Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.3 Texcoco Abr./Mai. 2018
https://doi.org/10.29312/remexca.v9i3.1213
Articles
Isolated native Bacillus thuringiensis from southeastern Mexico
1División de Estudios de Posgrado e Investigación-Instituto Tecnológico de Conkal. Avenida Tecnológico s/n, Conkal, Yucatán. CP. 97345. (alexgara24@gmail.com; arturo.reyes@itconkal.edu.mx; esau.ruiz@itconkal.edu.mx).
2Centro de Investigación y de Estudios Avanzados-Instituto Politécnico Nacional-Unidad Irapuato. Libramiento norte carretera Irapuato-León km 9.6, Irapuato, Guanajuato. CP. 36500. (jibarra@ira.cinvestav.mx).
In order to find native isolates of Bacillus thuringiensis, in the present work isolations were made from different materials obtained from the southeast of Mexico, in the period from March 2014 to July 2015. Bacterial colonies were selected based on the typical characteristics of the Bacillus genus and were identified as Bacillus thuringiensis, based on the presence of parasporal crystals, cry gene, hag gene, protein profile and insecticidal activity. An isolate of soil samples (ITCBT34) and two larvae of Chlosyne lacinia (ITCBT61, ITCBT62) was obtained. These isolates presented amplification of the cry and hag genes. Isolate ITCBT34 presented crystals with oval morphology, while isolates ITCBT61 and ITCCBT62 presented dipyramidal crystals. Differences were observed in the size of the fragment produced by the amplification of the hag gene and in the protein profile of the different isolates. The insecticidal activity was determined by crude bioassay with a concentration of 10 μg cm-2. The three isolates caused 100% mortality of Manduca sexta larvae at 96 h of exposure. Isolates ITCBT61 and ITCBT62 showed morphological characteristics and size of the hag gene similar to the reference strain B. thuringiensis svar. kurstaki HD1, the isolate ITCBT34 showed no similarity, so it could be an isolate not related to serovar kurstaki HD1.
Keywords: Chlosyne lacinia; Manduca sexta; gene cry; gene hag
Con el objetivo de encontrar aislados nativos de Bacillus thuringiensis, en el presente trabajo se realizaron aislamientos a partir de diferentes materiales obtenidos del sureste de México, en el periodo de marzo 2014 a julio 2015. Se seleccionaron colonias bacterianas con base a las características típicas del genero Bacillus y se identificaron como Bacillus thuringiensis, con base a la presencia de cristales parasporales, gen cry, gen hag, perfil de proteínas y actividad insecticida. Se obtuvo un aislado de muestras de suelo (ITCBT34) y dos de larvas de Chlosyne lacinia (ITCBT61, ITCBT62). Estos aislados presentaron amplificación de los genes cry y hag. El aislado ITCBT34 presentó cristales con morfología oval, mientras que los aislados ITCBT61 e ITCCBT62 presentaron cristales bipiramidales. Se observaron diferencias en el tamaño del fragmento producido por la amplificación del gen hag y en el perfil de proteínas de los diferentes aislados. La actividad insecticida se determinó mediante bioensayo burdo con una concentración de 10 µg cm-2. Los tres aislados causaron 100% de mortalidad de larvas de Manduca sexta a las 96 h de exposición. Los aislados ITCBT61 e ITCBT62 mostraron características morfológicas y tamaño del gen hag similares a la cepa de referencia B. thuringiensis svar. kurstaki HD1, el aislado ITCBT34 no presentó similitud, por lo que podría tratarse de una aislado no relacionado al serovar kurstaki HD1.
Palabras clave: Chlosyne lacinia; Manduca sexta; gen cry; gen hag
Introduction
Bacillus thuringiensis is the most used entomopathogen in the biological control of agricultural pests due to its high insecticidal specificity, which is conferred by the ability to form parasporal inclusions of a protein nature, called Cry proteins (Schnepf et al., 1998). The nomenclature of Cry proteins is based on the amino acid sequence, this allows to classify the closely related toxins (Crickmore et al., 1998), Cry proteins have insecticidal activity against insect larvae of the orders Lepidoptera, Diptera and Coleoptera (Burges, 1982) and in some cases also against hemiptera (Torres-Quintero et al., 2015), hymenoptera (van Frankenhuyzen and Tonon, 2013), mites (Erban et al., 2009) and nematodes of agricultural importance (Li et al., 2008).
There are reports of B. thuringiensis isolates from diseased or dead insect larvae due to pathogenesis, phylloplane or internal parts of plants (Monnerat et al., 2009), in this case it has the property of using B. Thuringiensis against insects difficult to control for their feeding habits, such as suckers or borers. Therefore, there is an interest in the search for new isolates, with the aim of finding strains that show novel insecticidal activity or with greater activity than isolates and strains of known references (Saadaoui et al., 2010). B. thuringiensis isolated from soil were significantly more toxic against Pieris brassicae, Ephestia kuehniella (Senfi et al., 2012), Heliothis armigera and Plutella xylostella (Xavier et al., 2007), than the reference strains.
Strains of B. thuringiensis native to Mexico showed high toxicity towards Spodoptera frugiperda when compared with the commercial standard strain HD1 (Vázquez-Ramírez et al., 2015). Strain LBIT-1200, isolated from soil showed greater toxicity activity against Manduca sexta and Trichoplusia ni compared to strain HD-73 (Reinoso-Pozo et al., 2016). The characterization of isolates is important to determine if they are known varieties or to determine new strains of B. thuringiensis with their own characteristics. In this regard, several reports present characterization of B. thuringiensis isolates that include the morphology of the parasporal body (Azizoglu et al., 2011), insecticidal activity (Alper et al., 2014) and molecular characterization (Sauka et al., 2010; Patel et al., 2012).
Recently, the sequencing of the hag genes that encode the proteins responsible for the formation of flagellin, which is an alternative to the serotyping technique and that allows the relationship between serotypes of B. thuringiensis (Reinoso-Pozo et al., 2016). Based on previous studies, the objective of this work was to isolate native strains of B. thuringiensis from different types of samples, which have potential to be used in the biological control of agricultural pests of economic importance in the southeast region of Mexico.
Materials and methods
Isolation of B. thuringiensis
Samples obtained from soil, larvae of Spodoptera frugiperda J.E.Smith (Lepidoptera: Noctuidae), larvae of Chlosyne lacinia ssp. lacinia Geyer (Lepidoptera: Nymphalidae), adults of Aidemona azteca Saussure (Orthoptera: Acrididae), leaves of Nerium oleander L. and stubble of Zea mays L. These materials were obtained from different regions of the Mexican Southeast the soil samples were collected eight in the state of Tabasco, eight in Yucatan, one in Campeche and one in Quintana Roo. Regarding the other materials were obtained, 23 samples of larvae of C. lacinia, two samples of adults of A. azteca and four samples of leaves of N. oleander in the municipality of Conkal, six samples of larvae of S. frugiperda and five samples of corn stubble in the municipality of Tekax, both in the state of Yucatán (Table 1). For the isolation of bacteria from soil, 5 g of the sample were weighed and placed in a 15 mL Falcon tube, which was calibrated with sterile distilled water until said volume and the mixture was resuspended by vigorous vortexing for 1 min. It was pasteurized in a water bath at 80 °C for 15 min and cooled immediately on ice.
Table 1 Description of the type and number of samples collected, Municipality and State, climate and soil characteristics.
| Samples (number) | Collection place | Climate characteristics* | Type of soil* |
| Soil (2) | Huimanguillo, Tabasco | Warm humid with rain all year | Plintosol |
| Soil (1) | Jalapa, Tabasco | Warm humid with rain all year | Leptocol |
| Soil (1) | Teapa, Tabasco | Warm humid with rain all year | Luvisol |
| Soil (1) | Cunduacán, Tabasco | Warm humid with rains in summer | Phaeosem |
| Soil (1) | Cárdenas, Tabasco | Warm humid with rains in summer | Vertisol |
| Soil (1) | Paraíso, Tabasco | Warm humid with rains in summer | Gleysol |
| Soil (1) | Centro, Tabasco | Warm humid with rains in summer | Solonchak |
| Soil (1) | Baca, Yucatán | Warm subhumid with rain in summer | Phaeosem |
| Soil (2) | Conkal, Yucatán | Warm subhumid with rain in summer | Histosol |
| Soil (1) | Dzidzantun, Yucatán | Very warm and warm semi-dry | Histosol |
| Soil (1) | Acanceh, Yucatán | Warm subhumid with rain in summer | Histosol |
| Soil (1) | Chabihau, Yucatán | Very warm and warm semi-dry | Lixisol |
| Soil (1) | Tekax, Yucatán | Warm subhumid with rain in summer | Solonchak |
| Soil (1) | DB, Yucatán | Very warm and warm semi-dry | Lixisol |
| Soil (1) | BJ, Quintana Roo | Warm subhumid with rain in summer | Leptosol |
| Soil (1) | Calakmul, Campeche | Warm subhumid with rain in summer | Phaeosem |
| Corn stubble (5) | Tekax, Yucatán | Warm subhumid with rain in summer | Solonchak |
| N. oleander (4) | Conkal, Yucatán | Warm subhumid with rain in summer | Histosol |
| S. frugiperda (10) | Tekax, Yucatán | Warm subhumid with rain in summer | Solonchak |
| C. lacinia (23) | Conkal, Yucatán | Warm subhumid with rain in summer | Histosol |
| A. azteca (2) | Conkal, Yucatán | Warm subhumid with rain in summer | Histosol |
*= INEGI (2017a, b, c, d); DB= Dzilam de Bravo; BJ= Benito Juárez.
The 5 mL of the suspension was taken and added to a flask containing 50 mL of nutrient broth (BD Bioxon®) and 0.25 M sodium acetate (Fermont) (to inhibit the germination of B. thuringiensis spores), incubated for 4 h at 200 rpm and at 29 °C. Then 1 mL of the culture was taken and diluted in 1 mL of sterile distilled water. This sample was again subjected to the pasteurization process, which is recommended to inactivate the growth of other microorganisms in the culture medium (Travers et al., 1987). At the end of the second pasteurization process, an aliquot of 100 μL was taken and evenly distributed in a Petri dish with nutritive agar. The Petri dishes were incubated at 30 °C for 24 h to favor the germination of B. thuringiensis spores and the development of bacterial colonies.
For the bacterial isolation from larvae and adult insects, 3 to 5 individuals were processed per sample depending on the size of these, which were macerated directly in the tube with the help of a glass rod and then hardened with sterile distilled water until 15 mL, vigorously shaken (Aldebis et al., 1994). In the case of isolates from plant leaves, 2 g of the sample was weighed, disinfected in 2% sodium hypochlorite solution, for 10 min and double washing was applied with distilled water, the sample was finely macerated in a mortar and 15 mL of sterile distilled water were added, the solution was transferred to a 15 mL Falcon tube (Monnerat et al., 2009). In both cases, a pasteurization process was carried out and the described process was continued to obtain isolates from soil samples.
Individual colonies were selected according to the typical characteristics reported for Bacillus (Sneath, 1986). Subsequently tests were performed to verify positive Gram stain and positive catalase reaction. Selected isolates were individually cultured on nutrient agar (Bioxon®) and stored at 4 °C for further studies.
Morphological characterization of the parasporal body
The presence and parasporal morphology of the selected isolates was carried out by direct observation of the crystals under DM500 optical microscope (Leica Microsystems, Switzerland) (1000X), morphology was confirmed by direct smear in phase contrast and staining with Coomassie blue (Sharif and Alaeddinoĝlu, 1988).
Extraction of genomic DNA
Total DNA extraction was performed according to the modified method reported by Rosso and Delécluse (1997). A bacterial culture of 2 mL was obtained in Luria-Bertani® medium (Invitrogen) at 28 °C with constant agitation at 250 rpm overnight, centrifuged at 8 000 rpm for 5 min, the supernatant and the cell pack were removed it was resuspended in 500 μL of buffer J (1.0 M TrisHCl, 0.1 M EDTA and 0.15 M NaCl, pH 8). Again, it was centrifuged under the same conditions as above the cell pack was resuspended in 300 μL of buffer J containing 40 mg mL of lysozyme (Sigma Aldrich Quimica) and incubated at 37 °C for 1 h. Subsequently, 20 μL of 10% SDS was added, it was homogenized gently and incubated at 70 °C for 20 min.
The 5 μL of RNAse (Promega) (10 mg mL), 10 μL of proteinase K (10 mg mL) (Thermo Fisher Scientific) were added and incubated at 60 °C for 90 min. 50 μL of 5 M NaCl (Baker, J. T.) was added and incubated on ice while stirring constantly. It was centrifuged at 13 000 rpm for 20 min, the supernatant was recovered, precipitated with the same volume of isopropanol (Sigma) and incubated at -70 °C for 30 min. Subsequently, it was centrifuged under the above conditions and the supernatant was removed, the pellet was washed with 200 μL of 70% ethanol (Fermont) and allowed to dry at room temperature, the DNA was resuspended in 20 μL of sterile distilled water and preserved at -20 °C until use.
Amplification of cry and hag genes
To determine the presence of cry genes in the isolates, PCR amplification was performed with the universal primers of block 1 (5’ TATGCWCAAGCWGCCAATYTWCATYT3’) and block 5 (5’ GGRATAAATTCAATTYKRTCWA 3’) (Sigma Aldrich Química) according to Noguera and Ibarra (2010).
The presence of the hag gene of flagellin in the native isolates was determined by PCR with the specific primers Bthag-F1 (5’-AGTACATGCGCCAAAACCAAG) and Bthag-R1 (5’-GTTTGCTTGAGAAAGCATGCT) (Sigma Aldrich Chemistry) according to Xu and Cote (2006). The PCR products were verified by 0.8% agarose gel electrophoresis (Invitrogen) and visualized in a Biorad Molecular Imager Gel Doc XR photodocument (Bio-Rad®).
Protein profile
The protein profile was obtained by SDS-PAGE electrophoresis of the spore-crystal complex of the isolates according to the reported methodology (Laemmli 1970). For this, each isolate was inoculated in 5 mL of LB medium and incubated in an orbital shaker at 30 °C with 200 rpm for 72 h. The spore-crystal complex was centrifuged at 10 000 rpm for 10 min and washed with sterile distilled water, this operation was repeated three times. The tablet was recovered and stored at 4 °C. A mixture was prepared with 5 μL of loading buffer with 5% β-mercaptoethanol (Sigma Aldrich Química), 3 μL of the spore-crystal complex sample and 2 μL of water, incubated at 95 °C for 10 min.
For the electrophoresis a 12% separating gel and the 4% polyacrylamide compactor (Sigma Aldrich Química) were prepared, the components used were 30% polyacrylamide, Tris-HCL buffer pH 8.8 (Sigma Aldrich Química), Tris-HCL buffer pH 6.8, 10% SDS, sterile distilled water, TEMED (Invitrogen®) and 10% ammonium persulphate (Sigma Aldrich Química), a volume of 5 mL was prepared for each gel and the concentrations were calculated according to the instructions in the manufacturer’s manual. Electrophoresis was performed in a modular vertical electrophoresis chamber Enduro™ (Bio-Rad), using the Page Ruler™ Plus Prestained Protein Ladder molecular weight marker (Bio-Rad®).
The electrophoresis was carried out in two phases, that of compaction at 40 V and 100 A for 1 h and the separation phase was carried out at 90 V and 100 A for 3 h. Subsequently the gel was stained with a solution of Coomassie blue dye G-250 0.1% for 1 h and stained in methanol/acetic acid. The molecular weight of the protein bands present in each sample was estimated according to the molecular weight marker used. The protein profiles observed were compared with the profile of the reference strain B. thuringiensis subsp. kurstaki HD1.
Detection of insecticide activity
A preliminary test was carried out at a high concentration with the isolates obtained to determine the insecticidal activity against Manduca sexta, since several studies report low lethal doses for several lepidopteran insects ranging from 1 to 164 ng cm-2 (Uribe et al., 2003; Sharma et al., 2010). The lyophilisate of the spore-crystal complex was obtained in a Labconco Lyph-Lock 4.5 lyophilizer (Labconco®), a final concentration of 10 μg cm-2 was used. The sample was weighed and diluted in 200 μL of Tween 80 (Sigma Aldrich Chemistry) (0.02%), added to the Petri dish with the artificial diet (Yamamoto, 1969) for the insect and distributed evenly over the entire surface of the diet, the surface was allowed to dry for 45 min and 20 neonatal larvae were placed for each isolate with two repetitions and the mortality was recorded at 96 h, as a negative control, 0.02% Tween 80 was used.
Results and discussion
Isolation of B. thuringiensis
Sixty-two samples of various collected materials were processed, including 18 soil samples, 10 of Spodoptera frigiperda, 23 of Chlosyne lacinia ssp. lacinia, two of Aidemona azteca, four of Nerium oleander leaves and five samples of stubble of Zea mays. A total of 210 colonies were selected, of which only 27 were observed in the presence of structures in the sporangia, among which were observed spherical, amorphous, bipyramidal, cubic, ovoid and some bodies adhered to the spores. The Coomassie blue staining test (Figure 1) confirmed the presence of parasporal crystals in only three isolates, which were designated with the ITCBT34, ITCBT61 and ITCBT62 keys (Table 2). These selected isolates were used in subsequent tests.
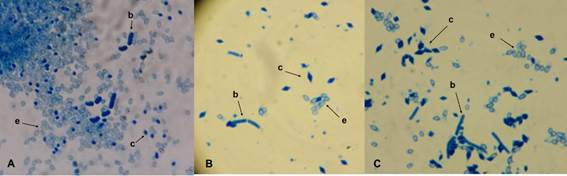
Figure 1 Morphology of the parasporal body by staining with Coomassie blue. A= ITCBT34. B= ITCBT61. C= ITCBT62; b) vegetative cell; c) parasporal crystal; and e) spore.
Table 2 Characteristics of the native isolates of Bacillus thuringiensis.
| Isolated | Habitat | Collection site | Location of the site (GPS) | Parasporal morphology | Genes cry | Genes hag |
| ITCBT34 | Soil | Huimanguillo, Tabasco | 17°53’26.31” 93°26’32.48” | Ovoid | + | + |
| ITCBT61 | Chlosyne lacinia ssp. lacinia | Conkal, Yucatán | 21°4’45.85” 89°29’59.46” | Dipyramidal | + | + |
| ITCBT62 | Chlosyne lacinia ssp. lacinia | Conkal Yucatán | 21°4’16.55” 89°30’24.16” | Dipyramidal | + | + |
Previous studies have reported obtaining native B. thuringiensis isolates from habitats such as soil (Adbullah et al., 2014), plants (Alper et al., 2014) and insects (Alquisira-Ramírez et al., 2014). In this diversity of environments, it is possible to find isolates that have dipyramidal, spherical, cubic crystals (Cicero et al., 2009, Azizoglu et al., 2011) and irregular ones (Assaeedi et al., 2011). In relation to southeastern Mexico, there are reports of B. thuringiensis isolates only from soils in the states of Tabasco (Bravo et al., 1998) and Yucatán (Ornelas-Pérez et al., 2016).
In the present work the selected isolates that were confirmed as B. thuringiensis presented oval crystals, sample obtained from soil, and dipyramidal samples obtained from larvae of Chlosyne lacinia (Table 2). The isolation index of B. thuringiensis obtained was 0.11. This result is within the values previously reported, since it is possible to find reports with indexes of 0.04 to 1 (Rosas-García et al., 2008; Alper et al., 2014).
Cry gene amplification
The three isolates showed the presence of cry genes, presented amplification bands with approximate sizes of 1400 bp (ITCBT61), 1500 bp (ITCBT62), 1650 bp (ITCBT34) and 1300 bp in the reference strain (HD1) (Figure 2a). The use of universal primers is common to determine the presence of cry genes (Patel et al., 2012; Reinoso-Pozo et al., 2016). According to the dipyramidal morphology of the parasporal crystals of the ITCBT61 and ITCBT62 isolates, it is likely that the amplification bands are correlated with cry1 and cry2 genes, which encode toxic proteins mainly towards Lepidoptera larvae (Sun et al., 2007; Sauka and Benintende, 2008). On the other hand, the fragment amplified in the ITCBT34 isolate shows a different band size (Figure 2a), so it could be another type of cry gene.
Determination of hag genes
The three isolates presented hag gene amplification products by PCR (Figure 2b). Isolates ITCBT61 and ITCBT62 presented a band of approximately 700 bp in size, similar to the band that showed the reference strain HD1. The isolate ITCBT34 presented a band of different size of approximately 1000 bp. The amplified fragments of the hag genes reported in B. thuringiensis show polymorphism with bands with sizes from 700 bp up to 1.9 kb (Xu and Cote, 2006). The fragment size of the hag gene of the isolate ITCBT34 reinforces that it is an isolate different from the other two (ITCBT61 and ITCBT62) and to the reference strain HD1. The determination of the presence of flagellin genes is a method that is gaining use as an alternative to serotyping in B. thuringiensis (Hendriksen and Hansen, 2006; Reinoso-Pozo et al., 2016).
Protein profile
By means of the SDS-PAGE analysis, the protein profile of the three isolates under study was determined. The profiles observed were different among the isolates and only the isolate ITCBT61 coincided with the profile of the reference strain HD1. In the case of the isolate ITCBT62, the presence of a band of 45 kDa was observed and the absence of a band of 130 kDa that is present in the isolate ITCBT61 and the strain HD1, the ITCBT34 presented a profile of bands different from 100, 85, 70 and 50 kDa (Figure 3).

Figure 3 SDS-PAGE of the protein profile presented by the native isolates of B. thuringiensis. PM= Molecular weight marker PageRuler ™ Plus Prestained Protein Ladder (Thermo Scientific), lane 1: HD1, lane 2: ITCBT61, lane 3: ITCBT62, lane 4 = ITCBT34.
We report mainly bands of 130 and 65 kDa for the strain HD1, which are product of proteins encoded by the cry1 and cry2 genes (Alper et al., 2014), so that the bands of 130 and 65 kDa of molecular weight that presented the native isolates, they can belong to the group of proteins Cry1 and Cry2. The protein profile that has been observed in other works varies, but the molecular weight range of the bands is 130 to 44 kDa (Silva et al., 2010; Barathi et al., 2012). Previous studies have reported the presence of proteins of sizes similar to those obtained in the present work, which showed toxicity mainly against pests of the order Lepidoptera (Valicente et al., 2010; Li and Bouwer, 2012).
Insecticidal activity
The test of insecticidal activity was carried out with a concentration of 10 μg cm-2, the three isolates showed insecticidal activity against Manduca sexta larvae. At 96 h, 100% mortality of larvae was observed (Table 3). The larvae exposed to the isolates showed symptoms of general necrotic and internal liquefied, characteristics typical of the toxicity caused by the Cry proteins of B. thuringiensis (Silva et al., 2010) (Figure 4).
Table 3 Qualitative bioassay of insecticidal activity of the native isolates of Bacillus thuringiensis against Manduca sexta at 96 h, with a concentration of 10 μg cm-2.
| Isolated | Average* | Mortality (%) |
| ITCBT34 | 20 | 100 ±0 |
| ITCBT61 | 20 | 100 ±0 |
| ITCBT62 | 20 | 100 ±0 |
| Witness** | 20 | 0 ±0 |
*= average number of dead larvae of two replicates, with 20 larvae each; **= Tween 80 (0.02%).

Figure 4 Qualitative test of native isolates of B. thuringiensis against newborn larvae of Manduca sexta at a concentration of 10 μg cm-2. A) negative control (Tween 80), B)= ITCBT34, C)= ITCBT61 and D)= ITCBT62.
The activity observed in the ITCBT61 and ITCBT62 isolates responds to expectations, since both isolates have a dipyramidal parasporal morphology and protein profile typical of the Cry1 and Cry2 proteins, which have insecticidal activity against Lepidoptera larvae. Isolate ITCBT34 also had activity towards these larvae. This result is not common, since this isolate presented parasporal body morphology and protein profile very different from the type of proteins that act on Lepidoptera larvae. This activity may be related to the presence of cry2 genes, which were reported in B. thuringiensis isolates with only oval morphology crystals (Arrieta and Espinoza, 2006).
The presence of cry1 and cry2 genes and activity against Lepidoptera larvae is characteristic of B. thuringiensis, and is frequently reported in studies on the search and characterization of native isolates. Were used in these works, several species of Lepidoptera such as Cadra cautella and Thaumetopoea wilkinsoni (Yilmaz et al., 2013), Spodoptera littoralis (Assaeedi et al., 2011), Heliothis armígera and Plutella xylostella (Xavier et al., 2007) and even against Coleoptera larvae Carpophilus hemipterus (Alper et al., 2014).
Conclusions
In general, similarity was observed in the tests between the native isolates of the present work and the reference strain B. thuringiensis svar. kurstaki HD1, as dipyramidal morphology and size in the amplification of the hag genes in the ITCBT61 and ITCBT62 isolates, in addition to the protein profile and amplification of cry genes in the ITCBT61 isolate. This shows that isolates ITCBT61 and ITCBT62 are highly related to strain B. thuringiensis svar. kurstaki HD1. Regarding the isolate ITCBT34, in the results of all the tests carried out, no similarity was observed with the HD1 strain, indicating that this isolate belongs to a different variety. Finally, the result of the toxicity test shows that the three isolates have potential for the control of pests of the order Lepidoptera.
Gratefulness
The authors thank Miguel Angel Mejía Bautista, Javier Luevano Borrroel and Leandro Gabriel Ordoñez for the technical support provided.
REFERENCES
Abdullah, R. R.; Sukar, N. A. and Ghanim, N. M. 2014. Improving efficacy of Bacillus thuringiensis against insects of different feeding habits by plasmid transfer technique of chitinase. Egypt. J. Biol. Pest Control. 24(1):37-44. [ Links ]
Aldebis, H. K.; Osuna, E. V. y Álvarez, C. S. 1994. Caracterización serológica de cepas de Bacillus thuringiensis Berliner aisladas de insectos españoles. Bol. San. Veg. Plagas. 20(3):765-769. [ Links ]
Alper, M.; Güneş, H.; Tatlipinar, A.; Çöl, B.; Civelek, H. S; Özkan, C. and Poyraz, B. 2014. Distribution, occurrence of cry genes, and lepidopteran toxicity of native Bacillus thuringiensis isolated from fig tree environments in Aydın Province. Turk. J. Agric. For. 38(6):898-907. [ Links ]
Alquisira, R. E. V.; Paredes, G. J. R.; Hernández, V. V. M.; Ramírez, T. J. A. and Peña, C., G. 2014. In vitro susceptibility of Varroa destructor and Apis mellifera to native strains of Bacillus thuringiensis. Apidologie. 45(6):707-718. [ Links ]
Arrieta, G. and Espinoza, A. M. 2006. Characterization of a Bacillus thuringiensis strain collection isolated from diverse Costa Rican natural ecosystems. Rev. Biol. Trop. 54(1):13-27. [ Links ]
Assaeedi, A. S. A.; Osman, G. E. H. and Abulreesh, H. H. 2011. The occurrence and insecticidal activity of Bacillus thuringiensis in the arid environments. Aust. J. Crop. Sci. 5(10):1185-1190. [ Links ]
Azizoglu, U.; Yilmaz, S.; Ayvaz, A.; Karaborklu, S. and Akbulut, M. 2011. Characterization of local Bacillus thuringiensis isolates and their toxicity to Ephestia kuehniella (zeller) and Plodia interpunctella (hubner) larvae. Egypt. J. Biol. Pest Control . 21(2):143-150. [ Links ]
Barathi, S.; Sangeetha, P.; Karthick, C.; Govindaraju, S. and Arulselvi, P. I. 2012. Diversity in protein profile of Bacillus thuringiensis strains isolated from varied soil environments. J. Pharm. Res. 5(9):4645-4647. [ Links ]
Bravo, A.; Sarabia, S.; López, L.; Ontiveros, H.; Abarca, C.; Ortiz, A.; Ortiz, M.; Lina, L.; Villalobos, F. J.; Peña, G. and Núñez, V. M. E. 1998. Characterization of cry genes in a Mexican Bacillus thuringiensis strain collection. Appl Environ Microbiol. 64(12):4965-4972. [ Links ]
Burges, H. D. 1982. Control of insects by bacteria. Parasitology. 84(4):79-117. [ Links ]
Cicero, E. A. S.; Ferraudo, A. S. and Lemos, M. V. F. 2009. Identification of cry genes from Bacillus thuringiensis effective against Sphenophorus levis, the sugar-cane borer. Bragantia. 68(4):817-823. [ Links ]
Crickmore, N.; Zeigler, D. R.: Feitelson, J.; Schnepf, E.; van Rie, J.; Lereclus, D.; Baum, J. and Dean, D. H. 1998. Revision of the Nomenclature for the Bacillus thuringiensis Pesticidal Crystal Proteins. Microbiol. Mol. Rev. 62(3):807-813. [ Links ]
Erban, T.; Nesvorna, M.; Erbanova, M. and Hubert, J. 2009. Bacillus thuringiensis var. tenebrionis control of synanthropic mites (Acari: Acaridida) under laboratory conditions. Exp. Appl. Acarol. 49(4):339-346. [ Links ]
Hendriksen, N. B. and Hansen, B. M. 2006. Detection of Bacillus thuringiensis kurstaki HD1 on cabbage for human consumption. FEMS Microbiol. Lett. 257(1):106-111. [ Links ]
INEGI. 2017a. Instituto Nacional de Estadística, Geografía e Informática. Anuario estadístico y geográfico de Campeche 2017. http://internet.contenidos.inegi.org.mx/contenidos /productos/prod-serv/contenidos/espanol/bvinegi/productos/nueva-estruc/anuarios-2017/ 702825095109.pdf. [ Links ]
INEGI. 2017b. Instituto Nacional de Estadística, Geografía e Informática. Anuario estadístico y geográfico de Quintana Roo 2017. http://internet.contenidos.inegi.org.mx/contenidos/productos/prod-serv/contenidos/espanol/bvinegi/productos/nueva-estruc/anuarios-2017/702825095130.pdf. [ Links ]
INEGI. 2017c. Instituto Nacional de Estadística, Geografía e Informática. Anuario estadístico y geográfico de Tabasco 2017. http://internet.contenidos.inegi.org.mx/contenidos/productos/prod-serv/contenidos/espanol/bvinegi/productos/nueva-estruc/anuarios-2017/702825095123.pdf. [ Links ]
INEGI. 2017d. Instituto Nacional de Estadística, Geografía e Informática. Anuario estadístico y geográfico de Yucatán 2017. http://internet.contenidos.inegi.org.mx/contenidos/Productos/prod-serv/contenidos/espanol/bvinegi/productos/nueva-estruc/anuarios-2017/702825095116.pdf. [ Links ]
Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227(5259):680-685. [ Links ]
Li, H. and Bouwer, G. 2012. Toxicity of Bacillus thuringiensis Cry proteins to Helicoverpa armigera (Lepidóptera: Noctuidae) in South Africa. J. Invertebr. Pathol. 109(1):110-116. [ Links ]
Li, X. Q.; Tan, A.; Voegtline, M.; Bekele, S.; Chen, C. S. and Aroian, R.V. 2008. Expression of Cry5B protein from Bacillus thuringiensis in plant roots confers resistance to root-knot nematode. Biol. Control. 47(1):97-102. [ Links ]
Monnerat, R. G.; Soares, C. M.; Capdeville, G.; Jones, G.; Martins, É. S.; Praça, L.; Cordeiro, B. A.; Braz, S. V.; dos-Santos, R. C. and Berry, C. 2009. Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Microbial Biotechnol. 2(4):512-520. [ Links ]
Noguera, P. A. and Ibarra, J. E. 2010. Detection of new cry genes of Bacillus thuringiensis by use of a novel PCR primer system. Appl. Environ. Microbiol. 76(18):6150-6155. [ Links ]
Ornelas, P. J. F.; Maldonado, B. M. G.; Elías, S. M.; Flores, G. M. D. S.; Lozano, C. M. G. and Wong, L. J. G. 2016. Evaluación de la mortalidad causada por cepas nativas de Bacillus thuringiensis y formulados preparados contra Trichoplusia ni, Spodoptera exigua, y Helicoverpa zea. Southwest Entomol. 41(4):1095-1110. [ Links ]
Patel, K. D.; Chudasama, C. J. and Ingle, S. S. 2012. Molecular characterization of Bacillus thuringiensis isolated from diverse habitats of India. J. Basic Microbiol. 52(4):437-445. [ Links ]
Reinoso, P. Y.; del Rincón, C. M. C. and Ibarra, J. E. 2016. Characterization of a highly toxic strain of Bacillus thuringiensis serovar kurstaki very similar to the HD-73 strain. FEMS Microbiol. Lett. 363(17), fnw188. [ Links ]
Rosas, G. N. M.; Mireles, M. M.; Hernández, M. J. L. and Ibarra, J. E. 2008. Screening of cry gene contents of Bacillus thuringiensis strains isolated from avocado orchards in México, and their insecticidal activity towards Argyrotaenia sp. (Lepidoptera: Tortricidae) larvae. J. Appl. Microbiol. 104(1):224-230. [ Links ]
Rosso, M. L. and Delécluse, A. 1997. Distribution of the insertion element IS240 among Bacillus thuringiensis strains. Curr. Microbiol. 34(6):348-353. [ Links ]
Saadaoui, I.; Al-Thani, R.; Al-Saadi, F.: Belguith-Ben, H. N.; Abdelkefi-Mesrati, L.; Schultz, P.; Rouis, S. and Jaoua, S. 2010. Characterization of Tunisian Bacillus thuringiensis strains with abundance of kurstaki subspecies harbouring insecticidal activities against the Lepidopteran insect Ephestia kuehniella. Curr. Microbiol. 61(6):541-548. [ Links ]
Sauka, D. H. y Benintende, G. B. 2008. Bacillus thuringiensis: generalidades: un acercamiento a su empleo en el biocontrol de insectos lepidópteros que son plagas agrícolas. Rev. Argent. Microbiol. 40(2):124-140. [ Links ]
Sauka, D. H.; Basurto, R. R. E.; Ibarra, J. E. and Benintende, G. B. 2010. Characterization of an Argentine isolate of Bacillus thuringiensis similar to the HD-1 strain. Neotrop. Entomol. 39(5):767-773. [ Links ]
Schnepf, E.; Crickmore, N. V.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D. R. and Dean, D. H. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62(3):775-806. [ Links ]
Senfi, F.; Safaralizadeh, M. H.; Safavi, S. A. and Aramideh, S. 2012. Isolation and characterization of native Bacillus thuringiensis strains from apple orchards at Urmia, Iran and their toxicity to lepidopteran pests, Ephestia kuehniella Zeller and Pieris brassicae L. Egypt. J. Biol. Pest Control . 22(1):33-37. [ Links ]
Sharif, F. A. and Alaeddinoĝlu, N. G. 1988. A rapid and simple method for staining of the crystal protein of Bacillus thuringiensis. J. Ind. Microbiol. 3(4):227-229. [ Links ]
Sharma, P.; Nain, V.; Lakhanpaul, S. and Kumar, P. A. 2010. Synergistic activity between Bacillus thuringiensis Cry1Ab and Cry1Ac toxins against maize stem borer (Chilo partellus Swinhoe). Lett. Appl. Microbiol. 51(1):42-47. [ Links ]
Silva, N. D.; Thuler, A. M. G.; Abreu, I. L. D.; Davolos, C. C.; Polanczyk, R. A. and Lemos, M. V. F. 2010. Characterization and selection of Bacillus thuringiensis isolates effective against Sitophilus oryzae. Sci. Agric. 67(4):472-478. [ Links ]
Sneath, P. H. A. 1986. Section 13. Endospore-forming gram-positive rods and cocci. In: Bergey’s manual of systematic bacteriology. P. H. A. Sneath, (Ed.). Williams and Wilkins, Baltimore, MD. 2:1104-1138. [ Links ]
Sun, Y.; Wei, W.; Ding, X.; Xia, L. and Yuan, Z. 2007. Detection of chromosomally located and plasmid-borne genes on 20 kb DNA fragments in parasporal crystals from Bacillus thuringiensis. Arch. Microbiol. 188(4):327-332. [ Links ]
Torres, Q. M. C.; Peña, C. G.; Hernández, V. V. M. and Arenas, S. I. 2015. Signs of Bacillus thuringiensis (Bacillales: Bacillaceae) infection in Myzus persicae (Hemiptera: Aphididae): Koch’s postulates. Fla. Entomol. 98(2):799-802. [ Links ]
Travers, R. S.; Martin, P. A. W. and Reichelderfer, C. F. 1987. Selective process for efficient isolation of soil Bacillus spp. Appl. Environ. Microbiol. 53(6):1263-1266. [ Links ]
Uribe, D.; Martínez, W. and Cerón, J. 2003. Distribution and diversity of cry genes in native strains of Bacillus thuringiensis obtained from different ecosystems from Colombia. J. Invertebr. Pathol. 82(2):119-127. [ Links ]
Valicente, F. H.; de Toledo, P. E. A.; de Vasconcelos, M. J. V.; Carneiro, N. P.; Carneiro, A. A.; Guimarães, C. T. and Lana, U. G. 2010. Molecular characterization and distribution of Bacillus thuringiensis cry1 genes from Brazilian strains effective against the fall armyworm, Spodoptera frugiperda. Biol. Control. 53(3):360-366. [ Links ]
van Frankenhuyzen, K. and Tonon, A. 2013. Activity of Bacillus thuringiensis cyt1Ba crystal protein against hymenopteran forest pests. J. Invertebr. Pathol. 113(2):160-162. [ Links ]
Vázquez-Ramírez, M. F.; Rangel-Núñez, J. C.; Ibarra, J. E. y del Rincón-Castro, M. C. 2015. Evaluación como agentes de control biológico y caracterización de cepas mexicanas de Bacillus thuringiensis contra el gusano cogollero del maíz Spodoptera frugiperda (Lepidoptera: Noctuidae). Interciencia. 40(6):397-402. [ Links ]
Xavier, R.; Nagarathinam, P.; Murugan, V. and Jayaraman, K. 2007. Isolation of Lepidopteran active native Bacillus thuringiensis strains through PCR panning. Asia Pac. J. Mol. Biol. Biotechnol. 5(2):61-67. [ Links ]
Xu, D. and Coté, J. C. 2006. Sequence diversity of the Bacillus thuringiensis and B. cereus sensu lato flagellin (h antigen) protein: comparison with h serotype diversity. Appl. Environ. Microbiol. 72(7):4653-4662. [ Links ]
Yamamoto, R. T. 1969. Mass rearing of the tobacco hornworm. II. Larval rearing and pupation. J. Econ. Entomol. 62(6):1427-1431. [ Links ]
Yilmaz, S.; Karabörklü, S.; Azizoglu, U.; Ayvaz, A.; Akbulut, M. and Yildiz, M. 2013. Toxicity of native Bacillus thuringiensis isolates on the larval stages of pine processionary moth Thaumetopoea wilkinsoni at different temperatures. Turk. J. Agric. For. 37(2):163-172. [ Links ]
Received: February 00, 2018; Accepted: April 00, 2018











 texto em
texto em 



