bornovanus, which was identified in the Phytopathology Laboratory of the University of Almeria, Spain prior to the realization of the experiment, and later corroborated by the Phytopathology laboratory of the University Center of Biological and Agricultural Sciences (CUCBA) of the University of Guadalajara. The texture of the soil is sandy loam with 3.44% organic matter, pH 5.2, and electrical conductivity (CE) in the saturation extract of 0.9 dS m-1. In the two experiments applications of bottom fertilizer 0N-34.8P-72.8K (superphosphate and potassium sulfate) were made. Additionally, throughout the cycle, 187N-14.2P-176K was added through the irrigation system.
Vegetal material. Experiment 1 and experiment 2 used the variety of watermelon (Citrullus lanatus) triploid “Mielheart” of the mini type, and as a pollinator the diploid watermelon “Minipool”. The interspecific hybrids of Cucurbita maxima × Cucurbita moschata “RS-841” and “Ercole” were additionally used rootstocks of wild watermelons (Citrullus lanatus var citroides) “Robust”, “RS0272” and “RS1833” for experiment 1, generating So a total of five treatments. In experiment 2 it was not possible to use the rootstocks of wild watermelon “RS0272” and “RS1833” due to its disappearance from the market, so only the rootstocks “RS-841”, “Ercole” and “Robust” were used.
In both experiments the rootstock “RS-841” was used as a control, because this rootstock is used in the region to produce watermelons of the variety Mielheart. The seedlings were grafted by the approach method using the method described by Lee (1994). The substrate that was used to germinate and develop the plant was a mixture of pearlite and peat-moss at a ratio of 2:1 (v/v).
Crop management. In both experiments the grafted plants of watermelons were planted at a distance of 0.7 m above the bed and 3.6 m between beds. The plantation beds were covered with silver/black polyethylene plastic for quilting. The irrigation was by drip, for this a strip with dripper separation of 45 cm and an expense of 1.2 L h-1 was used (Rain Bird, California, United States of America). For each treatment four repetitions were made, for a total of 20 experimental plots for experiment 1, and 12 for experiment 2. Each experimental unit was made up of five culture beds, with a width of 18 m and a length of rows of 10 m, giving a total of 180 m2 per plot.
The transplant was performed between 14 and 17 days after grafting, with a 2:1 distribution between triploid/diploid watermelons on the same row (Huitrón-Ramírez et al., 2009). Weed control was done manually and chemicals were used to control pests (imidacloprid, abamectin, cypermethrin) and diseases (chlorothalonil, boscalid + pyraclostrobin). For a suitable pollination, bees (Apis mellifera) were used which were introduced to the plot 30 d after the transplant, using 4 hives ha-1
Determination of quality and performance. The harvest was carried out by specialized personnel, who harvested only fruit with quality standards acceptable to the market. A count was made of all marketable fruits of the Mielheart variety, of which five fruits were selected for each experimental plot, by cut (four cuts in each experiment); subsequently, they were weighed on a scale (Mettler, Toledo Wildcat 3009, DF, Mexico) to estimate the yield and total production. In order to measure quality attributes, determinations were made on three representative fruits (by harvest), which were measured the total content of soluble solids, firmness, size, shape and thickness of the bark. The soluble solids were measured from the juice of the fruit with a digital refractometer (Atago Pal-1, Tokyo, Japan).
A penetrometer (Bertuzzi FT-011, Milan, Italy) was used to determine the firmness of the fruit pulp, for this the fruit was split in half, and three penetrations were made, one in the center of the fruit and two more in the perimeter zone of the pulp, approximately 2 cm from the fruit’s crust, obtaining three readings, of which the average was calculated. A metric tape was used to determine the equatorial and longitudinal perimeter; for the shape the equatorial perimeter was divided between the longitudinal perimeter and finally the thickness of the crust was measured with a digital vernier. At the end of the cycle, a percentage of survival of the plants was estimated to assess the resistance of the rootstocks to the MNSV.
Statistical analysis. A randomized block design was used. In both experiments, each block had a total of 72 plants (48 triploids and 24 diploids). The non-normal data were transformed by various procedures suggested by the Box-Cox transformation, the means were compared with Fisher’s LSD test. The statistical analysis was carried out with the Minitab 16 computer program (Minitab Inc., 2010).
Results and discussion
In experiments 1 and 2, most of the plants grafted onto the rootstocks of wild watermelons (C. lanatus cv. citroides) withered after the first harvest, causing their death (Figure 2). The symptoms observed in plants grafted on wild watermelons correspond to an infection caused by MNSV, which have been described and reported for watermelon and other cucurbits (Komuro, 1972; Moya et al., 2009). More than 80% of these plants had necrosis in the stems and numerous small necrotic spots throughout the foliage.
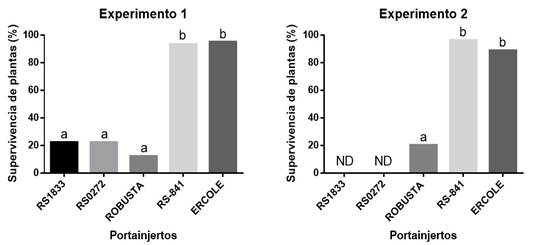
Figure 2 Survival cup (%) of watermelon plants grafted on rootstocks of interspecific hybrids of Cucurbita maxima x Cucurbita moschata (“RS-841” and “Ercole”) and wild watermelon rootstocks (“RS1833”, “RS0272” and “Robust”) in soils infested with MNSV associated with O. bornovanus.
The presence of Olpidium bornovanus was confirmed by means of microscopic observation. Infections caused by MNSV can be asymptomatic in young plants, so symptoms can appear suddenly during the stage of fruit ripening and can cause damage reaching 100% of the plants when there is the presence of the vector fungus O. bornovanus (Kwak et al., 2015). The rootstocks of C. lanatus cv. citroids have been used commercially due to their high compatibility with varieties of watermelons, high yields, resistance to nematodes (Meloidogyne spp.) and some pathogens such as Fusarium oxysporum f. sp. niveum (Huitrón-Ramírez et al., 2007; Thies et al., 2010; Thies et al., 2015), but they are very susceptible to MNSV.
Performance. Statistical difference was observed in both experiments in the average fruit weight (PUF) among the rootstocks of interspecific hybrids of C. maxima×C. moschata and wild watermelon rootstocks (Table 1). The watermelon PMF normally increases when grafting on wild watermelon rootstocks in soils without the presence of the MNSV and its vector fungus O. bornovanus (Rodríguez et al., 2008). On the other hand, Bekhradi et al. (2011) observed that the weight of watermelon fruits is not affected by the type of rootstock that is used.
Table 1 Yield parameters in watermelons grafted with different rootstocks.
| Treatments | Fruits per plant | PMF (kg) | Yield (t ha-1) |
| Experiment 1 (2011-2012) | |||
| RS1833 | 1.6 a | 1.4 a | 12.1 a |
| RS0272 | 1.7 a | 1.7 a | 13.5 a |
| Robust | 1.9 a | 1.4 a | 13.5 a |
| RS-841 | 5.1 b | 2.4 b | 49.5 b |
| Ercole | 5.2 b | 2.3 b | 51.9 b |
| Significance | *** | *** | *** |
| Experiment 2 (2012-2013) | |||
| Robust | 0.9 a | 2.4 a | 9 a |
| RS-841 | 3.8 b | 3.4 b | 52 b |
| Ercole | 3.7 b | 3.4 b | 50 b |
| Significance | *** | *** | *** |
PMF= average weight of the fruit. ***= significant at p≤ 0.001. Different letters indicate significant difference at p<0.05.
Similarly, Alan et al. (2007) reported no difference in the PMF in an open-field crop in which he compared three types of rootstocks and one control without grafting. However, the presence of pathogens such as MNSV can reduce the weight and size of the fruit in non-resistant cultivars (Huitrón-Ramírez et al., 2009). In the two experiments of this study, the rootstocks of wild watermelon produced less fruit, with statistical difference compared to the rootstocks of the interspecific hybrids.
Some authors have attributed a greater development of watermelon fruits to the vigorous root system of some rootstocks, as is the case of the rootstocks of C. maxima×C. moschata, because they absorb water and nutrients more efficiently and increase the tolerance of grafted plants to biotic and abiotic factors (Rouphael et al., 2008; Rouphael et al., 2012; Borgognone et al., 2013). It was observed in this study, that the yield of plants grafted on “RS-841” and “Ercole” surpassed the plants grafted on wild watermelon by 430% in experiment 1 and by 578% in experiment 2.
The plants grafted onto the rootstocks of wild watermelon began to wither a few days before the first harvest in both experiments, which reduced the development of the fruit. While the plants grafted on “RS-841” and “Ercole” remained healthy during the development of the fruit and the stage of maturation; these rootstocks allowed the fruit to achieve an optimum state of maturation (Figure 3). Plants infested with MNSV usually suffer a drastic decrease in yield compared to plants grafted on interspecific hybrids of C. maxima×C. moschata (Huitrón-Ramírez et al., 2009). In the same way in this study the plants grafted on wild watermelons were of a lower yield than the plants grafted on “RS-841” and “Ercole”.

Figure 3 Development of watermelon plants grafted on soils infested with MNSV. Watermelon plants grafted on “Robust” (C. lanatus cv. citroides) before (A) and during the ripening of the fruit affected by the MNSV (B). Watermelon plants grafted onto “RS-841” (C. maxima×C. moschata) before (C) and during the ripening of the fruit (D).
Quality of the fruit. In experiment 1 and 2, statistical differences were detected in the firmness of the fruit pulp (Table 2) between the treatments with rootstocks of interspecific hybrids and wild watermelons. The use of different rootstocks can generate variability in the firmness of the fruit (Bruton et al., 2009). In most cases, the firmness of the fruit benefits the watermelon producers, since the fruits with greater firmness increase their shelf life. Additionally, the content of soluble solids (SS) is one of the most important aspects in the quality of the fruit, because the presence of sugars is responsible for the sweetness of the fruit (Sánchez-Rodríguez et al., 2012).
Table 2 Quality parameters in grafted watermelon.
| Treatments | Firmness (kg) | SS (°Brix) | GC (mm) | PE (cm) | PL (cm) | Form PE/PL |
| Experiment 1 (2011-2012) | ||||||
| RS1833 | 2.7 a | 9.8 a | 11 b | 46.7 a | 48.9 a | 0.98 |
| RS0272 | 2.8 a | 9.6 a | 10.6 b | 48.8 a | 50.7 a | 0.96 |
| Robust | 2.7 a | 9.7 a | 10.7 b | 48.1 a | 49.4 a | 0.97 |
| RS-841 | 3.2 b | 10.3 b | 9.9 a | 52.7 b | 54.4 b | 0.97 |
| Ercole | 3.2 b | 9.9 a | 10.7 b | 52.2 b | 53.3 b | 0.98 |
| Significance | ** | *** | *** | *** | *** | ns |
| Experiment 2 (2012-2013) | ||||||
| Robust | 2.68 a | 9.7 a | 10.6 b | 48 a | 49.4 a | 0.96 |
| RS-841 | 3.16 b | 10.3 b | 9.9 a | 53 b | 54.3 b | 0.96 |
| Ercole | 3.14 b | 9.9 a | 10.7 b | 52 b | 53.3 b | 0.95 |
| Significance | * | * | ** | *** | *** | ns |
SS= soluble solids; GC= bark thickness; PE= equatorial perimeter; PL= longitudinal perimeter. The symbols. ns, *, **, ***= not significant, significant at p≤ 0.05, 0.01, 0.001, respectively.
The treatment with the rootstock “RS-841” showed the highest content of SS in both experiments, registering significant difference with respect to the other treatments, even with respect to the rootstock “Ercole”. However, most authors affirm that the SS content does not seem to be altered by the use of different types of rootstocks (Rouphael et al., 2008; Han et al., 2009; Bekhradi et al., 2011; Miceli et al., 2016). The rind of the fruits of “RS-841” was significantly thinner in the two experiments.
A thicker crust may be associated with a reduction in pulp content for the consumer. While watermelons with a very thin crust are more susceptible to mechanical damage during packaging and transport (Alexopoulos et al., 2007). Therefore, the preference in the thickness of the bark may vary depending on the purpose for which the fruits are intended. No variation in the shape of the fruit was found in either of the two experiments.
It is notable that the previously mentioned results showed variation between the two experiments studied. In experiment 2, a higher mortality was observed in the treatment with the “Robust” rootstock, as well as an increase in the severity of the MNSV symptoms, with respect to Experiment 1. Kido et al. (2008) indicate that MNSV symptoms increase when the temperature is below 20 °C, and viral propagation and dissemination increase at temperatures above 25 °C.
However, under the conditions in which the experiments were established it was not possible to determine if the oscillation in temperature was responsible for the increase in the severity of MNSV infection. On the other hand, in the quality variables it was possible to appreciate that the rootstock “RS-841” showed superior characteristics in comparison with the other rootstocks. Özmen et al. (2015) observed a similar phenomenon when comparing different cycles of grafted watermelons these authors mentioned that the yield was not affected by seasonal variation. However, it is well known that rootstocks can have effects on quality, although in most cases it is usually harmful, except for the size of the fruit (Lee, 1994).
Conclusions
The rootstocks of the interspecific hybrids are an excellent option for the production of “Mielheart” watermelon in soils infested with the melon necrotic spot virus (MNSV) and its vector fungus Olpidium bornovanus. Additionally, these rootstocks produce fruits with acceptable quality standards for the United States market. The rootstocks of wild watermelons should not be used in soils infested with the MNSV because they are very susceptible to this virus and can cause considerable losses in yield. However, under other conditions, rootstocks of wild watermelons can be very useful, due to their high resistance to pests such as nematodes and other pathogens that occur in the soil. Fruit quality was affected when wild watermelon rootstocks were used in soils infested with MNSV.
Literatura citada
Alan, O.; Ozdemir N. and Gunem, Y. 2007. Effect of grafting on watermelon plant growth, yield and quality. Pakistán. J. Agron. 6(2):362-365. [ Links ]
Alexopoulos, A. A.; Kondylis, A. and Passam, H. C. 2007. Fruit yield and quality of watermelon in relation to grafting. Finlandia. J. Food Agric. Environ. 5(1):178-179. [ Links ]
Bekhradi, F.; Kashi, A. and Deishad, M. 2011. Effect of three cucurbits rootstocks on vegetative and yield of ‛Charleston Gray’ watermelon. Irán. Int. J. Plant Prod. 5(2):105-110. [ Links ]
Borgognone, D.; Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E. and Schwarz, D. 2013. Effect of nitrogen form and nutrient solution pH on growth and mineral composition of self-grafted and grafted tomatoes. Holanda. Scientia Hort. 149(1):61-69. [ Links ]
Bruton, B. D.; Fish, W. W.; Roberts, W. and Popham, T. W. 2009. The Influence of Rootstock Selection on Fruit Quality Attributes of Watermelon. Emiratos Árabes Unidos. Open Food Sci. J. 3(1):15-34. [ Links ]
Colla, G.; Rouphael, Y.; Cardarelli, M. and Rea, E. 2006. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. Estados Unidos de América. HortScience. 41(3):622-627. [ Links ]
Crinò, P.; Lo Bianco, C.; Rouphael, Y.; Colla, G.; Saccardo F. and Paratore, A. 2007. Evaluation of rootstock resistance to fusarium wilt and gummy stem blight and effect on yield and quality of a grafted “inodorus” melon. Estados Unidos de América. HortScience. 42(3):521-525. [ Links ]
Davis, A. R.; Perkins, V. P.; Hassell, R.; Levi, A.; King, S. R. and Zhang, X. 2008. Grafting effects on vegetable quality. Estados Unidos de América. HortScience. 43(6):1670-1672. [ Links ]
Davis, A. R. and Perkins, V. P. 2005. Rootstock Effects on Plant Vigor and Watermelon Fruit Quality. Estados Unidos de América. Cucurbit Genet Coop Rep. 28(1):39-42. [ Links ]
FAOSTAT. 2016. Crops production. <http://faostat.fao.org/default.aspx>. [ Links ]
García, R. M. R.; Chiquito, A. E.; Loeza, L. P. D.; Godoy, H. H.; Pineda, E. V.; Pons, H. J. L.; González, Ch. M. M. y Anaya, L. J. L. 2010. Producción de chile ancho injertado sobre criollo de Morelos 334 para el control de Phytophthora capsici. Agrociencia. 44(6):701-709. [ Links ]
Guirado, M.; Sáez, E.; Serrano, Y. y Gómez, J. 2009. Obtención y caracterización de aislados monoesporangiales de Olpidium bornovanus. España. Bol. San. Veg. Plagas. 35(4):629-644. [ Links ]
Han, J. S.; Park, S.; Shigaki, T.; Hirschi, K. D. and Kim, C. K. 2009. Improved watermelon quality using bottle gourd rootstock expressing a Ca2+/H+ antiporter. Holanda. Mol. Breed. 24(3):201-211. [ Links ]
Herrera, V. J.; Córdoba, S. M.; Cebrián, M.; Rosselló, J.; Alfaro, H. A. and Jordá, C. 2010. Genetic diversity of Melon necrotic spot virus and Olpidium isolates from different origins. Reino Unido. Plant Pathol. 59(2):240-251. [ Links ]
Huitrón, R. M. V.; Diaz, M.; Diánez, F. y Camacho, F. 2007. The effect of various rootstocks on triploid watermelon yield and quality. Finlandia. J. Food, Agric. Environ. 5(3/4):344-348. [ Links ]
Huitrón, R. M. V.; Ricárdez, S. M. and Camacho, F. F. 2009. Influence of grafted watermelon plant density on yield and quality in soil infested with melon Necrotic Spot Virus. Estados Unidos de América. HortScience . 44(7):1838-1841. [ Links ]
INIFAP. 2013. Red de estaciones del INIFAP. 30 de mayo 2016. < http://clima.inifap.gob.mx/redinifap/ >. [ Links ]
Kido, K.; Tanaka, C.; Mochizuki, T.; Kubota, K.; Ohki, T.; Ohnishi, J.; Knight, L. M. and Tsuda, S. 2008. High temperatures activate local viral multiplication and cell-to-cell movement of Melon necrotic spot virus but restrict expression of systemic symptoms. Estados Unidos de América. Phytopathology. 98(2):181-186. [ Links ]
King, S. R.; Davis, A. R.; Zhang, X. and Crosby, K. 2010. Genetics, breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Holanda. Scientia Hort. 127(2):106-111. [ Links ]
Komuro, Y. 1972. Cucumber green mottle mosaic virus on cucumber and watermelon and melon necrotic spot virus on muskmelon. Japón. Jpn. Agr. Res. Q. 6(1):41-45. [ Links ]
Kwak, H. R.; Kim, J. S.; Cho, J. D.; Lee, J. H.; Kim, T. S.; Kim, M. K. and Choi, H. S. 2015. Characterization of melon necrotic spot virus occurring on watermelon in Korea. Pakistán. Plant Pathol. J. 31(4):379-387. [ Links ]
Kyriacou, M. C.; Soteriou, G. A.; Rouphael, Y.; Siomos, A. S. and Gerasopoulos, D. 2016. Configuration of watermelon fruit quality in response to rootstock‐mediated harvest maturity and postharvest storage. Reino Unido. J Sci Food Agriculture. 96(7):2400-2409. [ Links ]
Lee, J. M. 1994. Cultivation of grafted vegetables. I. Current status, grafting methods, and benefits. Estados Unidos de América. HortScience . 29(4):235-239. [ Links ]
Miceli, A.; Romano, C.; Moncada, A.; Piazza, G.; Torta, L.; D’Anna, F. and Vetrano F. 2016. Yield and quality of mini-watermelon as affected by grafting and mycorrhizal inoculum. J. Agr. Sci. Tech. 18:(2):505-516. [ Links ]
Minitab 16. 2010. Minitab Inc.Versión 16.1.0. [ Links ]
Moya, M. G.; Sáez, E.; Serrano, Y. y Gómez, J. 2009. Etiología de la muerte súbita de la sandía en invernaderos del sureste peninsular de España. España. Bol. San. Veg. Plagas . 35(4):617-628. [ Links ]
Osuna, Á. P.; Aguilar, S. J.; Fernández, P. S.; Godoy, H. H.; Corral, D. B.; Flores, M. J. P.; Borrego, P. A. y Olivas, E. 2012. Injertos en chiles tipo Cayene, jalapeño y chilaca en el noroeste de Chihuahua. México. Rev. Mex. Cienc. Agríc. 3(1):739-750. [ Links ]
Özmen, S.; Kanber, R.; Sarı, N. and Ünlü, M. 2015. The effects of deficit irrigation on nitrogen consumption, yield, and quality in drip irrigated grafted and ungrafted watermelon. Holanda. J. Integr. Agric. 14(5):966-976. [ Links ]
Ricárdez, S. M.; Huitrón, R. M. V.; Tello, M. J. C. and Camacho, F. F. 2010. Planting density for grafted melon as an alternative to methyl bromide use in Mexico. Holanda. Scientia Hort. 126(2):236-241. [ Links ]
Rodríguez, N.; Huitrón, M.V.; Díaz, M. and Camacho, F. 2008. Effect of different rootstocks on the production and quality of watermelon cv. Reina de Corazones. Bélgica. Acta Hort. 797(3/4):437-442. [ Links ]
Rouphael, Y.; Cardarelli, M.; Colla, G. and Rea, E. 2008. Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. Estados Unidos de América. HortScience . 43(3):730-736. [ Links ]
Rouphael, Y.; Cardarelli, M.; Rea, E. and Colla, G. 2012. Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Holanda. Photosynthetica. 50(2):180-188. [ Links ]
Sánchez, R. E.; Leyva, R.; Constán, A. C.; Romero, L. and Ruiz, J. M. 2012. Grafting under water stress in tomato cherry: improving the fruit yield and quality. Reino Unido. Ann. Appl. Biol. 161(3): 302-312. [ Links ]
Thies, J. A.; Ariss, J. J.; Hassell, R. L.; Olson, S.; Kousik, C. S. and Levi, A. 2010. Grafting for management of southern root-knot nematode, Meloidogyne incognita, in watermelon. Estados Unidos de América. Plant Dis. 94(10):1195-1199. [ Links ]
Thies, J. A.; Ariss, J. J.; Hassell, R. L.; Buckner, S. and Levi, A. 2015. Accessions of Citrullus lanatus var. citroides are valuable rootstocks for grafted watermelon in fields infested with root-knot nematodes. Estados Unidos de América. HortScience . 50(1):4-8. [ Links ]
USDA. 2006. United States standards for grades of watermelon. http://www.ams.usda.gov/amsv1.0/getfile?ddocname=stelprdc5050334/. [ Links ]
Received: January 00, 2018; Accepted: February 00, 2018











 text in
text in 


