Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.3 Texcoco abr./may. 2018
https://doi.org/10.29312/remexca.v9i3.1215
Articles
Chemical treatment of corn seed for control of trips (Frankliniella occidentalis) Pergande (Thysanoptera:Thripidae)
1Departamento de Parasitología Agrícola-Universidad Autónoma Agraria Antonio Narro. Calzada Antonio Narro 1923, Saltillo, Coahuila, México. CP 25315.
The objective of the present investigation was to evaluate the chemical control of trips Frankliniella occidentalis Pergande (Thysanoptera:Thripidae) with the application of four seed products (thiodicarb, acephato, bifenthrin + imidacloprid and thiametoxan + lambda cyalotrina) with two doses; one according to the dosage of the commercial house and the other 50% smaller, in the variety Roque 1. A randomized block design with four repetitions, 10 treatments and two controls, a hybrid (Ares®-Unisem) was used and a white one. The sampling of the density of trips was carried out by means of a manual aspirator and blue traps, the samplings were made from the emergence of the plants and during eight weeks, carrying out two samplings per week. The results show that the treatment with thiamethoxane + lambda cyalotrine presented the lowest density of trips with a value of 1.53 trips per plant, while the absolute control was the one with the highest value with 2.14.
Keywords: Zea mays L.; Frankliniella occidentalis; Pergande; Thysanoptera: Thripidae
El objetivo de la presente investigación fue evaluar el control químico de trips Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) con la aplicación de cuatro productos a la semilla (thiodicarb, acephato, bifentrina + imidacloprid y thiametoxan + lambda cyalotrina) con dos dosis; una de acuerdo a la dosificación de la casa comercial y la otra al 50% menor, en la variedad Roque 1. Se usó un diseño de bloques al azar con cuatro repeticiones, 10 tratamientos y dos testigos, un hibrido (Ares®-Unisem) y uno blanco. El muestreo de la densidad de trips se realizó mediante un aspirador manual y trampas azules, los muestreos se realizaron desde la emergencia de las plantas y durante ocho semanas, efectuando dos muestreos por semana. Los resultados muestran que el tratamiento con thiametoxan + lambda cyalotrina presentó la densidad de trips más baja con un valor de 1.53 trips por planta, mientras que el testigo absoluto fue el que presentó el valor más alto con 2.14.
Palabras clave: Zea mays L.; Frankliniella occidentalis; Pergande; Thysanoptera: Thripidae
Introduction
Maize (Zea mays L.) is cultivated practically throughout Mexico, in diverse climatic and soil conditions and its variety is such that the country has been considered the center of origin and diversity of this crop (Kato et al., 2009). In the state of Guanajuato, the Spring-Summer cycle is the one with the largest sowing surface and the environmental conditions are characterized by long periods of drought and high temperatures, which make the trips (Frankliniela occidentalis) the most important pest in the crop of corn (Castresona et al., 2008). The trips of the flower, is one of the most important agricultural pests in the world (Zhao, 2013) and initiate its damage during the first 20 to 25 days since the plant emerges, in some cases the damage can be so severe that the plant can present the symptom of lack of moisture (CESAVEG, 2008). This pest feeds, oviposits and transmits viruses in plants, which causes alterations in plant tissue as scars as well as other imperfections (Kay and Herron, 2010). If there is no control, the attack continues until the plant finishes growing, but the real damage causes it in the first days of emergency (Castresona et al., 2008).
The use of insecticides has been the main strategy used to control F. occidentalis (Bielza et al., 2007), presenting difficulties in their control due to their behavior, since the nymphs are found in the foliage, the pupae in the Soil, and the adult has great mobility (Lopes da Silva et al., 2003; Helyer and Brobyn, 2008). The high pressure to insecticides has led to the development of resistance to the main groups of insecticides (Zhang et al., 2008). Since the mid-1980s, resistance in populations of F. occidentalis has been the subject of research after its establishment and propagation in greenhouses and outdoor production (Lewis, 1998; Bielza et al., 2007).
The first reports of resistance were associated with toxaphene applied to cotton (Race, 1961; Bielza et al., 2007). While several studies have shown that populations of F. occidentalis in different parts of the world, have developed resistance to insecticides such as diazinon, methomyl, cypermethrine, permethrin, fenvalerate and imidacloprid (Guangyu et al., 1995; Kontsedalov et al., 1998; Bustillo, 2009) since that time, there have been cases of lack of efficacy in insecticides of the main chemical products (Herron and James, 2007); which shows that chemical combat must be conducted carefully (Rodríguez et al., 2003).
From this point of view, it is interesting to study the efficacy of new active materials and the form or period of application for the control of this pest. An alternative is the treatment of seeds, which is the application of biological, physical and chemical techniques and agents that provide the seed and the plant protection against the attack of insects and diseases in the early stages of cultivation (ISF, 2007). Seed treatments can be used as primary tools in a successful Integrated Pest Management plan for sustainable agriculture, because the pest is controlled with lower amounts of active ingredient per hectare and are not released into the atmosphere (FAO, 2012). Therefore, the objective of this research was to evaluate the biological effectiveness of different insecticides applied to corn seed for the control of trips (Frankliniella occidentalis).
Materials and methods
The research was conducted in the spring-summer 2013 agricultural cycle in the experimental field of Technological Institute of Roque, (ITR), located in Celaya, Guanajuato, at 20° 31’ 49” north latitude and 100° 48’ 16.8” west longitude, at a height of 1 713 meters above sea level (Fundacion Guanajuato Produce, 2014). Four chemical products were used to treat the seed; thiamethoxan + lambda cyalotrine, bifenthrin + imidacloprid, thiodicarb and acephate, of which two doses were applied; commercial dose (DC) and 50% of the commercial dose (-50% DC) applied to the seed of the variety Roque I, a commercial control, the hybrid Ares (Unisem®) treated with fipronil and an absolute (Table 1). A randomized complete block design with 4 repetitions and 10 treatments was used. Each experimental unit consisted of three rows of 5 m long and 75 cm of separation for a total area of 2 500 m2.
Table 1 Distribution of treatments applied to seed and doses.
| Trata | Active ingredient | Chemical group | Dose L-¹ | |
| T1 | Bifentrina + Imidacloprid | Pyrethroid/Neonicotinoid | 0.584 g | Commercial |
| T2 | Bifentrina + Imidacloprid | Pyrethroid/Neonicotinoid | 0.295 g | -50% DCb |
| T3 | Acephato | Organophosphorus | 0.966 g | Commercial |
| T4 | Acephato | Organophosphorus | 0.488 g | -50% DCb |
| T5 | Thiametoxan + lambda cyalotrina | Neonocotinoid/Pyrethroid | 0.943 mL | Commercial |
| T6 | Thiametoxan + lambda cyalotrina | Neonocotinoid/Pyrethroid | 0.481 mL | -50% DCb |
| T7 | Thiodicarb | Carbamates | 9.55 mL | Commercial |
| T8 | Thiodicarb | Carbamates | 5 mL | -50% DCb |
| T9 | Fipronil (commercial control) | Phenylpyrazoles | 0.51 mL | - |
| T10 | Absolute control | - | 0 | - |
a= treatments; b= commercial dose.
The application of the chemicals to the seed was done manually, using a plastic container with a capacity of 4 L. The seed was placed inside the container to which was added the active ingredient plus water for humidify the seed and impregnate the product, it was stirred for a period of 10, later the seed was placed in plastic trays to remove excess moisture.
For the agronomic management of the crop, a fallow was made at a depth of 30 cm, then two steps of harrowing and furrowing were carried out. The sowing was carried out dry and manually. An irrigation was applied after sowing and an irrigation of aid when the plant presented the VT stage. Only one weeding was performed mechanically in stage V3. At the time of planting, it was fertilized at a dose of 240-40-00, where it was used as a source of urea and ammonium phosphate. For weed control Sanson® selective herbicide (Nicosulfuron: 2- (4,6-dimethoxypyrimidin-2-licarbomoylsulfamoyl) -N, N-dimethylnicotinamide) was applied at a dose of 1 L ha-1. Finally, the secant herbicide Paraquat® for narrow leaf and 2-4-D® for wide leaf was applied when the crop was in physiological maturity.
The sampling of trips was made from the emergency, through the use of a manual vacuum cleaner and blue traps. For this, four plants were taken at random for each experimental unit. The sampling consisted of sweeping the entire plant with the vacuum cleaner from the neck, and behind the leaves and the head. Samples were taken every four days for eight weeks. All the collected insects were placed in transparent plastic bottles of 200 mL with water, identifying them with the data of treatment and date of collection for their later identification, which was done by taxonomic keys of Mound and Marullo (1996).
For the samples with blue traps two traps of 40 x 60 cm were placed for each experimental unit, which were placed inside a transparent plastic bag that was impregnated with glue (Spaider®) and placed at random. The counts of the trips in each trap were made twice a week for 8 weeks; which were made in the field. After the counts, the plastic bags were removed and new ones were placed.
For all the registered data, an analysis of variance (Anva) was performed using the proc Anova procedure of the statistical package SAS system for Windows ver. 9.0 (2002). The multiple comparison of the means was performed using the Tukey test (p≤ 0.05).
Results and discussion
In Table 2 the Anva is presented for the density of F. occidentalis in which a significant effect (p≤ 0.01) is observed for treatment, week of sampling and treatment interaction per week. The coefficient of variation was less than 22% and a coefficient of determination R2 of 0.823 indicating that there is 82% validity in the results.
Table 2 Squares of means for the density of trips (Frankliniella occidentalis) in corn plants.
| Source of variation | GL | Trips density (individuals plant-1) | ||
| Repetitions | 3 | 0.116 | ||
| Treatment | 9 | 1.107** | ||
| Week | 7 | 16.662** | ||
| Treatment x week | 63 | 0.302** | ||
| Error | 27 | 0.272 | ||
| R² | - | 0.823 | ||
| CV (%) | - | 21.495 |
**= significant at 0.01 probability.
In the Table 3 and 4 show the average squares for the samples taken in eight weeks, where a highly significant effect (p≤ 0.01) of the treatments evaluated in weeks 1, 2 and 8 is observed depending on the incidence of trips per plant, while in weeks 3, 4, 5, 6 and 7 the effect of the treatment was not observed.
Table 3 Mean squares for the behavior of trips in corn with chemical treatment of insecticides of different toxicological groups to the seed, from week 1 to 4.
| FV | GL | CM | ||||||||
| Weeks (trips plant-1) | ||||||||||
| 1 | 2 | 3 | 4 | |||||||
| Treatment | 9 | 0.635** | 1.166** | 0.518** | 0.284** | |||||
| EE | 30 | 0.225 | 0.394 | 0.255 | 0.117 | |||||
| Total | 39 | - | - | - | - | |||||
| CV (%) | - | 19.351 | 22.807 | 20.376 | 18.003 | |||||
**= statistical significance of 0.02 probability; ns= not significant.
Table 4 Mean squares for the behavior of trips in corn with chemical treatment of insecticides of different toxicological groups to the seed, from week five to week eight.
| FV | GL | CM | ||||||||
| Weeks (trips plant-1) | ||||||||||
| 5 | 6 | 7 | 8 | |||||||
| Treatment | 9 | 0.159ns | 0.282ns | 0.018ns | 0.157** | |||||
| EE | 30 | 0.101 | 0.139 | 0.056 | 0.059 | |||||
| Total | 39 | - | - | - | - | |||||
| CV (%) | - | 22.336 | 28.842 | 20.508 | 19.798 | |||||
**= statistical significance of 0.02 probability; ns= not significant.
Next, the results obtained in weeks 1, 2 and 8 are shown, which was where the experiment showed differences between treatments. For week 1, treatment with fipronil and acephate at -50% DC were those with the highest density with 3.09 and 2.82 trips per plant. Whereas, treatment with thiamethoxane + lambda cyalotrine to DC had the lowest density with 1.64 trips per plant (Table 5). These values are similar to those reported by Arias and Adrian (2012) in the cultivation of soybeans who used thiamethoxane + lambda cyalotrine at a dose of 200 mL ha-1, which presented a density of 1.6 trips per plant at 11 days later to the emergency.
Table 5 Mean behavior of trips density in maize for the week one sampling.
| Treatments | Active ingredient | Dose | Average (trips plant-1) | ||||
| 9 | Fipronil | - | 3.0928 | a | |||
| 4 | Acephato | 50% | 2.8205 | a | |||
| 10 | Witness | - | 2.7113 | ab | |||
| 8 | Thiodicarb | 50% | 2.5853 | ab | |||
| 3 | Acephato | Commercial | 2.4943 | ab | |||
| 2 | Bifentrina + Imidacloprid | 50% | 2.445 | ab | |||
| 1 | Bifentrina + Imidacloprid | Commercial | 2.2893 | ab | |||
| 7 | Thiodicarb | Commercial | 2.2658 | ab | |||
| 6 | Thiametoxan + lambda cyalotrina | 50% | 2.177 | ab | |||
| 5 | Thiametoxan + lambda cyalotrina | Commercial | 1.64 | b | |||
Stocks with the same letters are not statistically different.
On the other hand, in relation to the treatments that presented a higher incidence, our results are superior to those reported by Beltran et al. (2004) in the control of Frankliniella schultzei in the cultivation of cotton using thiamethoxan in a dose of 200 g of eg. in 100 kg of seeds, where a density of 0.2 trips per plant occurred 15 days after emergence. For Fipronil the results are lower than those reported by Bustillo (2009) in the asparagus crop, which presented a density of 11.4 trips per plant at 11 days after emergence. In this sense, we can mention that the best results with the product thiamethoxan + lamda cyhalothrin are due to the thiamethoxan adheres and penetrates quickly to the seed which allows stability and bioavailability of the active ingredient (Valarezo and Loor, 2013).
In week two the highest values of trips density were for the control treatments and thiamethoxan + lambda cyalotrine at -50% DC with values of 3.4 and 3.32 respectively. While the lowest value was observed in the thiodicarb treatment to DC with 1.85 trips per plant, there was a reduction in the population of 54.63% between the treatments with higher and lower density (Table 6). In relation to the thiodicarb product, our values are higher than those reported by Beltran et al. (2004) for the control of Frankliniella schultzei in cotton, where they observed a density of trips of 0.87 per plant 20 days after emergence. In this regard Valarezo and Loor (2013) mention that thiodicarb is an insecticide with rapid and residual action for the treatment of the seed, protecting it from the sowing until the first days after its germination.
Table 6 Behavior of the means in trips density in corn for the week 2 sampling.
| Treatments | Active ingredient | Dose | Average (trips plant-1) | ||||
| 10 | Witness | - | 3.4005 | a | |||
| 6 | Thiametoxan + lambda cyalotrina | 50% | 3.3253 | ab | |||
| 4 | Acephato | 50% | 3.32 | ab | |||
| 2 | Bifentrina + Imidacloprid | 50% | 3.0183 | ab | |||
| 9 | Fipronil | - | 2.82 | ab | |||
| 8 | Thiodicarb | 50% | 2.664 | ab | |||
| 1 | Bifentrina + Imidacloprid | Commercial | 2.646 | ab | |||
| 3 | Acephato | Commercial | 2.5295 | ab | |||
| 5 | Thiametoxan + lambda cyalotrina | Commercial | 1.9705 | ab | |||
| 7 | Thiodicarb | Commercial | 1.858 | b | |||
Stocks with the same letters are not statistically different.
For week 8, in Table 7 it can be observed that the thiodicarb treatment to DC was the one that presented the highest density of trips per plant (1.71) and the treatments bifenthrin + imidacloprid at -50% DC, bifenthrin + imidacloprid and acephate at DC expressed lower density of trips (1, 1.1, 1.1 and 1.18, respectively), there being a difference of 41.84% of trips incidence among the treatments that reported the highest and lowest values in week 8 of evaluation.
Table 7 Behavior of the means in trips density in corn for the week 8 sampling.
| Treatments | Active ingredient | Dose | Average (trips plant-1) | ||||
| 8 | Thiodicarb | 50% | 1.7195 | a | |||
| 5 | Thiametoxan + lambda cyalotrina | Commercial | 1.39 | ab | |||
| 7 | Thiodicarb | Commercial | 1.207 | ab | |||
| 10 | Witness | - | 1.207 | ab | |||
| 9 | Fipronil | - | 1.207 | ab | |||
| 6 | Thiametoxan + lambda cyalotrina | 50% | 1.207 | ab | |||
| 4 | Acephato | 50% | 1.183 | ab | |||
| 3 | Acephato | Commercial | 1.1035 | b | |||
| 1 | Bifentrina + imidacloprid | Commercial | 1.1035 | b | |||
| 2 | Bifentrina + imidacloprid | 50% | 1 | b | |||
Stocks with the same letters are not statistically different.
These results are in contrast to those reported by Machaca (2012) in the control of trips (Trips tabaci L.) in the cultivation of onion using imidacloprid at a dose of 50 mL per L in which an average of 2.88 of Trips tabaci was observed per plant at 63 days after the application. While, Larral and Ripa (2007) reported a presence of zero Heliotrips haemorrhoidalis 60 days after application in avocado plants (Persea amaricana Mill) using imidacloprid at a dose of 90 mL hL-1.
On the other hand, Table 8 shows the behavior of the means in the density of trips at eight weeks of sampling; where thiamethoxane + lambda cyalotrine treatment to DC was the one that reported the lowest density of trips per plant with 1.53, followed by thiodicarb treatment of high dose with value of 1.62, while the control was the one with the highest value with 2.14, followed by thiodicarb at -50% DC with 1.97 trips per plant. A difference of 28.31% can be observed between the highest and the lowest value. These results differ from those reported by Larral and Ripa (2007) in a study carried out on the chemical control of Heliotrips haemorrhoidalis in avocado cultivation (Persea amaricana Mill) using thiamethoxan at a dose of 20 g hL-1 who reported a null presence of H. haemorrhoidalis 60 days after application.
Table 8 Behavior of means in the incidence of trips in corn during a period of eight weeks of sampling.
| Treatments | Active ingredient | Dose | Average (trips plant-1) | ||||
| 10 | Witness | - | 2.14134 | A | |||
| 8 | Thiodicarb | 50% | 1.97634 | Ab | |||
| 9 | Fipronil | - | 1.96809 | Abc | |||
| 4 | Acephato | 50% | 1.91847 | Abcd | |||
| 2 | Bifentrina + imidacloprid | 50% | 1.88878 | Abcd | |||
| 6 | Thiametoxan + lambda cyalotrina | 50% | 1.88766 | Abcd | |||
| 3 | Acephato | Commercial | 1.78666 | Bcde | |||
| 1 | Bifentrina + imidacloprid | Commercial | 1.65956 | Cde | |||
| 7 | Thiodicarb | Commercial | 1.62434 | De | |||
| 5 | Thiametoxan + lambda cyalotrina | Commercial | 1.53494 | E | |||
Stocks with the same letters are not statistically different.
The effectiveness of thiamethoxan + lamda cyhalothrin in the study we can mention that it is an effective insecticide to control this pest (Shan et al., 2012), and is considered a second generation nicotinoid (Maienfisch et al., 2001; Naun et al., 2003), which act on the central nervous system, specifically on the nicotinic acetylcholine receptor (Domínguez, 2014).
Neonicotinoids are widely used in the treatment of seeds (Proietto et al., 2013), since their distribution in the seed and in the plant, is slowly metabolized and is available for a long period of time close to 30 days (Clavijo, 2008).
In the Figure 1 shows the reported results for the density of trips per week, week 2 is observed to have the highest number of trips, with an average of 2.75 trips per plant, followed by week 1 and 3 with values of 2.45 and 2.47 respectively. While week 7 was the one that presented the lowest density with 1.16 trips per plant, there is a difference of 57.78% between week 2 and week 7. These results agree with those reported by Díaz (1994) where it mentions that the attack more severe trips (F. occidentalis) is mainly during 15 to 25 days after the plant has emerged.
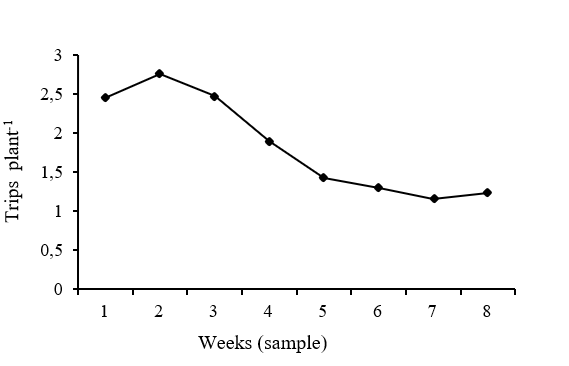
Figure 1 Behavior of the means in the density of trips in corn per week, during eight weeks of sampling.
In Figure 2, the behavior of the density of trips in relation to the climatic conditions during the sampling period is presented, where it can be observed that in weeks 1, 2 and 8 the highest values of maximum temperature were presented with 29.97, 29.69 and 27.83 °C respectively, also this week the highest density of trips per plant (2.45, 2.75 and 1.23). Regarding Ascencio (2000), he points out that high temperatures and absence of rains (Valenzuela-García et al., 2010), favor the increase in the population density of trips, for which it is considered that temperature and precipitation are two abiotic factors that interact on the development of trips populations, since there is a highly positive correlation between air temperature, soil temperature, and relative humidity with the population density of trips (Driutti, 2000).
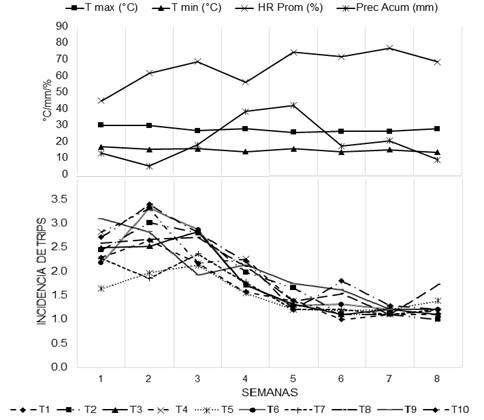
Figure 2 Behavior of the climatic conditions (maximum temperature, minimum temperature, relative humidity and accumulated precipitation) and the density of trips in a sampling period of eight weeks.
On the other hand, minimum temperatures were reported in weeks 1, 2, 3 and 5 with 16.7, 15.2, 15.6 and 15.5 °C respectively. And the accumulated precipitation presented the highest values for weeks 3, 4, 5, 6 and 7 with 18, 38.4, 42, 17.2 and 20.4 mm. These conditions favored the lower density of trips with 2.47, 1.9, 1.42, 1.29 and 1.16 respectively, because rainfall is an important factor for the control of trips as it cleans the leaves causing the trips to fall to the ground (Valle et al., 2003), which meant that the sampling in these weeks did not show significant differences between treatments in the density of trips, since together with the temperature, the number of hours of light interacts negatively in the population development of trips (Drutti, 2000).
According to the data shown in Figure 2, in week two the climatic conditions were presented with values of a maximum and minimum temperature (29.69 and 15.21 °C), a relative humidity of 61.66% and a cumulative rainfall of 5 mm, which allowed this week to have the highest incidence of trips in corn plants, these conditions affected insect populations, resulting in a decrease in the reproductive rate. In this sense, it has been established that temperature is the main environmental condition that interacts in the population development of trips (Driutti, 2004).
Conclusions
The lower density of F. occidentalis in the maize crop was presented with the Thiametoxan + lambda cyalotrina treatment in commercial dose (0.943 mL L-1) applied to the seed, with an average of 1.53 trips per plant, exceeding 28.62% to the absolute witness.
At 15 days after corn germination, the highest density of trips per plant was found with 2.7552.
The chemical treatment of corn seed reduces the density of F. occidentalis during the 45 days after germination.
When temperatures are high and precipitation is low it helps that trips populations increase in the plant, on the other hand, if the precipitation is high it has a positive effect on the control of F. occidentalis, since it is the lava of the plant.
Literatura citada
Arias, N. y Adrián, M. 2012. Control de trips en el cultivo de soja. INTA EEA Concepción del Uruguay. División tratamiento de semillas. GLEBA SA. [ Links ]
Asencio, B. G. 2000. Fluctuación poblacional, daño e identificación de trips del aguacate cv. Hass en Michoacán, México. Tesis de Maestría en Ciencias. [ Links ]
Bayer Crop Sciencie. 2007. Semevin 350 FS. http://www.bayercropsciencie.com.ec./productdesc.aspx?prodid=11 . [ Links ]
Beltrán, R.; Helman, S. y Peterlin, O. 2004. Control de Caliothrips phaseoli Hood y Frankliniella schultzei Trybon y Aphis gossypii Glover con insecticidas sistémicos aplicados a las semillas de algodón. INTA, Argentina. [ Links ]
Bielza, P.; Quinto, V.; Contreras, J.; Torne, M.; Martín, A. and Espinosa, P. J. 2007. Resistance to spinosad in the western flower thrips, Frankliniella occidentalis (Pergande), in greenhouses of south-eastern Spain. Pest Manag. Sci. 63:682-687. [ Links ]
Bustillo, P. A. E. 2009. Evaluación de insecticidas químicos y biológicos para controlar Frankliniella occidentalis (Thysanoptera: Thripidae) en cultivos de espárragos. Rev. Colomb. Entomol. 35(1):12-17. [ Links ]
Catresana, J.; Gagliana, E.; Puhl, L.; Bado, S.; Vianna, L. y Castresana, M. 2008. Atracción del Trips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) con trampas de luz en un Cultivo de Gerbera jamesonii (G). IDESIA. 26(3). [ Links ]
Cesaveg, 2008. Comite estatal de sanidad vegetal Guanajuato. Manual de plagas y enfermedades del maíz. Campaña Manejo Fitosanitario del Maíz. [ Links ]
Clavijo, J. 2008. Tiamethoxam: Un Nuevo concepto en vigor y productividad. Ed. M. Manrique. Bogota. DC. Co. 196 p. [ Links ]
Driutti, A. 2004. Control biológico natural de trips, Thrips tabaco Lindeman 1888 (Thysanoptera: Thripidae) por surtidos depredadores en el cultivo de cebolla (Allium cepa L.) por el cultivo de bordaduras y/o entrelineas. Instituto Agronómico “Pedro M. Fuentes Godo”. UNNE. Las Heras 727, Chaco, Argentina. [ Links ]
Drutti, A. A. 2000. Control biológico natural de Trips, Thrips tabaci Lindeman 1888 (Thysanoptera: Thripidae) por sírfídos predadores en cultivo de cebolla (Allium cepa L.) por el cultivo de borduras y/o entrelineas. Universidad Nacional Del Nordeste. Comunicaciones Científicas y Tecnológicas 2000. Instituto Agrotécnico “Pedro M. Fuentes Godo” - UNNE. [ Links ]
FAO. 2012. (Food and Agriculture Organization). http://www.fao.org. [ Links ]
Fundación Guanajuato Produce. 2014 a. Fundación Guanajuato Produce http://www.fundacionguanajuato.com/CGI-BIN/Clima/index.php. [ Links ]
Guangyu, Z.; Wei, L.; Brown, J. M. and Knowles, C. O. 1995. Insecticide resistance in field and laboratory strains of western flower trips (Thysanoptera: Thripidae). J. Econ. Entomol. 88(5):1164-1170. [ Links ]
Helyer, N. L. and Brobyn, P. J. 2008. Chemical control of western flower thrips (Frankliniella occidentalis Pergande). Ann. Appl. Biol. 121(2):219-231. [ Links ]
Herron, G. A. and James, T. M., 2007. Insecticide resistance in Australian populations of western flower thrips, Frankliniella occidentalis Pergande (Thysanoptera: Thripidae). Gen. Appl. Ent. 36:1-5. [ Links ]
ISF. 2007. El tratamiento de semillas, una herramienta para la agricultura sostenible. Federación internacional de semillas. Federation internationale du commerce des semences. Chemin du reposir 7. CH-1260 NYON/Suiza. [ Links ]
Kato, Y. T. A.; Mapes, S. C.; Mera, O. L. M.; Serratos, H. J. A. y Bye B., R. 2009. Origen y diversificación del maíz: una revisión analítica. Universidad Nacional Autónoma de México (UNAM)-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). México, D. F. 116 p. https://www.biodiversidad.gob.mx/genes/pdf/origen_div_maiz.pdf. [ Links ]
Kay, I. R. and Herron, G. A. 2010. Evaluation of existing and new insecticides including spirotetramat and pyridalyl to control Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) on peppers in Queensland. Australian J. Entomol. 49(2):175-181. [ Links ]
Kontsedalov, S.; Weintraub, P. G.; Horowitz, A. R. and Ishaaya, I. 1998. Effects of insecticides on immature and adult western flower thrips (Thysanoptera: Thripidae) in Israel. J. Econ. Entomol. 91(5):1067-1071. [ Links ]
Larral, P. y Ripa, R. 2007. Evaluación de la efectividad de pesticidas para el control de Heliotrips haemorrhoidalis (Thysanptera: Thripidae) sobre el Palto (Persea americana Mill). Instituto de Investigaciones Agropecuarias, casilla 3 La Cruz. [ Links ]
Lewis T, 1998. Chemical control, in thrips as crop pests. (Ed.). Lewis, T. CAB International, New York, NY. 567-594 pp. [ Links ]
Lopes Da, S. A.; Da Silva, N. F.; Pires, L. L.; Ferreira, H. De J.; Caetano, B. V. e Peixoto, Dos S. L. 2003. Eficiência agronômica de inseticidas no controle do Thrips tabaci Lind., 1888 (Thysanoptera, Thripidae) na cultura do alho. Pesquisa Agropecuária Tropical. 33(1):39-42. [ Links ]
Manchaca, V. A. 2012. Comparación de efectividad de distintos insecticidas en el control de “trips” Thrips tabaci L., en el cultivo de cebolla (Allium cepa L.) cultivar sivan, en el proter- sama. Universidad Nacional Jorge Basadre. Facultad de Ciencias Agropecuarias Escuela Académico Profesional de Agronomía. Perú. [ Links ]
Mound, L. A. and Marullo, R. 1996. The thrips of central and south America: an introduction (Insecta; Thysanoptera). Memoirs on Entomol. Int. vol. 6. 487 p. [ Links ]
Nadal, A. y Wise, T. A. 2005. Los costos ambientales de la liberación agrícola: el comercio del maíz en México y Estados Unidos en el marco del NAFTA. Globalización y medio ambiente. Lecciones desde las Américas. RIDES-GDAE. Santiago de Chile. 49-92 pp. [ Links ]
Nauen, R. U.; Ebbinghaus, K.; Salgado, V. L. and Kaussmann, M. 2003. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants, Pestic. Biochem. Physiol. 76:55-69. [ Links ]
Proietto, M.; Scordino, M.; Sabatino, L.; Pantò, V.; Morabito, G.; Chiappara, E.; Traulo, P. and Gagliano, G. 2013. UHPLC/MS-MS analysis of six neonicotinoids in honey by modified QuEChERS: method development, validation, and uncertainty measurement. International J. Food Sci. 1:1-8. [ Links ]
Race, S. R. 1961. Early-season trips control on cotton in New Mexico. J. Econ. Entomol. 54:974-976. [ Links ]
Rodríguez, I; Duran, I; Morales, H. y Cardona, C. 2003. Líneas base, dosis diagnóstico y medición periódica de resistencia a imidacloprid, spinosad y carbosulfan en poblaciones de adultos de Trips palmi (Thysanoptera: Thripidae) en el Valle del Cauca, Colombia. Rev. Colomb. Entomol. 29(1):29-33. [ Links ]
SAS Institute Inc. 2002. Guide for personal computers. SAS Institute, Cary. NC. USA. [ Links ]
Shan, C.; Ma, S.; Wang, M. and Gao, G. 2012. Evaluation of insecticides against the western flower thrips, Frankliniella occidentals (Thysanoptera: Thripidae), in the laboratory, Fla. Entomol. 95:454-460. [ Links ]
Syngenta 2006. Powered by crusier inversión que si crece. Manual 17 p. [ Links ]
Valarezo, C. O. y Loor, A. L. 2013. Efecto del tratamiento insecticida a la semilla de maíz antes de la siembra.Revista la Técnica.26-33 [ Links ]
Zhang, S.Y.; Kono, S.; Murai, T. and Miyata, T. 2008. Mechanisms of resistance to spinosad in the western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Insect Sci 15:125-132. [ Links ]
Zhao, M.; Ho, H.; Wu, Y.; He, Y. and Li, M. 2014. Western flower thrips (Frankliniella occidentalis) transmits Maize chlorotic mottle virus. J. Phytopathol. 162(7-8):532-536. [ Links ]
Received: February 00, 2018; Accepted: March 00, 2018











 texto en
texto en 


