Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.2 Texcoco Fev./Mar. 2018
https://doi.org/10.29312/remexca.v9i2.1083
Articles
Metabolic diversity of edaphic microorganisms associated with corn cultivation in the Yaqui Valley, Sonora
1Campo Experimental Norman E. Borlaug-Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Norman Borlaug km 12, Ciudad Obregón, Sonora, CP. 85000. (escalante.daniela@inifap.gob.mx).
2Instituto Tecnológico de Sonora. 5 de febrero 818 Sur, Col. Centro, Ciudad Obregón, Sonora. CP. 85000. (b.rize@hotmail.com; el-aby-carlos@hotmail.com).
3CONACYT- Instituto Tecnológico de Sonora. 5 de febrero 818 Sur, Col. Centro. CP. 85000, Ciudad Obregón, Sonora (sergio.delossantos@itson.edu.mx).
In the Yaqui Valley, like other sites with intensive agriculture, soils have been generated with low content of organic matter (< 1.5%), high levels of erosion due to wind events, saline or alkaline soils and low efficiency of fertilizer use. Therefore, new technologies are necessary to maintain the sustainability of the agricultural system through rational exploitation of natural resources and preserving the environment. One of these technologies is the application of biofertilizers based on microorganisms that promote plant growth, which have beneficial effects on the growth and health of plants. Therefore, the objective of this work was to isolate and metabolically characterize edaphic microorganisms associated with the cultivation of corn in said agricultural area. The isolated microorganisms were characterized based on their ability to synthesize indoles, produce siderophores and solubilize phosphates, as well as the presence of ACC deaminase and hemolytic activity. The 96% of isolates synthesized indoles, 78% had the ability to produce siderophores and only 31% solubilized phosphates. Sixty-six promising strains were selected, of which 73 had ACC deaminase activity and 64 were non-hemolytic. Finally, growth promotion trials were carried out with the individual inoculation of 7 bacteria (22B45, 11B20, 53A2, 52B4, 22A67, 13B41 and 41B1), promoting the development of plants with some strains, mainly with the 53A2 bacteria that produced increases greater than 50% in the aerial dry weight and diameter of the stem in the inoculated plants.
Keywords: Zea mays L.; indoles; promotion of plant growth; siderophores
En el Valle del Yaqui, al igual que otros sitios con agricultura intensiva, se han generado suelos con bajo contenido de materia orgánica (<1.5%), altos niveles de erosión por eventos eólicos, suelos salinos o alcalinos y baja eficiencia de aprovechamiento de fertilizantes. Por lo cual son necesarias nuevas tecnologías para mantener la sostenibilidad del sistema agrícola mediante explotación racional de los recursos naturales y preservando el ambiente. Una de estas tecnologías es la aplicación de biofertilizantes a base de microorganismos promotores del crecimiento vegetales, los cuales tienen efectos benéficos sobre el crecimiento y salud de plantas. Por ello, el objetivo del presente trabajo fue aislar y caracterizar metabólicamente microorganismos edáficos asociados al cultivo del maíz en dicha zona agrícola. Los microorganismos aislados fueron caracterizados en base a su capacidad de sintetizar índoles, producir sideróforos y solubilizar fosfatos, así como la presencia de actividad ACC desaminasa y hemolítica. El 96% de los aislados sintetizó índoles, el 78% tuvo la capacidad de producir sideróforos y solamente 31% solubilizó fosfatos. Se seleccionaron 76 cepas promisorias, de las cuales 73 presentaron actividad ACC desaminasa y 64 resultaron no hemolíticas. Finalmente se realizaron ensayos de promoción de crecimiento con la inoculación individual de 7 bacterias (22B45, 11B20, 53A2, 52B4, 22A67, 13B41 y 41B1), logrando promover el desarrollo de las plantas con algunas cepas, principalmente con la bacteria 53A2 que produjo incrementos mayores a 50% en el peso seco aéreo y diámetro del tallo en las plantas inoculadas.
Palabras clave: Zea mays L.; índoles; promoción de crecimiento vegetal; sideróforos
Introduction
The green revolution emerged in the 1940s, during which important increases in crop yields were achieved, using a combination of improved varieties, high amounts of fertilizers and pesticides, as well as irrigation systems (Ahrens et al., 2008; Duchemin et al., 2015). This process began in the Yaqui Valley, an agricultural region with approximately 225 000 hectares of irrigated fields located in the state of Sonora, between the mountains of the Sierra Madre and the Gulf of California.
The climate in the Yaqui Valley is semi-arid, with variable precipitation rates with an average of 317 mm per year and an average temperature in the autumn-winter cycle of 21 °C and 30 °C in the spring-summer cycle (Luers et al., 2003). The main crops produced are wheat (173 495 ha), soybean (48 695 ha), corn (21 082), safflower (17 923) and sorghum (10 327) (SIAP, 2016). Like many other post-revolution green agricultural regions in the world, the Yaqui Valley has to face the impacts of intensive fertilizer use, growing competition for water in other sectors, current economic and political conditions (McCullough and Matson, 2016).
On the other hand, in addition to the excessive use of agrochemicals, the agronomic management (mainly the intensive use of agricultural machinery, and the little or no vegetal coverage) has generated soils with low content of organic matter (<1.5%), high levels of erosion due to wind events due to its dry climate (>200 t ha-1 year-1), saline or alkaline soils, and with low efficiency of applied fertilizers (Ibarra-Villareal et al., 2016). Therefore, new technologies are needed that are oriented to maintain the sustainability of the agricultural system through the rational exploitation of natural resources and the application of adequate measures to preserve the environment (Shankar Singh et al., 2011).
One of these technologies is the application of biofertilizers based on microorganisms that promote plant growth (MPCV), which have beneficial effects on the growth and health of plants (Nihorimbere et al., 2011). The MPCV can facilitate the growth and development of plants through direct and indirect mechanisms. Direct stimulation can involve the supply of substances synthesized by the bacteria itself and help to take nutrients from the environment, which can be nitrogen, phytohormones, iron and phosphorus (Verma et al., 2010), while indirect stimulation it includes the prevention or elimination of phytopathogens by the production of inhibitory substances or by the increase in the resistance of the plant (Saleem et al., 2007). It is very common for a single MPCV to show more than one mode of action, as it is also common in the rhizosphere, MCV having a single mode of action to act synergistically to stimulate the growth of the host plant (Vessey, 2003).
Unlike the adverse effects of the continuous use of chemical fertilizers, when the MPCV are applied to the soil they improve its structure without leaving toxic residues. In this way, microbial inoculants or biofertilizers are an important component in sustainable agriculture (Shankar Singh et al., 2011). However, problems of variability in colonization efficiency, performance in the field and competition in the rhizosphere may be controversial issues. This variability depends considerably on microorganisms, soil type, plant species, and environmental conditions. Therefore, for the use of MPCV as biofertilizers requires the identification and selection of competent microorganisms in the rhizosphere with attributes of growth promotion (Armenta-Bojorquez et al., 2010; Babalola, 2010).
The objective of the present work was to metabolically characterize the edaphic microbiota associated with the maize crop in the Yaqui Valley, in order to identify native microorganisms with agrobiotechnological potential.
Materials and methods
Soil sampling and isolation of microorganisms
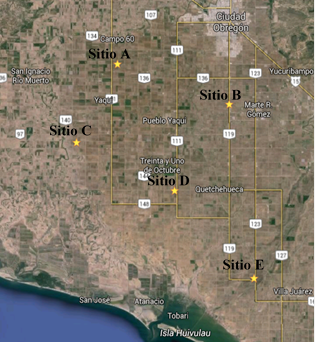
Soil was sampled at five sites (27° 25’ 38.3” latitude north 110° 06’ 27.8” longitude west, 27° 22’ 30.3” latitude north 109° 55’ 56.4” longitude west, 27° 19’ 29.7” latitude north 110° 10’ 18.2” west longitude, 27° 15’ 43.7” north latitude 110° 01’ 06.1” west longitude, 27° 08’ 50.4” north latitude 109° 53’ 39.5” west longitude) where it is cultivated corn in the Yaqui Valley, Sonora (Figure 1). At each site, a composite sample of 10 kg of soil was collected for microbiological analysis. Isolation of microorganisms was performed using the technique of serial dilutions in enriched culture media, nutrient agar supplemented with 80 µg ml-1 of cycloheximide for isolation of bacteria and potato dextrose agar supplemented with 80 µg ml-1 of carbenicillin, the isolation of fungi and actinomycetes. The cultures were incubated at 28 ±2 °C for 7 days, determining at 72 h the colony forming units (UFC) per gram of dry weight of soil (gss). The isolations were made in triplicate (de los Santos-Villalobos et al., 2013).
Metabolic characterization of the isolates obtained
Production of indoles. 1 × 105 UFC of each isolate was inoculated in 100 ml of nutrient broth supplemented with 100 ppm tryptophan. These cultures were incubated at 28 °C for 3 days at 100 rpm. Then, 1 ml of culture was centrifuged at 13 000 rpm for 3 min, to the supernatant obtained was added 2 ml of Salkowski reagent. Said reaction was incubated in the dark for 20 min at room temperature (Glickmann and Dessaux, 1995). The quantification of indoles was determined at 540 nm in a spectrophotometer. This trial was carried out with three independent replicas.
Production of siderophores. The chromium azurol S technique (CAS agar) reported by Schwyn and Neilands (1987) was used to determine the production of siderophores by the strains under study. Each isolate was inoculated in the center of a Petri dish containing the CAS culture medium and incubated at 28 ±2 °C for 7 days. The capacity to produce siderophores is represented by a transparent halo around the microorganism, the production efficiency (EP) was determined, related the measurements of the diameters of the halo (DH) and the colony (DC), EP= (DH-DC/ DC)*100. The siderophore production experiments will be carried out in duplicate.
Solubilization of phosphates. The ability of the isolated microorganisms to solubilize phosphate was determined using the PVK medium (Pikovskaya, 1948) which contains insoluble tricalcium phosphate. Said medium was supplemented with bromophenol blue to facilitate the observation of the solubilization haloes. Each isolate was inoculated in the center of a Petri dish with PVK medium and incubated at 28 ±2 °C for 7 days (Nautiyal, 1999). The solubilization efficiency (ES), related to the measurements of the diameters of the halo (DH) and the colony (DC), ES= (DH-DC/DC) *100, was determined. These tests were performed in duplicate.
The ACC activity (1-aminociclopropane-1-carboxylate) deaminase. The ACC deaminase activity was determined according to Penrose and Glick (2003). To this end, a pre-inoculum of each microorganism was prepared in broth with minimal DF salts (Dworkin and Foster). Subsequently, the culture was seeded in Petri dishes added with ACC (3 mM). Plates were incubated at 30 °C for 3 days. As control, the same strain was grown in a minimum medium DF with the presence of nitrogen source (NH4)2SO4, the capacity of a strain to use ACC was determined if it presented growth in DF medium with AA as the sole nitrogen source. They were made with three independent replicas.
Hemolytic activity. It was determined according to the technique followed by Figueroa et al. (2016), with modifications. The microorganisms were seeded on nutrient agar and incubated at 28 °C for 24 h and subsequently transferred to boxes with blood agar, where they were sown by stinging. Finally, the boxes were incubated at 28 °C for 24 h to monitor the appearance of hemolytic activity, classifying them as alpha hemolytic (partial hemolysis), betta hemolytic (complete hemolysis) and gamma hemolytic (no hemolytic activity). The evaluations were done in duplicate.
Trials of plant growth promotion in corn
Promising bacterial strains were selected based on their metabolic characteristics and tests were carried out to promote plant growth with greenhouse maize plants. The corn seeds were inoculated at the time of sowing with 1 × 106 UFC g-1 of substrate of each of the selected strains and in consortium, the tests were carried out in pots of 2.5 L a mixture formed by two parts of agricultural soil of the Valley of the Yaqui (clay texture), a part of peat and a part of pearlite. The physiological characteristics (height, stem diameter, fresh air weight and fresh root weight, total biomass, number of leaves) were quantified at 6 weeks after sowing (Parra-Cota et al., 2014).
Two trials were carried out at different times of the year: In the first trial (summer) 6 isolates from the Yaqui Valley were evaluated (11B20, 13B41, 52B4, 22A67, 53A2 and 22B45) while in the second trial (autumn) they were evaluated four strains (53A2, 52B4, 13B41, 41B1). In both trials seeds of hybrid H-431 (Ortega et al., 1993) were used, which stands out for its resistance to diseases, high temperatures, adaptation to different dates and ecological planting areas among other factors.
Statistical analysis
A completely randomized design was used with five or seven treatments including the control (plants not inoculated) depending on the number of strains to be evaluated in each experiment. Consisting of each experimental unit of five plants, two repetitions were made per treatment. All statistical analyzes of the physiological data were performed using the statistical program JMP8 (SAS Institute Inc., Cary, NC). The data were analyzed by analysis of variance (Anova) and Tukey test with a level of α= 0.05. In all the figures the average values are shown and the vertical bars represent standard deviation.
Results and discussion
From the soil samples, the total cultivable population in each site was determined. Site E, located to the south of the Valley (Figure 1), had a larger population, while site C had the smallest population, with 6 × 106 UFC gss-1 and 1.9 × 106 UFC gss-1, respectively (Figure 2). Subsequently, when isolating the microorganisms, it was found that there is no direct relationship between the population and the number of isolates, since all the sites with the exception of site D, presented a very similar number of isolated microorganisms (Table 1). A total of 216 microorganisms were isolated at the five sites.
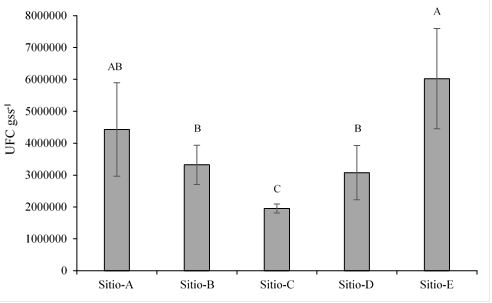
Figure 2 Population of microorganisms per site in the Yaqui Valley. Different letters indicate statistically significant differences (α= 0.05).
Table 1 Isolated microorganisms in each site sampled in the Yaqui Valley.
| Site | Bacteria | Actinomycetes | Fungi | Total |
| A | 25 | 14 | 9 | 48 |
| B | 24 | 20 | 1 | 45 |
| C | 17 | 28 | 0 | 45 |
| D | 16 | 3 | 4 | 23 |
| E | 11 | 44 | 0 | 55 |
| Total | 93 | 109 | 14 | 216 |
Based on their micro and macroscopic characteristics, the isolates were classified as bacteria (proteobacteria and firmicutes), actinomycetes and fungi. The number of bacterial and actinomycete isolates was greater than the fungal isolates, which may be an indication of the edaphoclimatic conditions of the sampling sites, because the soils of the Yaqui Valley generally have a lower organic matter content than the 1.5% (Lobell et al., 2002), in addition to sampling during the month of July, when temperatures are very high (22-41 °C) decreasing soil moisture.
Metabolic characterization
To characterize metabolically isolated microorganisms, the ability to synthesize indoles, solubilize phosphates and produce siderophores was determined.
In the tests to determine the production of indoles it was found that 207 isolates (96%) had the ability to produce indoles, 80% of the strains had a production of 1 to 4 ppm, while five strains had a production greater than 6 ppm, being the bacterial strain 22B45 the main producer with a synthesis of 11.47 ppm (Figure 3a, Table 2). Arruda et al. (2013) carried out the isolation and characterization of 292 bacteria of the maize rhizosphere, also finding that a high percentage (98%) of their isolates had the capacity to synthesize indoles. It has been reported that auxins produced by bacteria increase the area and length of the root surface and therefore provide the plant with greater access to soil nutrients (Glick, 2012).
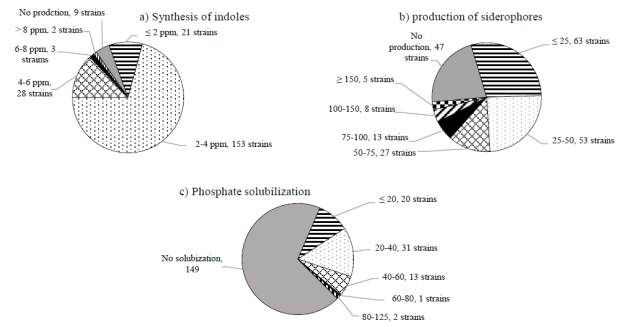
Figure 3 Metabolic capacities of isolated microorganisms; a) synthesis of indoles; b) production of siderophores; and c) Solubilization of phosphates.
Table 2 Microorganisms with the best metabolic capacities evaluated.
| ID strain | Microorganism | Indoles (ppm) | Siderophores (IEP) | Phosphates (IES) |
| 22B45 | Bacterium | 11.47 | 28.6 | 42.9 |
| 23A300 | Actinomycete | 8.54 | 20.5 | 0 |
| 43B3 | Actinomycete | 7.86 | 38.8 | 30.5 |
| 11B20 | Actinomycete | 7.05 | 37.5 | 0 |
| 51B3 | Actinomycete | 6.25 | 24.3 | 29.9 |
| 43B1 | Actinomycete | 5.88 | 39.3 | 15.6 |
| 23A800 | Actinomycete | 5.47 | 20 | 29.2 |
| 13B400 | Actinomycete | 5.42 | 108.3 | 47.2 |
| 53A2 | Bacterium | 2.52 | 333.3 | 0 |
| 31B5 | Bacterium | 2.7 | 300 | 0 |
| 22A50 | Fungus | 3.64 | 190 | 0 |
| 11B800 | Actinomycete | 2.12 | 161.1 | 18.2 |
| 12A5 | Actinomycete | 3.95 | 158.3 | 0 |
| 52B4 | Bacterium | 3.33 | 150 | 0 |
| 42A8 | Actinomycete | 2.44 | 150 | 0 |
| 43A2 | Actinomycete | 3.44 | 123.8 | 58.3 |
| 53A7 | Actinomycete | 2.16 | 8.7 | 105.4 |
| 11B700 | Actinomycete | 5.1 | 95 | 66 |
| 11B20 | Bacterium | 3.06 | 25 | 60 |
| 12B2 | Actinomycete | 2.65 | 100 | 58.3 |
| 22A67 | Bacterium | 2.88 | 40 | 58.3 |
| 51A4 | Actinomycete | 4.02 | 12.5 | 57.5 |
| 41B3 | Actinomycete | 2.72 | 0 | 125 |
Regarding the determination of strains with the ability to produce siderophores, molecules with high affinity to iron, 22% of the strains lack this characteristic. Approximately 100 strains had an efficiency lower than 50, identifying 10 strains with an efficiency higher than 100, the bacterial strain 53A2 presented the highest efficiency with 333.3 (Figure 3b, Table 2). Sharma and Johri reported in 2003 that two strains of Pseudomonas producers of siderophores had the ability to promote the growth of corn plants under iron-limiting conditions. The bacteria that synthesize siderophores can also act as biological control agents, De los Santos et al. (2013) conducted a study where it was found that through the production of siderophores, Burkholderia cepacia XXVI could inhibit the growth of the pathogen Colletotrichum gloeosporioides.
Subsequently the ability to solubilize phosphates for each of the isolated microorganisms was determined. This characteristic was the most particular, since only 67 isolates presented it, the majority with low efficiencies, only in 3 microorganisms a solubilization efficiency greater than 50 was determined.
The actinomycete 53A4 presented the highest efficiency (105.4). No fungal isolate was positive for this characteristic (Figure 3c, Table 2). The solubilization efficiencies of the isolates studied are low, but the maximum efficiencies coincide with those obtained in other studies (Kannapiran and Ramkumar, 2011). It has been reported that, in grain corn, it is possible to reduce the rate of phosphorus fertilizer by 50% without reducing the yield, if the plants are inoculated with the appropriate phosphate solubilizing microorganisms (Antoun, 2012).
A total of 54 isolates presented the three characteristics evaluated, 163 isolates were able to produce siderophores and indoles, 65 synthesized indoles and solubilized phosphates, while 55 isolates were able to synthesize siderophores and solubilize phosphates, unlike other studies (Arruda et al., 2013) where the number of strains with both capacities have not been found or has been limited. The potential of phosphorus-solubilizing microorganisms and with the simultaneous synthesis of pathogen-suppressing metabolites, mainly siderophores, phytohormones and lytic enzymes, has been demonstrated in several studies (Vassilev et al., 2006).
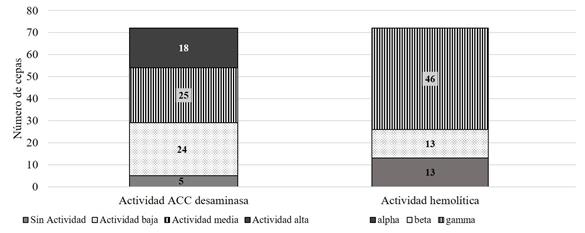
Subsequently, the strains with the best results in each of the determinations were selected, selecting a total of 76 strains of bacteria and actinomycetes, which were analyzed to qualitatively determine their ACC deaminase activity and haemolytic activity. It was found that 96% of the microorganisms analyzed have ACC deaminase activity, identifying 18 strains with high activity (Figure 4). The presence of ACC deaminase activity is an indication of the strain's potential to help plants mitigate the effects of certain stress conditions, since it eliminates the ACC, the precursor of ethylene synthesis and reduces the toxic effect of this hormone in high concentrations in plants (Glick, 2007).
Finally, the hemolytic capacity of the microorganisms was determined, which allows us to identify potentially pathogenic isolates (Forbes et al., 2007). The 64% of the strains have hemolytic gamma activity while only 18% have beta hemolytic activity (Figure 4). The percentage of beta hemolytic isolates was low, its use should not be ruled out completely since its products or metabolites can be used without applying the microorganism directly in the field.
Promotion of plant growth
To evaluate the growth promotion capacity of the isolated microorganisms, trials were carried out with maize plants under greenhouse conditions. Seven bacterial strains were selected, five of them (22B45, 11B20, 53A2, 52B4, 22A67) based exclusively on their results in the metabolic determinations, while the two remaining strains (13B41 and 41B1) were further selected for their tolerance to conditions of abiotic stress such as high temperatures, drought and salinity. The first trial was conducted in summer (June July), the results obtained in each treatment are shown in Table 3.
Table 3 Effect of inoculation of native edaphic bacteria on corn plants in Summer.
| Strains | Aerial dry weight (g) | Root dry weight (g) | Height (cm) | Stem diameter (mm) | Number of leaves |
| 53A2 | 9.5 a | 10.25 a | 26 a | 6.75 a | 6.75 a |
| 11B20 | 7.5 b | 8.5 a | 24 a | 5.25 abc | 5.25 b |
| 13B41 | 7.25 bc | 5.75 b | 22.75 a | 6 ab | 5.75 ab |
| 52B4 | 6 bcd | 9.5 a | 26 a | 4.5 bc | 5.5 ab |
| 22A67 | 6 bcd | 9.75 a | 23.5 a | 4.5 bc | 5.75 ab |
| 22B45 | 4.5 d | 3 c | 23.75 a | 4 c | 6 ab |
| Witness | 5.5 cd | 9 a | 23 a | 4.5 bc | 5.25 ab |
Different letters per column denote statistically significant differences between treatments (α= 0.05).
The inoculation with the 53A2 and 11B20 bacteria significantly promoted the growth of the aerial part of the plant, being the highest effect in the treatment with 53A2 with 2.37 g surpassing the control treatment that weighed 1.37 g. Similar results were found by Viruel et al. (2014) where they attribute the increase in biomass due to the increase in the availability of nutrients such as iron and phosphorus. Unlike the effect on the aerial part, the inoculation of the bacteria did not cause a statistically significant effect on the dry weight of the root or on the height of the plants in the treatments evaluated. The inoculation with the 53A2 bacteria increased the size of the diameter, which had an average size of 6.75 mm, increasing the stem thickness of the non-inoculated plants by 50%.
In the second trial, carried out during autumn (September October), a greater effect of the inoculation of growth-promoting bacteria was observed in maize plants (Table 4). The plants inoculated with strains 53A2 and 13B41 had a significantly higher air dry weight than the control treatment, with increases of 71.7 and 48.5%, respectively. A similar result was obtained in determining the dry weight of the root, where the best results were obtained with inoculations with strains 53A2 and 13B41, weighing 1.72 and 1.86, respectively. The inoculation of the four strains evaluated significantly increased the number of leaves (25%), compared to the control.
Table 4 Effect of inoculation of native edaphic bacteria on maize plants in Autumn.
| Strains | Fresh air weight (g) | Root fresh weight (g) | Aerial dry weight (g) | Root dry weight (g) | Height (cm) | Stem diameter (mm) | Number of leaves |
| 53A2 | 10 a | 14.5 a | 2.37 a | 1.72 ab | 27.5 a | 5.5 a | 8.75 a |
| 13B41 | 9.25 ab | 12 ab | 2.05 ab | 1.86 a | 29.5 a | 5.75 a | 8.75 a |
| 41B1 | 7.5 ab | 10.5 b | 1.74 bc | 1.43 bc | 23 a | 5.25 a | 8.75 a |
| 52B4 | 6.75 b | 9 b | 1.54 c | 1.03 c | 24.7 a | 4.25 ab | 8.75 a |
| Witness | 8.25 b | 10.25 b | 1.38 c | 1.28 c | 23.5 a | 3.25 b | 7 b |
Different letters per column denote statistically significant differences between treatments (α= 0.05).
Finally, no treatment had a significant effect on the height of the plants, but on the stem thickness (except 41B1) where increments of 61 to 77% were obtained with diameters of approximately 5.5cm compared to 3.25 of the non-inoculated control. Obtaining stems with greater thickness and not increasing the height with the inoculation of the bacterial strains is a good indicator of their potential in reproductive stages of the plants, since a reduced height of the plant is considered desirable in tropical maize, since plants that are generally tall, have a lot of foliage and a low harvest index, in addition, plants with thicker stems frequently have greater resistance to lodging or tipping (Forero and Fernández, 2010).
A better performance of the microorganisms was observed in the test carried out during the months of September and October, only strain 53A2 was consistent in both evaluations, which is inferred that it is a result of the capacity of each microorganism to adapt to different conditions, since It has been reported that their performance and effects on plants may be influenced by factors that include adaptation to different types of soils, climatic conditions and plant genotypes (Arruda et al., 2013; Saharan and Nehra, 2011).
Conclusions
However, the intensive agriculture that has been carried out in the Yaqui Valley, in the edaphic microbiota associated with the cultivation of corn, there is a diversity of microorganisms that have characteristics of agricultural interest, such as the synthesis of various compounds (auxins, siderophores and enzymes/organic acids for the solubilization of phosphates) that impact the development and health of plants. It is important the study and characterization of microorganisms adapted to soil and climatic conditions, to select those that have the greatest potential to maintain and multiply in the sites of interest and in this way, reduce the use of agrochemicals and in turn improve the fertility of the soil and increase/maintain crop yields by reducing negative effects on the environment.
Gratefulness
The authors thank the National Forestry, Agriculture and Livestock Research Institute (INIFAP) for financing the present study through the Fiscal Resources Research Project No. 2315932912: isolation and characterization of promising microorganisms to strengthen corn cultivation in Southern Sonora and Northern Sinaloa
REFERENCES
Ahrens, T. D.; Beman, J. M.; Harrison, J. A.; Jewett, P. K. and Matson, P. A. 2008. A synthesis of nitrogen transformations and transfers from land to the sea in the Yaqui Valley agricultural region of northwest Mexico. Water Res. Res. 44(7):1-13. [ Links ]
Antoun, H. 2012. Beneficial microorganisms for the sustainable use of phosphates in agriculture. Procedia Engineering. 46:62-67. [ Links ]
Armenta, B. A. D.; García, G. C.; Camacho, B. J. R.; Apodaca, S. M. Á.; Gerardo, M. L. y Nava, P. E. 2010. Biofertilizantes en el desarrollo agrícola de México. Ra Ximhai. 6(1):51-56. [ Links ]
Arruda, L.; Beneduzi, A.; Martins, A.; Lisboa, B.; Lopes, C.; Bertolo, F.; Passaglia, L. M. P. and Vargas, L. K. 2013. Screening of rhizobacteria isolated from maize (Zea mays L.) in Rio Grande do Sul State (South Brazil) and analysis of their potential to improve plant growth. Appl. Soil Ecol. 63:15-22. [ Links ]
Babalola, O. O. 2010. Beneficial bacteria of agricultural importance. Biotechnology letters. 32(11):1559-1570. [ Links ]
de los Santos, V. S.; Barrera, G. G. C.; Miranda, S. M. A. and Peña, C. J. J. 2012. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol. 28(8):2615-2623. [ Links ]
de los Santos, V. S.; Folter, S.; Délano, F. J.; Gómez, L. M.; Guzmán, O. D. and Peña, C. J. J. 2013. Growth promotion and flowering induction in mango (Mangifera indica L. cv “Ataulfo”) trees by Burkholderia and Rhizobium inoculation: morphometric, biochemical, and molecular Events. J. Plant Growth Reg. 32(3):615-627. [ Links ]
Duchemin, B.; Fieuzal, R.; Rivera, M. A.; Ezzahar, J.; Jarlan, L.; Rodríguez, J. C.; Hagolle, O. and Watts, C. 2015. Impact of sowing date on yield and water use efficiency of wheat analyzed through spatial modeling and FORMOSAT-2 images. Remote Sensing. 7(5):5951-5979. [ Links ]
Forbes, B. A.; Sahm, D. F. and Weissfeld, A. S. 2007. Bailey & Scott’s. Diagnostic microbiology. 12th edition, Mosby Elsevier. pp. 93-115. [ Links ]
Forero, F. E. and Fernández, J. P. 2010. Effect of different doses of fresh filter cake in the corn crop (Zea mays). Revista UDCA Actualidad Div. Científ. 13(1):77-86. [ Links ]
Glick, B. R. 2007. Promotion of plant growth by bacterial ACC deaminase. Critical Rev. Plant Sci. 26(5-6):227-242. [ Links ]
Glick, B. R. 2012. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012:1-15. [ Links ]
Glickmann, E. and Dessaux, Y. 1995. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61(2):793-796. [ Links ]
Kannapiran, E. and Ramkumar, V. S. 2011. Isolation of phosphate solubilizing bacteria from the sediments of Thondi coast, Palk Strait, Southeast coast of India. Ann. Biol. Res. 2(5):157-163. [ Links ]
Lobell, D. B.; Ortiz, M. J. I.; Addams, C. L. and Asner, G. P. 2002. Soil, climate, and management impacts on regional wheat productivity in Mexico from remote sensing. Agric. Forest Meteorol. 114(1):31-43. [ Links ]
Luers, A. L.; Lobell, D. B.; Sklar, L. S.; Addams, C. L. and Matson, P. A. 2003. A method for quantifying vulnerability, applied to the agricultural system of the Yaqui Valley, Mexico. Global Environ. Change. 13(4):255-267. [ Links ]
McCullough, E. B. and Matson, P. A. 2016. Evolution of the knowledge system for agricultural development in the Yaqui Valley, Sonora, Mexico. Proceedings of the National Academy of Sciences. 113(17):4609-4614. [ Links ]
Nihorimbere, V.; Ongena, M.; Smargiassi, M. and Thonart, P. 2011. Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol. Agron. Soc. Environ. 15(2):327-337. [ Links ]
Parra, C. F. I.; Peña, C. J. J.; de los Santos, V. S.; Martínez, G. N. A. and Délano, F. J. P. 2014. Burkholderia ambifaria and B. caribensis promote growth and increase yield in grain amaranth (Amaranthus cruentus and A. hypochondriacus) by improving plant nitrogen uptake. PloS one. 9(2):e88094 [ Links ]
Saleem, M.; Arshad, M.; Hussain, S. and Bhatti, A. S. 2007. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 34(10):635-648. [ Links ]
Saharan, B. S. and Nehra, V. 2011. Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res. 21(1):1-30. [ Links ]
Schwyn, B. and Neilands, J.B. 1987. Universal chemical assay for the detection and determination of siderophores. Analytical Biochem. 160(1):47-56. [ Links ]
Singh, J. S.; Pandey, V. C. and Singh, D. P. 2011. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosys. Environ. 140(3):339-353. [ Links ]
Sharma, A. and Johri, B. N. 2003. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol. Res. 158(3):243-248. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). Secretaría de Agricultura Ganadería Desarrollo Rural Pesca y Alimentación (SAGARPA) . 2016.. Producción anual agrícola. http://nube.siap.gob.mx/cierre-agricola/. [ Links ]
Vassilev, N.; Vassileva, M. and Nikolaeva, I. 2006. Simultaneous P-solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl. Microbiol. Biotechnol. 71(2):137-144. [ Links ]
Verma, J. P.; Yadav, J.; Tiwari, K. N. and Lavakush, S. V. 2010. Impact of plant growth promoting rhizobacteria on crop production. Inter. J. Agric. Res. 5(11):954-983. [ Links ]
Vessey, J. K. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil 255(2):571-586. [ Links ]
Viruel, E.; Erazzú, L. E.; Martínez, C. L.; Ferrero, M. A.; Lucca, M. E. and Siñeriz, F. 2014. Inoculation of maize with phosphate solubilizing bacteria: effect on plant growth and yield. J. Soil Sci. Plant Nutr. 14(4):819-831. [ Links ]
Received: November 00, 2017; Accepted: January 00, 2018











 texto em
texto em