Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.3 Texcoco abr./may. 2018
https://doi.org/10.29312/remexca.v9i3.335
Articles
In vitro characterization of rhizobacteria and their antagonism with fungi that cause damping off in chili
1Colegio de Postgraduados-Campus San Luis Potosí. Agustín de Iturbide núm. 73, Salinas de Hidalgo, SLP, México. CP. 78600. Tel. 01(496) 9630240. (hernandez.eyra@colpos.mx; ismaelhr@colpos.mx; franciscojmf@colpos.mx).
2Microbiología-Colegio de Postgraduados-Campus Montecillo. Carretera México-Texcoco km 36.5, Montecillo, Texcoco, Edo. de México. CP. 56230. Tel. 01(595) 9520200, ext. 1280 y 1269. (jalmaraz@colpos.mx).
3Centro de Investigación en Genética y Ambiente-Universidad Autónoma de Tlaxcala. Autopista Tlaxcala-Texmelucan km 10.5, Ixtacuixtla, Tlaxcala. CP. 90120. Tel. 01(248) 4815500. (alopezlo@conacyt.mx).
The production of chili seedlings (Capsicum annuum L.) in seedlings is affected by the disease known as “damping off ”. Its prevention and treatment is done regularly by fungicides, whose effects on the fungi that cause this disease are not always effective, but they do represent environmental problems. Given this situation, biological control acquires greater relevance. In the present work three strains of phytopathogenic fungi of the genus Fusarium spp. (FP, FN and FA, by the colors purple, orange and yellow developed, respectively). As for bacterial strains, 18 strains of rhizobacteria (B) were selected, which were compared in vitro with the three strains of Fusarium and one of Rhizotocnia (R). Strain B23 was the most efficient in inhibiting the growth of the phytopathogens studied, reducing the growth of FP, FN and FA (50, 44 and 47%, respectively) and R (56%); the production of AIA varied between 1.24 and 11.32 μg mL-1, the highest solubilization of inorganic phosphate (104 μg mL-1) was obtained with B8. When evaluating the effect of B7, B9, B15 and B23 on the survival of chili seedlings inoculated with Fusarium sp. and Rhizotocnia sp., it was observed that the B7 and B23 strains were the ones that had the greatest effect on the survival of the seedlings during the first 34 days, with values of between 80 to 100%, while in the control seedlings it was between 30 and 50%. On the other hand, strains B8 and B9 were identified molecularly as Pseudomonas chlororaphis; B7 and B14 as Pseudomonas sp.; B15 as Pseudomonas protegens and B23 as Bacillus sp. The presented results show the potential of the use of bacteria as growth promoters and biocontrol agents of fungi causing the damping off in “guajillo” type chili seedlings.
Key words: Capsicum annuum; Fusarium; Rhizoctonia; damping off; rhizobacteria
La producción de plántulas de chile (Capsicum annuum L.) en almácigos es afectada por la enfermedad conocida como “damping off”. Su prevención se hace regularmente mediante fungicidas, cuyos efectos sobre los hongos causantes de esta enfermedad no son siempre efectivos, pero representan problemas de tipo ambiental. Ante esta situación, el control biológico adquiere mayor relevancia. En el presente trabajo se aislaron tres cepas de hongos fitopatógenos del género Fusarium spp. (FP, FN y FA; por los colores púrpura, naranja y amarillo, respectivamente). En cuanto a cepas bacterianas, se seleccionaron 18 cepas de rizobacterias (B), las cuales fueron confrontados in vitro con las tres cepas de Fusarium y una de Rhizotocnia (R). La cepa B23 fue la más eficiente en la inhibición del crecimiento de los fitopatógenos estudiados, al reducir el crecimiento de FP, FN y FA (50, 44 y 47%, respectivamente) y de R (56%); la producción de AIA varió entre 1.24 y 11.32 µg mL-1, la mayor solubilización de fosfato inorgánico (104 µg mL-1) se obtuvo con B8. Al evaluar el efecto de B7, B9, B15 y B23 sobre la supervivencia de plántulas de chile inoculadas con Fusarium sp. y Rhizotocnia sp., se observó que las cepas B7 y B23 son las que tuvieron mayor efecto sobre la supervivencia de las plántulas durante los primeros 34 días, con valores de entre 80 a 100%, mientras que las plántulas control fue de entre 30 y 50%. Por otra parte, las cepas B8 y B9 se identificaron molecularmente como Pseudomonas chlororaphis; B7 y B14 como Pseudomonas sp.; B15 como Pseudomonas protegens y B23 como Bacillus sp. Los resultados muestran la potencialidad del uso de bacterias como promotoras del crecimiento y agentes de biocontrol de hongos causantes del damping off en plántulas de chile tipo “guajillo”.
Palabras clave: Capsicum annuum; Fusarium; Rhizoctonia; damping off; rizobacterias
Introduction
In Mexico, the disease known as early drying or “damping off ” is considered the main phytosanitary problem in the seedling phase of the pepper crop (Velásquez-Valle et al., 2007) and is attributed to a complex of pathogenic fungi that includes to Phytophthora capsici, Fusarium spp., Rhizoctonia solani, Sclerotium rolfsii and Pythium sp. (Velázquez-Valle et al., 2013). Seedlings can be affected in two ways: wilting of the plant as a consequence of rotting of its roots and involvement at the base of the stem (neck); both symptoms cause the collapse of the seedlings (Goldberg, 1995). The incidence of diseased seedlings in nurseries can reach up to 29% and constitute a source of inoculum that farmers transfer to the field during the transplant; crop losses in nursery, field and harvest can vary between 10 to 100% (Montero-Tavera et al., 2013).
The use of chemical fungicides for the control of phytopathogens is not always effective (Huang et al., 2012), in addition to causing pollution and harmful effects for human health and the environment (Gomathi and Ambikapathy, 2011). An alternative for the control of diseases caused by these fungi is the use of plant growth promoting rhizobacteria (PGPR) (Kloepper and Schroth 1981), which do not have negative effects on the environment (Mehetre and Kale et al., 2011).
The PGPR can act as: a) biocontrol agents of diseases, through competition for space or food, or through the production of antibiotics and induction of plant defense systems (Matar et al., 2009), decreasing or preventing disease (Saravanakumar et al., 2008); b) growth promoters, through the production of growth regulators, as in the case of auxin-3-acetic acid (AIA) (Goswami et al., 2015), synthesized by approximately 80% of the rhizobacteria (Ahmad et al., 2005), having as an effect a greater nutrient uptake and consequently promoting the growth and yield of the crops; and c) inorganic phosphate solubilizers through the secretion of phosphatases and organic acids that release the unavailable mineral or organic P, estimating that 40% of the PGPR present this capacity (Richardson et al., 2011).
The objective of this study was to characterize in vitro 18 bacterial isolates based on their ability to inhibit the growth of four fungal wilt fungi in chili pepper seedlings, their ability to produce indoleacetic acid and the solubilization of inorganic phosphate.
Materials and methods
Microbial isolations. Sampling of rhizospheric maize soil (Zea mays L.) established in an agroforestry module (22° 43’ 08.4” north latitude, 101° 48’ 17.7” west longitude) located in The Palma Pegada, municipality of Salinas, SLP was carried out, with an elevation of 2 222 meters above sea level, where the previous crop was chili. Isolation of the strains was performed as described by Kavitha et al. (2005), but the culture medium used was yeast-glucose-agar peptone (YPGA) (De Leon et al., 2008), incubating at 28 °C for 24 h. Subsequently, the morphological and cultural characterization was carried out in a period of 24 h, where the color, shape, elevation and edge of the colony was considered.
On the other hand, in seedlings of “guajillo” chili seedlings located in La Dulce Grande (23° 00’ 00.1” north latitude 102° 10’ 29.1” west longitude), municipality of Villa of Ramos, SLP, a sampling of seedlings that had symptoms of “damping off ”. In the laboratory of the Postgraduate College, Campus San Luis Potosi, the roots and neck of diseased seedlings were washed; they were then dissected in small portions (2 mm length) and placed in a culture medium potato-dextrose-agar (PDA, DIBICO) at 28 °C for seven days, after which the purification of the fungal colonies was carried out, obtaining three strains belonging to the genus Fusarium spp. (F). Additionally, a strain of Rhizoctonia spp. (R) provided by the Edaphology Laboratory of the Postgraduate College, Campus Montecillo. The strains were conserved in PDA.
Determination of in vitro microbial antagonism. Reactivation of bacterial and fungal isolates was performed in YPGA and PDA media, respectively. To evaluate the antifungal activity of 18 bacterial isolates against four pathogenic fungi, the dual culture method was used (Coskuntuna and Özer, 2008). The one-day-old bacterial isolates were placed 2 cm from the periphery of the Petri dish, each in triplicate, and incubating at 28 °C for 24 h; after that period, a 5 mm diameter disc with fungal mycelium was placed at a distance of 4 cm from the bacteria. The cultures were incubated at 28 °C, until the control (without bacteria) filled the box. The results were expressed as the percentage of inhibition corresponding to each of the bacterial strains. The inhibition percentage was calculated according to Mehetre and Kale (2011).
Production of indoleacetic acid (AIA). The Luria-Bertani liquid medium (Bric et al., 1991) modified according Goswami et al. (2014), which was incubated at 25 ±2 °C in rotating agitation (New Brunswick Scientific Edison) for five days. The 2 ml of each isolate was taken and centrifuged at 8 000 rpm; the supernatant of each culture was mixed with the Salkowski reagent (Balota et al., 1998) in a ratio of 2:1 and incubated in the dark for 30 min, to show the characteristic color development (pink-violet). Finally, the absorbance of the colored complex was read at 530 nm in a spectrophotometer (Bio Tek Laboratory Instrument, Synergy 2, USA). The concentration of AIA was determined by means of a standard curve in a range of 0 to 300 µg mL-1. For each isolation, three replications were evaluated using as reference the culture medium without bacterial inoculum.
Solubilization of inorganic phosphorus. The 18 bacterial strains were inoculated in flasks containing 20 ml of liquid culture medium Pikovskaya (Pikovskaya, 1948), which had insoluble tricalcium phosphate [Ca3(PO4)2] as the sole source of phosphorus, and incubated at 25 ±2 °C in constant agitation for 7 days. The 2 ml of culture were taken from each vial and centrifuged at 8 000 rpm. The soluble phosphorus of the supernatant was estimated by the blue colorimetric method of molybdenum (Jackson, 1967) at 480 nm. The corresponding amount of soluble phosphate was calculated at 7 days of the growth of the culture through a standard curve of soluble phosphate in a range of 0 to 300 µg mL-1 (Banerjee et al., 2010).
Seedling survival. This part of the research was conducted under greenhouse conditions. The guajillo chili seed was seeded in a mixture of peat moss substrates and vermiculite (1:1) previously sterilized twice at 121° for 1 h, with an interval of 3 days. Seven days after the emergence, the seedlings were transplanted to unicel trays. A period of 5 days was given for the acclimation of the seedlings and the inoculation was carried out with 1 mL of bacterial culture containing 109 cells mL-1 of each bacterial strain (B7, B9, B15 and B23) selected from the in vitro study.
The bacteria were applied with a micropipette at the base of the stem of the seedlings. An interval of 4 days was given to perform the inoculation of the pathogenic fungus (Hassan et al., 2015), the fungal mycelium came from a 7-day-old culture and was placed where the seedling roots began to develop. The survival of the seedlings was recorded and in the end the treatment with the greatest number of surviving plants was obtained, which was attributed to the effect of the inoculated antagonist bacteria against each phytopathogenic fungus.
Molecular identification of rhizobacteria and fungi. The isolation of genomic DNA from rhizobacteria B7, B8, B9, B14, B15 and B23 and fungi F. purple, F. orange and F. yellow from bacterial culture on YPGA nutritional agar for rhizobacteria and PDA for fungi was performed, using the extraction protocol CTAB and sodium acetate (LaMontagne et al., 2002). Amplification and sequencing of genomic DNA was carried out at the Potosino Institute for Scientific and Technological Research (IPICYT). A 16S rRNA gene fragment was amplified using oligonucleotides 533F (Weisburg et al., 1991) and 1391R (Turner et al., 1999) in a Verity thermocycler for endpoint PCR (Applied Biosystems). The products obtained were sequenced with the dideoxynucleotide method labeled in the 3130 Genetic Analyzer sequencer (Applied Biosystems). The percent identity of the sequences of the 16S rRNA gene fragment with the type strains was determined using BLASTN from the GenBank database.
Statistical analysis. The behavior of the bacteria in the inhibition of fungal growth, the production of indoleacetic acid, as well as the solubilization of phosphorus as a reflection of the activity to reduce the “damping off ” were analyzed identifying differences (at 95% reliability.) After to identify the relevant sources of variation using InfoStat (Di Rienzo, 2014).
Results and discussion
Microbial isolations. A total of 50 strains of rhizobacteria were isolated, of which 18 were selected taking their growth rate as a criterion in a period of 24 h. On the other hand, three fungal strains were isolated from chili seedlings, which belong to the genus Fusarium, based on their pigmentation of the fungal mycelium and the culture medium where they were grown, they were assigned the nomenclature FP, FN and FA (Fusarium purple, F. orange and F. yellow, respectively).
Determination of in vitro microbial antagonism. The results obtained in dual culture showed that strain B23 was statistically the most efficient, inhibiting the mycelial growth of FP (50.02%), FN (44.05%), FA (46.67%) and Rhizoctonia spp. (R; 55.56%) (Figure 1). These results are similar to those reported by Ríos-Velasco et al. (2016), who report a percentage of growth inhibition of F. oxysporum of 42 and 51.5% due to the effect of B. methylotrophicus and B. amyloliquefaciens, respectively, as well as those found by Mojica-Morin et al. (2009), when confronting F. oxisporum with the strains of B. thuringiensis GM-23 (43.02%) and HD-121 (42.04%) and observed higher percentages of inhibition for R. solani due to the effect of the GM-64 strains. (66.66%) and HD-203 (65.99%). On the other hand, Djordjevic et al. (2011), when evaluating 16 bacterial isolates as antagonists of F. oxisporum, report a higher inhibition percentage (70.98%) on the growth of this fungus with strain Ab23, with results similar to those found in the present study with the isolates Ab9 (59.74%), Ab17 (57.7%) and Ab1 (56.6%).
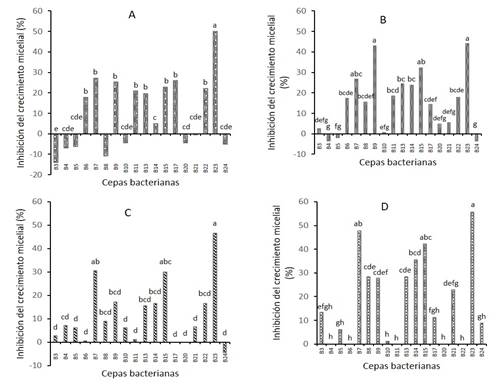
Figure 1 Inhibitory effect of 18 bacterial strains on the mycelial growth of four phytopathogenic fungi. A) Fusarium Purple, B) Fusarium Orange, C) Fusarium Yellow and D) Rhizoctonia. Negative values indicate growth stimulation.
In Figure 1A it can be seen that strains B6; B13; B11; B22; B15; B9; B17 and B7 exert a statistically similar antagonistic effect on Fusarium purple (FP), but different from the rest of the bacterial strains studied; B23 inhibited FP in 50%. When contrasting the 18 bacterial strains with Fusarium orange (FN) it was found that strains B23 and B9 showed a percentage of inhibition of 44.05 and 42.86%, respectively, which was higher than the rest of the treatments (Figure 1B).
While strains B7 and B15 inhibited fungal growth of Fusarium yellow (FA) by 30%, and with strain B23 almost reached 50% (Figure 1C). On the other hand, percentages of Rhizoctonia growth inhibition of 47.7, 42.2 and 35.5% were found when comparing strains B7, B15 and B14 respectively (Figure 1D). Finally, strains B3, B4, B5, B10, B20, B24 had no inhibitory effect on the four phytopathogens studied, which indicates that these strains were not able to produce a sufficient amount of substances that can inhibit the growth of pathogens, so that a threshold that allows them to exhibit an antagonistic response is not reached (Figueroa-López et al., 2016).
It is well documented that the antagonistic effect of rhizobacteria on the inhibition of the growth of phytopathogens is due to the synthesis of secondary metabolites (Khannous et al., 2014). Trivedi et al. (2008) showed that volatile metabolites produced by Pseudomonas corrugata play a predominant role in the inhibition of fungi F. oxysporum and A. alternata, while diffusible metabolites play only a secondary role. On the other hand, it has been shown that some species of Pseudomonas produce lytic enzymes, which can break the cell membranes of some phytopathogenic fungi; the best known are: chitinases, cellulases, proteases and 1,3-glucanases (Compant et al., 2005).
Production of indoleacetic acid (AIA). The production of AIA is a property of some rhizobacteria to stimulate and facilitate plant growth (Mohite, 2013). This auxin is a product derived from the metabolism of L-tryptophan produced by several soil microorganisms, including PGPR (Goswami et al., 2015). In the present work, when analyzing the AIA production of 18 bacterial isolates from the maize rhizosphere, a variation of 1.3 to 11.2 μg mL-1 was found. The production of AIA significantly higher (p≤ 0.05) corresponded to B14 and the lowest to B4 (Figure 2).
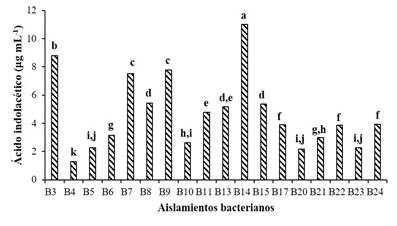
Figure 2 Production of indoleacetic acid from 18 bacterial isolates from the corn rhizosphere. Averages with different letters indicate significant differences (Tukey 0.05).
These results agree with those reported in several investigations, where the production of AIA in bacterial strains coming from the maize rhizosphere was evaluated. Noh et al. (2014) mention a production of 3.3 μg mL-1 of AIA. On the other hand, Josic et al. (2012) point out that when adding 5mM of tryptophan to the King B liquid medium, the concentration of AIA increased from 8.6 to 26 μg mL-1 in strain Q4m (Pseudomonas sp.). It has been observed that the production of AIA by rhizobacteria varies between different species and strains; which is influenced by the growing conditions, growth stage and substrate availability (Mirza et al., 2001).
According to the production of AIA in vitro, the rhizobacteria can be divided into three groups: low (1 to 10 μg mL-1), medium (11 to 20 μg mL-1) and high producing (21 to 30 μg mL-1) of AIA (Rojas et al., 2010). According to this classification, most of the strains analyzed in the present study are classified as low producers of indoleacetic acid. However, not all bacteria that produce a high amount of AIA stimulate plant growth (Noh et al., 2014). For example, Agrobacterium tumefaciens produces up to 78 μg mL-1 of AIA, which induces the development of the disease known as “crown gill” (Lara et al., 2011), while lower concentrations (1.1 to 12.1 µg mL-1) increased the growth of wheat plants in vitro (Khalid et al., 2004).
Ramírez-Malagon and Ochoa-Alejo (1996), in an in vitro study showed that the AIA has an effect on the induction and growth of adventitious buds of the pepper culture, being its concentration the one that determines the type of response. Peña et al. (2016) report an AIA production, between 4.0 to 24.3 μg mL-1 in five Bacillus species, which is sufficient to promote the growth of Capsicum annuum L. Therefore, in the present study eight strains can be considered promising for promote the growth of the chili crop.
Solubilization of inorganic phosphorus. The amount of inorganic phosphate (Pi) dissolved in the culture medium varied among the different isolates (Figure 3). Strain B8 was the one that solubilized the highest amount of Pi (100.4 μg mL-1), followed by strain B14, with which 80.92 μg mL-1 and then B17 and B21 were recorded (60.67 and 56.39 μg mL-1, respectively). The amount of phosphate solubilized by the rest of the strains, with the exception of B10 and B9 (null effect), was found in the range of 2.21 to 49.12 μg mL-1. The results recorded in strains B8, B14 and B17 are superior to those reported by Nautiyal (1999), who found a variation of 8 to 35 μg mL-1 in five species of Pseudomonas and from 8 to 17 μg mL-1 in three Bacillus species.
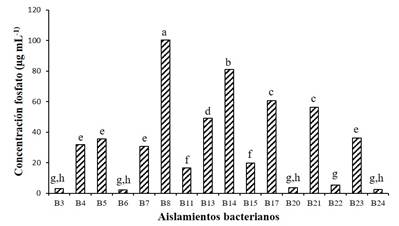
Figure 3 Phosphate solubilized by bacterial isolates. Columns with different letters indicate significant differences (Tukey 0.05).
It has been pointed out that bacteria solubilize Pi through the production of CO2, organic acids, proton excretion and NH4 + assimilation. The most important mechanism of solubilization of calcium phosphate is acidification through the biosynthesis and secretion of organic acids (Paredes and Espinosa, 2009). Such organic acids can directly dissolve the Pi as a result of the exchange of phosphate anions by an acid anion or by chelating the Fe, Al and Ca ions associated with P (Omar, 1998), which consequently increases the bioavailability of P, which can be exploited by the crops or the wild flora present.
Seedling survival. When evaluating the effect of strains B7, B9, B15 and B23 on the survival of chili seedlings inoculated with Fusarium spp. (FA, FP and FN) and Rhizotocnia sp. (R), we observe in Figure 4 (a, b and d) 100% survival in seedlings inoculated with B23 and a survival in control plants of FP, FA and R of 80, 77.8 and 83%, respectively (only inoculated with the pathogen) at eight days. In this same period, 100% survival was also observed when inoculating B9 and B15 for the case of FP (4a) and B15 for the case of Rhizoctonia (Figure 4d). It is observed that from 34 to 60 days, there is a decrease in the survival of the control seedlings in comparison with the seedlings inoculated with rhizobacteria. On the other hand, only strains B7 and B23 have an effect on the survival of FA infected seedlings up to 55 days (Figure 4b), this survival coincides with that reported by Abdel-Monaim (2013) who obtained 72% and 76.34% of survival by Bacillus megaterium in bean plants in two different seasons, whose plants were infested with the pathogens R. solani, F. solani, F. oxysporum and M. phaseolina causing damping off to the plant.
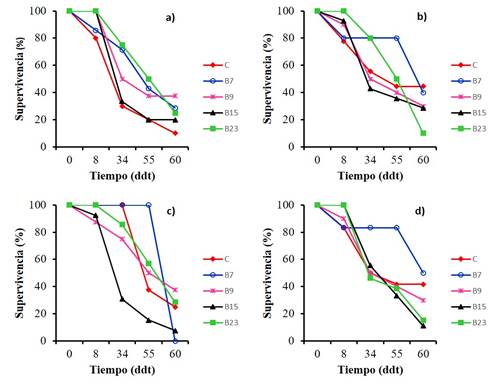
Figure 4 Effect of four antagonistic bacteria on the survival of “guajillo” chili seedlings inoculated with different phytopathogenic fungi. a) Fusarium Purple; b) F. yellow; c) F. orange; and d) Rhizoctonia.
With respect to the fungus FN (Figure 4c), both in control seedlings and in the seedlings inoculated with B7 and B23, 100% survival was recorded. This same trend is maintained until 34 days for the case of control seedlings and those inoculated with B7. The behavior of the control seedlings could possibly be due to the fact that the amount of fungal mycelium was not enough for this fungus to express virulence in the first 34 days or that its effect to cause the disease is later than the other pathogens used in the present study.
Finally, for the case of Rhizotocnia spp., Strain B7 had an effect on the survival of the seedlings, because it was 80% until 55 days, while the control seedlings were 41.4% (Figure 4d). In this regard, Toppo and Tiwari (2015), report a variation in the survival of tomato seedlings (of 30 days) infected with Rhizoctonia which varied between 93.33 to 46.67.6% when inoculating Pseudomonas alcaligenes PKJ25 and Pseudomonas PKS10. In the present study, at 34 days, the highest survival was 83.3 and the lowest was 46.2% when inoculating B7 and B23, respectively.
Molecular identification of rhizobacteria and fungi. Isolates B8 and B9 were identified as Pseudomonas chlororaphis; B7 and B14 as Pseudomonas sp. and B15 as Pseudomonas protegens. For its part, B23 was identified as Bacillus sp. All presented an identity 99%, with a coverage of 100%. The genus Pseudomonas is composed of gram-negative bacteria, which belong to the γ-Proteobacteria, are distributed in a wide variety of environments and are known for their metabolic versatility in the use of organic compounds as carbon and energy sources (Lessie and Phibbs, 1984; Wu et al., 2011) and in the production of various secondary metabolites (Gross and Loper, 2009). Pseudomonas is one of the most complex bacterial groups, with more than 100 species described so far, in which there is the presence of groups and subgroups.
The group of Pseudomonas flourescens contains several species (P. brassicacearum, P. protegens, P. chlororaphis and P. fluorescence), described as PGPR for its ability to suppress disease-causing pathogens (Raajimakers et al., 2009) and produce antibiotics or secondary metabolites (Gross and Loper, 2009) that make them very viable for biotechnological applications (Saravanakumar et al., 2008). Bacterial identification through the 16S ribosomal gene has been widely used; however, it is well known that nearby bacterial species cannot be differentiated by the use of this gene due to its high conservation rate (Fox et al., 1992; Kämpfer and Glaeser, 2012), so the use of the analysis of multilocus sequences by the use of other genes as molecular markers: rpoB, rpoD, gyrB (Yamamoto et al., 2000; Ait Toyeb et al., 2005), allowing identification up to the species level.
In this work, by using the 16S rRNA gene as the only molecular marker, it was possible to designate the obtained isolates, which belong to Pseudomonas and Bacillus, up to gender level. Members of these genera have been reported as PGPR, with different mechanisms such as production of phytohormones, suppression of pathogens or producers of secondary metabolites of biotechnological interest.
On the other hand, FP and FN fungal isolates correspond to Fusarium sp.; the first with 93% identity and coverage 84% and the second with 86% identity and 80 coverage. F. yellow corresponds to a non-cultivated fungus, with 94% identity and 33% coverage. Fusarium sp belongs to the family Nectriaceae. The genus Fusarium is a group of filamentous fungi widely distributed in soil and plants. Due to their ability to grow at 37 °C, they are considered opportunistic (Tapia and Amaro, 2014). Santos (2010) mentions Fusarium sp. as one of the main causal agents of the wilt of the chili that diminishes the yield of the crop.
Literatura citada
Abdel, M. M. F. 2013. Improvement of biocontrol of damping-off and root rot/wilt of Faba bean by salicylic acid and hydrogen peroxide. Mycobiology. 41(1):47-55. [ Links ]
Ahmad, F.; Ahmad, I. and Khan, M. S. 2005. Indole acetic acid production by the indigenous isolated of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 29:29-34. [ Links ]
Ait, T. L.; Ageron, E.; Grimont, F. and Grimont P. A. 2005. Molecular phylogeny of the genus Pseudomonas based on rpoB sequences and application for the identification of isolates. Res Microbiology. 156(5-6):763-773. [ Links ]
Balota, E. L.; Colozzi, F. A.; Andrade, D. S. e Hungria, M. 1998. Biomassa microbiana e sua atividade em solos sob diferentes sistemas de preparo e sucessã ode culturas. Rev. Bras. Ciencia do Solo. 22:641-649. [ Links ]
Banerjee, S.; Palit, R.; Sengupta, C. and Standing, D. 2010. Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Austr. J. Crop Sci. 4(6):378-383. [ Links ]
Bric, J. M.; Bostock, R. M. and Silverstone S. E. 1991. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 57(2):535-538. [ Links ]
Compant, S.; Duffy B.; J. Nowak, C. Clément and Barka E. A. 2005. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol . 71:4951-4959. [ Links ]
Coskuntuna, A. and Özer, N. 2008. Biological control of onion basal rot disease using Trichoderma harzianum and induction of antifungal compounds in onion set following seed treatment. Crop Prot. 27:330-336. [ Links ]
De León, L.; Rodríguez, A.; López, M. M. and Siverio, F. 2008. Evaluation of the efficacy of immunomagnetic separation for the detection of Clavibacter michiganensis subsp. michiganensis in tomato seeds. J. Appl. Microbiol. 104:776-786. doi:10.1111/j.1365-2672.2007.03595.x. [ Links ]
Di Rienzo, J. A.; Casanoves, R. F. M. G.; Balzarini, B.; González, M. L.; Tablada T. M. y Robledo S. C. W. 2014. InfoStat versión 2014. Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar. [ Links ]
Djordjevic, M.; Ugrinovic, M.; Sevic, M.; Djordjevic, R. and Mijatovic, M. 2011. Antagonistic effect of soil bacteria against fusarium wilt of pepper in vitro.Acta Agriculturae Serbica. 16:19-31. [ Links ]
Figueroa, L. A. M.; Cordero, R. J. D; Martínez, A. J. C; López, M. M; Lizárraga, S. G. J; Félix, G. R; Castro, M. C. and Maldonado, M. I. E. 2016. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. Springer Plus. DOI: 10.1186/s40064-016-1780-x. [ Links ]
Fox, G. E.; Wisotzkey, J. D. and Jurtshuk, P. Jr. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Inter. J. System. Bacteriol. 42(1):166-170. [ Links ]
Goldberg, N. P. 1995. Chile pepper diseases. Agricultural Experiment Station. College of Agriculture and Home Economics. New Mexico State University. Circular 549. Las Cruces, NM, USA. 20 p. [ Links ]
Gomathi, S. and V. Ambikapathy. 2011. Antagonistic Activity of Fungi Against Pythium debaryanum (Hesse) Isolated From Chilli Field Soil. Adv App Sci Res. 2:291-297. [ Links ]
Goswami, D., Dhandhukia P., Patel P. and Thakker J. N. 2014. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 169:66-75. [ Links ]
Goswami, D.; Thakker, J. N. and Dhandhukia, P. C. 2015. Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from L-tryptophan (Trp) using HPTLC. J. Microbiol. Methods. 110:7-14. [ Links ]
Gross, H. and Loper, J. E. 2009. Genomics of secondary metabolite productions by Pseudomonas spp. Natural Proceedings Reports. 26(11):1408-46 [ Links ]
Hassan, D. Gh.; Hassan, M. G.; Rashid, H.; Ali, D. W. and Majeed, M. 2015. Evaluation of microbial antagonists for the management of wilt/root rot and damping-off diseases in chilli (Capsicum annuum). Rev. Vegetos. 28(4):102-110. [ Links ]
Huang, X.; Zhang, N.; Yong, X.; Yang, X. and Shen, Q. 2012. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol. Res. 167:135-143. [ Links ]
Jackson, M. L. 1967. Soil chemical analysis. (Ed). Prentic Hall, Inc., Engle Wood Cliff. USA. [ Links ]
Josic, D.; Delic, D.; Rasulic, N.; Stajkovic, O.; Kuzmanovic, D.; Stanojkovic, A. and Pivic, R. 2012. Indigenous pseudomonads from rhizosphere of maize grown on pseudogley soil in Serbia. Bulgarian J. Agric. Sci. 18(12):197-206. [ Links ]
Kavitha, K.; Mathiyazhagan, S.; Senthilvel, V.; Nakkeeran, S. and Chandrasekar, G. 2005. Development of bioformulations of antagonistic bacteria for the management of damping off of Chilli (Capsicum annuum L.). Archives of Phytopathology and Plant Protection. DOI: 10.1080/03235400400008382. [ Links ]
Kämpfer, P. and Glaeser, S. P. 2012. Prokaryotic taxonomy inn the sequencing era-the poluphasic approach revisited. Environ. Microbiol. 14(2):291-317. [ Links ]
Khalid, A.; Arshad M. and Zahir, Z. A. 2004. Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol. 96:473-480. [ Links ]
Khannous, L.; Mouna, J.; Mouna, D.; Ramzi, M.; Nour, C.; Bassem, K.; Néji G. and Imen, F. 2014. Isolation of a novel amylase and lipase-producing Pseudomonas luteola strain: study of amylase production conditions. Lipids in Health and Disease. 13:9. [ Links ]
Kloepper, J. W. and Schroth M. N. 1981. Plant growth-promoting rhizobacteria and plant growth under gnotobiotic conditions. Phytopatology. 71:642-644. [ Links ]
LaMontagne, M. G.; Michel, Jr. F. C.; Holden, P. A. and Reddy, C. A. 2002. Evaluation of extraction and purification methods for obtaining PCR-amplifiable DNA from compost for microbial community analysis. J. Microbiol. Methods. 49(3):255-264. [ Links ]
Lessie, T. G. and Phibbs, P. V. Jr. 1984. Alternative pathways of carbohydrate utilization in Pseudomonads. Annual Rev. Microbiol. 38:359-388. [ Links ]
Lara, M. C.; Oviedo Z. L. y Betancur H. C. A. 2011. Bacterias nativas con potencial en la producción de ácido indolacético para mejorar los pastos. Zootec. Trop. 29:187-194. [ Links ]
Matar, S. M.; El-Kazzaz, S. A.; Wagih, E. E.; El-Diwan, A. I.; Moustafa, H. E.; Abo-Zaid, G. A.; Abd-Elsalam, H. E. and Hafez, E. E. 2009. Antagonistic and inhibitory effect of Bacillus subtilis against certain plant pathogenic fungi, I. Biotechnology. 8(1):53-61. [ Links ]
Matar, S. M.; El-Kazzaz, S. A.; Wagih, E. E.; El-Diwan, A. I.; Moustafa, H. E.; Abo-Zaid, G.A.; Abd-Elsalam, H. E. and Hafez E. E. 2009. Antagonistic and inhibitory effect of Bacillus subtilis against certain plant pathogenic fungi, I. Biotechnology. 8(1):53-61. [ Links ]
Mojica, M. V; Luna, O. H. A; Sandoval, C. C. F.; Pereyra, A. B; Morales, R. L. H.; González, A. N. A.; Hernández, L. C. E. y Alvarado, G. O. G. 2009. Control biológico de la marchitez del chile (Capsicum annuum L.) por Bacillus thuringiensis. Phyton. 78(2):105-110. [ Links ]
Mehetre, S. T. and Kale, S. P. 2011. Comparative efficacy of thermophilic bacterium, Bacillus licheniformis (NR1005) and antagonistic fungi, Trichoderma harzianum to control Pythium aphanidermatum-induced damping off in chilli (Capsicum annuum L.). Arch. Phytopathol. Plant Protec. 44(11):1068-1074. [ Links ]
Mirza, M. S.; Ahmad, W.; Latif, F.; Haurat, J.; Bally, R.; Normand P. and Malik, K. A. 2001. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant Soil 237:47-54. [ Links ]
Mohite, B. 2013. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 13(3):638-649. [ Links ]
Montero, T. V.; Guerrero, A. B. Z.; Anaya, L. J. L.; Martínez, M. T.0.; Guevara, O. L. y González, C. M. M. 2013. Diversidad genética de aislados de Rhizoctonia solani (Kuhn) de chile en México. Rev. Mex. Cienc. Agríc. 4(7):1043-1054. [ Links ]
Nautiyal, C. S. 1999. An eficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters. 170:265-270. [ Links ]
Noh, M. J.; Zúñiga, A. J. J.; Borges, G. L.; Yam, C. C. y Godoy, H. G. 2014. Aislados bacterianos con potencial biofertilizante para plántulas de tomate. Terra Latinoam. 32(4):273-281 [ Links ]
Omar, S. A. 1998. The role of rock phosphate solubilizing fungi and vesicular arbuscular mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotechnol. 14:211-219. [ Links ]
Paredes, M. M. y Espinosa, V. D. 2009. Ácidos orgánicos producidos por rizobacterias que solubilizan fosfato: una revisión crítica. Terra Latinoam. 28(1):61-70. [ Links ]
Peña, Y. L. P.; Ruíz, S. E.; Barbosa, C. J. E. and Reyes, R. A. 2016. Isolation of Mexican Bacillus species and their effects in promoting growth of chili pepper (Capsicum annuum L. cv. Jalapeño). Indian J. Microbiol. 56(3):375-378. DOI 10.1007/s12088-016-0582-8. [ Links ]
Pikovskaya, R. I. 1948. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 17:362-370. [ Links ]
Ramírez, M. R. and Ochoa, A. N. 1996. An improved and reliable chili pepper (Capsicum annuum L.) plant regeneration method. Plant Cell Rep. 16:226-231. [ Links ]
Raajimakers, J. M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C. and Moënne, L. Y. 2009. The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil. 321(1-2):20. [ Links ]
Richardson, A. E.; Lynch, J. P.; Ryan, P. R.; Delhaize, M.; Smith, F. A.; Smith, S. E.; Harvey, P. R.; Ryan, M. H.; Veneklaas, E. J.; Lambers, H.; Oberson, A.; Culvenor, R. A. and Simpson, R. J. 2011. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121-156. DOI 10.1007/s11104-011-0950-4. [ Links ]
Rios, V. C; Caro, C. J. N; Berlanga, R. D. I; Ruíz, C. M. F; Ornelas, P. J. J; Salas, M. M. A; Villalobos, P. E. and Guerrero, P. V. M. 2016. Identification and antagonistic activity in vitro af Bacillus spp. and Trichoderma spp. isolates againts common phytopathogenic fungi. Rev. Mex. Fitopatol. 34:84-99. [ Links ]
Rojas, C. A.; Rodríguez, D. A. M.; Montes, V. S. y Pérez J. S. 2010. Evaluación de la promoción de crecimiento de Cynodon dactylon L. por rizobacterias productoras de fitohormonas aisladas de un suelo contaminado con hidrocarburos derivados del petróleo. Rev. Polibotánica. 29:1-147. [ Links ]
Santos, J. P. 2010. Estrategias para el control de Phytophthora capsici Leo. y Fusarium solani Mart. en chile (Capsicum annum L.). Tesis de maestría. Colegio de Postgraduados. [ Links ]
Saravanakumar, D.; Lavanya, N.; Muthumeena, B.; Raguchander, T.; Suresh S. and Samiyappan R. 2008. Pseudomonas fluorescens enhances resistance and natural enemy population in rice plants against leaf folder pest. J. Appl. Entomol. 132:469-479. [ Links ]
Tapia, C. y Amaro J. 2014. Género Fusarium. Rev Chilena Infectol. 31(1):85-86. [ Links ]
Trivedi, P.; Pandey, A. and Palni, L. M. S. 2008. In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol. Res . 163:329-336. [ Links ]
Turner, S.; Pryer, K. M.; Miao, V. P. y Palmer, J. D. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryotic Microbiol. 46(4):327-338. [ Links ]
Toppo, S. R. and Tiwari, P. 2015. Biocontrol potentialities of native Pseudomonas isolates against plant pathogenic fungi Rhizoctonia spp., Fusarium spp. and Colletotricum spp. in tomato rhizosphere under greenhouse condition. The Bioscan. 10(1):373-377. [ Links ]
Velásquez, V. V. R; Amador R. M. D.; Medina, A. M. M. y Lara V. F. 2007. Presencia de Patógenos en Almácigos y Semilla de Chile (Capsicum annuum L.) en Aguascalientes y Zacatecas, México. Rev. Mex. Fitopatol. 25(1):75-79. [ Links ]
Velásquez, V. V. R.; Reveles, T. L. R. y Reveles, H. M. 2013. Manejo de las principales enfermedades del chile para secado en el norte centro de México. Campo Experimental Zacatecas. CIRNOC-INIFAP. Folleto técnico núm. 50. 57 p. [ Links ]
Weisburg, W. G.; Barns, S. M.; Pelletier, D. A. and Lane, D. J. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173(2):697-703. [ Links ]
Wu, X.; Monchy, S.; Taghavi, S.; Zhu, W.; Ramos, J. and van der Lelie, D. 2011. Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS microbiology Reviews. 35(2):299-323. [ Links ]
Yamamoto, S.; Kasai, H.; Arnold, D. L.; Jackson, R. W.; Vivian, A. y Harayamas, S. 2000. Phylogeny of the genus Pseudomonas: intergenic structure reconstructed from nucleotide sequences of gyrB and rpoD genes. Microbiology. 146:2385-2394. [ Links ]
Received: February 00, 2018; Accepted: March 00, 2018











 texto en
texto en 


