Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.1 Texcoco Jan./Fev. 2018
https://doi.org/10.29312/remexca.v9i1.858
Essays
Collection of edaphic microorganisms and native endophytes to contribute to national food security
1Instituto Tecnológico de Sonora-CONACYT. 5 de febrero 818 Sur, Col. Centro, Ciudad Obregón, Sonora, México. CP. 85000.
2Campo Experimental Norman E. Borlaug-INIFAP. Norman E. Borlaug km 12, Cd. Obregón, Sonora. México. CP. 85 000. (parra.fannie@inifap.gob.mx).
3Instituto Tecnológico de Sonora. 5 de febrero 818 Sur, Col. Centro, Ciudad Obregón, Sonora, México. CP. 85 000. (angelikaherrera76@gmail.com; brendavalara@hotmail.com).
4Colección Nacional de Cepas Microbianas y Cultivos Celulares (CINVESTAV-IPN). Av. Instituto Politécnico Nacional 2508, Col. San Pedro Zacatenco. Ciudad de México, México. CP. 07 360. (jestrada@cinvestav.mx).
COLMENA (http://www.itson.mx/colmena), is a collection of microorganisms focused on the conservation, classification, characterization, and transfer of native microorganisms isolated from diverse agro-systems, and other habitats. The objective of this collection is to protect the microbial diversity associated with changes in land use, reducing the degradation of soils. So far, soil microorganisms from two important agricultural regions in Mexico have been isolated, the Yaqui Valley, Sonora and the Valley of Fuerte, Sinaloa. Currently, COLMENA conserves approximately 1 446 strains of soil microorganisms associated with various agricultural crops, such as: wheat (448), corn (313), alfalfa (54), broccoli (51), beans (35), among others. Recently, the taxonomic classification of 353 bacterial and fungal strains-through the amplification of the 16S RNAr and 5.8S RNAr genes- has been concluded, noting that the most abundant bacterial genera are Bacillus (27%), Pseudomonas (8%) and Stenotrophomonas. (6%), while the most abundant fungal genera were Aspergillus (8%), Penicillium (3%) and Myrothecium (3%). On the other hand, the metabolic characterization of a fraction of the collection was also carried out, finding that 3% of the microbial collection has the capacity to produce indoles (> 5 mg/L), the solubilization of phosphorus and the production of siderophores it was observed in 36% and 61% of the strains analyzed (396), respectively. Only 3% of the total microbial collection has been identified as producing cellulases and 11% of a total of 258 strains analyzed showed β-hemolysis. These results show the versatility of these microbial strains as potential cost-effective alternatives for agro-industrial practices, focused on contributing to global food security.
Keywords: agriculture; microbial collections; food security; soil
COLMENA (http://www.itson.mx/colmena), es una colección de microorganismos enfocada en la conservación, clasificación, caracterización, y transferencia de microorganismos nativos aislados de diversos agro-sistemas, y otros hábitats. El objetivo de esta colección es resguardar la diversidad microbiana asociada a los cambios de uso de suelo, disminuyendo la degradación de los suelos. Hasta el momento, microorganismos del suelo de dos importantes regiones agrícolas en México han sido aislados, el Valle del Yaqui, Sonora y el Valle del Fuerte, Sinaloa. Actualmente, COLMENA conserva aproximadamente 1 464 cepas de microorganismos edáficos asociadas a diversos cultivos agrícolas, tales como: trigo (448), maíz (313), alfalfa (54), brócoli (51), frijol (35), entre otros. Recientemente, la clasificación taxonómica de 353 cepas bacterianas y fúngicas -mediante la amplificación de los genes 16S RNAr y 5.8S RNAr- ha sido concluida, observando que los géneros bacterianos más abundantes son Bacillus (27%), Pseudomonas (8%) y Stenotrophomonas (6%), mientras que los géneros fúngicos más abundantes fueron Aspergillus (8%), Penicillium (3%) y Myrothecium (3%). Por otra parte, también se llevó a cabo la caracterización metabólica de una fracción de la colección, encontrando que 3% de la colección microbiana tiene la capacidad de producir de índoles (>5 mg L-1), la solubilización de fósforo y producción de sideróforos fue observada en 36% y 61% de las cepas analizadas (396), respectivamente. Solo 3% de la colección microbiana total ha sido identificada como productora de celulasas y 11% de un total de 258 cepas analizadas presentaron β-hemolisis. Estos resultados muestran la versatilidad de estas cepas microbianas como alternativas potenciales costo-efectivas para prácticas agro-industriales, enfocadas a contribuir a la seguridad alimentaria global.
Palabras clave: agricultura; colecciones microbianas; seguridad alimentaria; suelo
Introduction
World food security is one of the main challenges facing humanity today, which “exists when all people have, at all times, physical, social and economic access to sufficient, safe and nutritious food that meets their daily and daily energy needs and food preferences, to lead an active and healthy life” (FAO, 1996).
Today, enough food is produced for the entire world population, 7 350 million people; however, it is projected that it will increase to 9 billion people by the year 2050 (Godfray et al., 2010; Naciones Unidas, 2015), so that the demand for food will increase by 70-100% (World Bank, 2008; FAO, 2016). In this context, since agriculture contributes 98% of global food production (Rao, 2013), it is crucial to increase agricultural productivity with the objective of satisfying the global demand for food, contributing to food security and sovereignty national.
In this way, several challenges must be addressed: i) soil fertility (high costs of agricultural production associated with the consumption of agrochemicals); ii) climate change; iii) growing market for biofuels; iv) availability of natural resources; v) degradation of natural resources; and vi) limited technology transfer (Friedrich, 2014; Hernández-Mondragón et al., 2016). Thus, based on agricultural activities that are susceptible to being modified and implemented by man, food production is strongly linked to the fertility of agricultural soils.
The soil, consisting of mineral material (45%), water (20-30%), gases (20-30%) and organic matter (1-5%) (McCauley et al., 2005), provides diverse ecosystem services, such as: i) social and ecological sustainability; ii) adaptation and mitigation of climate change; iii) biotechnological resource for humanity; iv) water and nutrient cycling; and v) food security (Nelson et al., 2013). However, at a global level, soil degradation rates suggest that we will only have a fertile layer for 60 more years (Dotterweich, 2013). Currently, land degradation affects 1 900 million hectares worldwide, rapidly increasing at a rate of 5 to 7 million hectares per year. This degradation impacts approximately 70% of the agricultural soils in moderate to severe levels, which generates an estimated cost of $400 billion dollars per year worldwide, affecting more than one billion people, especially in arid zones (Olafur, 2007).
One of the main causes of soil degradation is the intensive agricultural practices used for agricultural production, from mechanical tillage to the excessive and constant use of synthetic fertilizers and pesticides (Friedrich, 2014). This degradation of soils leads to a decrease in its physical (moisture and gas exchange), chemical (pH and cation exchange capacity) and biological (modifications in microbial communities involved in nutrient cycling) properties, leading to an increase in its apparent density, less stability of its aggregates, susceptibility to compaction, loss of fertility, leaching of nutrients, decrease in productivity, increase of greenhouse gas emissions and decrease of carbon sequestration and microbial activity (Friedrich, 2014).
The diversity of edaphic microorganisms is an important component involved in the maintenance of soil fertility, this diversity includes more than 105 species (Dohrmann et al., 2013), which are responsible for carrying out between 80-90% of the processes observed in the soil(Nannipieri et al., 2003), i. e. the cycling of nutrients (nitrogen, carbon, sulfur and phosphorus), decomposition of organic matter, solubilization of minerals (K, Ca, Mn, Mg, etc), photosynthesis, degradation of xenobiotic compounds, control of plant diseases, maintenance of the structure and function of the soil, among others (Pankhurst et al., 1997; Pal an McSpadden, 2006; Pankratova, 2006; Ryan et al., 2008). Currently, only a small fraction of the soil microbial communities (1-10%) has been able to be cultivated, so the study of this microbial resource will allow understanding the impact of anthropogenic and natural activities on microbial diversity and ecology (Kalia and Gupta, 2005), representing a tool to increase the productivity of agricultural crops in a way that is friendly to the environment and cost-effective.
Recently, the use of beneficial microorganisms as the basis of biofertilizers has acquired great relevance in the agricultural sector, offering a sustainable alternative focused on increasing crop production and soil fertility. These comprise a heterogeneous group of free-living microorganisms or those associated with various parts of the plant, with the ability to stimulate plant growth, protect plants against attack by pathogens or tolerate abiotic stress conditions (high temperatures, salinity, and low water availability) (Dimkpa et al., 2009; Grover et al., 2011; Glick, 2012). The above has been studied under various microbial mechanisms, which can be classified as direct and indirect.
Thus, the promotion of plant growth directly by microorganisms involves mechanisms that facilitate the intake of nutrients from the soil, such as: i) nitrogen fixation; ii) the solubilization of minerals, such as potassium and phosphorus, in a way that makes them available for plants; iii) phytohormone production; and iv) mineralization of organic compounds. On the other hand, indirect mechanisms involve the antagonistic action against pathogens, through the production of antibiotics, hydrolytic enzymes, siderophores, exopolysaccharides, and induction of systemic response (Gupta et al., 2015).
The use of microbial inoculants has led to the reduction of the economic costs of agricultural production, due to a lower application of synthetic fertilizers and pesticides or the efficient use of these by plants, i.e. nitrogen and phosphorus (Gyaneshwar et al., 2002). In this sense, diverse bacterial and fungal genera, such as: Pseudomonas, Bacillus, Penicillum, Aspergillus, among others, have been reported for their metabolic characteristics of producing phytohormones, involved in root elongation and greater use of nitrogen or in the production of organic acids that contribute to neutralize the pH of the soil, favoring the solubilization of insoluble phosphorus (Kathiresan et al., 1995).
In addition, it has been identified that the application of bioinoculants allows to reduce the use of synthetic fertilizers, Adesemoye et al. (2009) inoculated a consortium composed of Bacillus amyloliquefaciens, Bacillus pumilus and Glomus intraradices in tomato cultivation at greenhouse level, that the application of 75% of the recommended fertilization dose plus the microbial consortium evaluated positively impacted the growth of the plants, their yield and absorption of nutrients (nitrogen and phosphorus), results comparable to those obtained by the use of the recommended total fertilizer dose.
Thus, during the last decades one of the strategies focused on the partial or total substitution of synthetic agricultural inputs has been the application of microbial inoculants with diverse metabolic capacities of interest. However, most of the Mexican agriculture has opted for the consumption and application of microbial consortia from other countries, where the climatic and crop conditions are different from those registered in our country and generally have obtained unfavorable results by the use of microbial inoculants in the national agricultural productivity, thus promoting the discontent of the productive sector towards the use of these microorganisms, without mentioning the potential ecological damages by the introduction of exogenous microbial strains to these agrosystems.
The main limitations for the success in the field of the application of microbial inoculants, are summarized in: 1) identifying a strain or microbial consortium with significant impact on the desired characteristic in the agricultural crop of interest; 2) the method of mass propagation and reproduction of these microorganisms, determining the optimal growth conditions; and 3) formulating the bio-inoculant with the promising strain (s) and the application vehicle or carrier. Once these limitations have been successfully covered, several aspects of the microorganisms that are part of this bio-blocker must be considered, such as i) having the capacity to settle in the soil or the plant under the biotic and abiotic conditions of the target ecosystem ; ii) compete with the native microbiota; and iii) colonize the rhizosphere and the part of the plant of interest, expressing the desired metabolic characteristics of growth promotion / protection against pathogens (Köhl et al., 2011; Brahmaprakash and Sahu, 2012; Galindo et al., 2013, Bashan et al., 2014). In addition, the success of the commercialization of this bio-inoculant depends on its economic viability in comparison to the synthetic fertilizers and fungicides available in the market (Malusa et al., 2016).
In this sense, the great effort of the scientific community for field application has led to the isolation of thousands of microbial crops associated with various agricultural crops of great importance to our country. A small number of strains have been studied at the laboratory level and only a few have become part of microbial inoculant developments and form part of a variety of biological products on the market, focused on increasing agricultural productivity in a sustainable manner. However, the vast majority of isolated microbial strains that are not selected due to the absence or low levels of some metabolic activity of interest are separated from the group of viable microorganisms, without considering the various potential metabolic characteristics stored in their genomes.
Therefore, the isolated microbiota should be protected in certified microbial collections (regardless of the metabolic characteristics of particular interest) to preserve ex situ the native microbial diversity associated with the crops, as well as the potential agro-biotechnological resource that these represent for the scientific community, producers and public-private sector, which will allow to explore even more its ecology in our current and future agriculture of our country.
Thus, the microbial collections play an important role in the conservation and sustainable use of the microbial resource, providing authentically pure, stable biological material, useful for carrying out research and teaching, facilitating access to reference strains and useful reagents for the control of quality (Sharman and Shouche, 2014). In addition, they offer training and training services in techniques related to the conservation, development and identification of microorganisms (WFCC, 2014).
On the other hand, the continuous discovery of new microbial species generates the need to preserve them and transfer them to the scientific community, to carry out research, teaching and their potential biotechnological exploitation. However, these microbial collections are usually found in highly specialized institutions focused on safeguarding their agro-biotechnological potential to guarantee food security in countries with a solid economy, greater scientific and technological development and aware of the importance of protecting the soil microbial communities.
These countries preserve, in a certified manner, approximately 98% of the total of strains, which constitutes 45% of the total of microorganisms protected in the American continent, in comparison to Mexico that only maintains around 9,078 microbial cultures in 18 collections, which represents only 2%, and that corresponds only 0.4% of the total microorganisms protected worldwide (WFCC, 2014).
Collection of edaphic microorganisms and native endophytes
The Collection of Native Endogenous and Microorganisms (COLMENA) www.itson.mx/colmena) is a microbial collection focused on the preservation, classification, characterization and transfer of native microorganisms isolated from different agro-systems and other habitats in Mexico. The objective of this collection is to reduce the loss of microbial diversity, as a strategy for soil conservation, by isolating, protecting, characterizing and typifying the cultivable soil microbial resource, quantifying the potential environmental and economic benefits of its re-incorporation into the ecosystems.
Currently, COLMENA preserves and studies a collection of 1 464 microorganisms isolated from soil and associated with various agricultural crops of economic importance to Mexico, such as: wheat (448), corn (313), beans (35), broccoli (51), alfalfa (54), and others (Figure 1). The first stage of COLMENA began with the isolation of soil microorganisms in two of the main agricultural regions of Mexico, a) the Yaqui Valley, located in the state of Sonora in Mexico (length 108° 53’ and 110° 37’ E latitude 26° 53’ and 28° 37’ north latitude) constituted by 225 000 ha, which contributes approximately 35% of the national wheat production (CIMMYT, 2017; SAGARPA, 2017) and b) the Valley of Fuerte, located in the state of Sinaloa (longitude 108° 16’ 47” and 109° 04’ 42” longitude west; latitude 25° 53’ 29” and the 26° 38’ 47” north), being the first place in the production of corn of the state, with a planted area of 277 642 ha (SAGARPA, 2011; SIAP-SAGARPA, 2016).
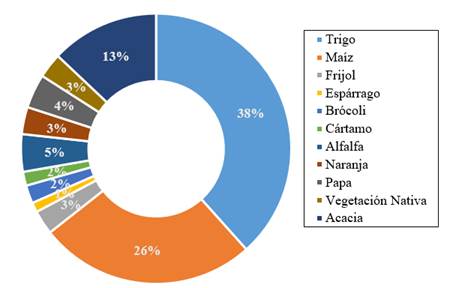
Figure 1 Percentage contribution of the number of microbial strains preserved in the COLMENA associated with the agricultural crops under study, located in the Yaqui Valley, Sonora and the Valley of Fuerte, Sinaloa, Mexico.
To date, 24% of the strains preserved in COLMENA have been characterized molecularly, by amplifying the gene 16S RNAr and 5.8S RNAr, identifying a bacterial diversity composed of 28 genera, the most abundant being: Bacillus (27%), Pseudomonas (8%) and Stenotrophomonas (6%) and 24 fungal genera, the most representative being Aspergillus (8%), Penicillium (3%) and Myrothecium (3%) (Figure 2). Thus, the detailed molecular identification of these strains is crucial to answer fundamental questions of systematics, taxonomy and evolution, which allows: i) to establish quality criteria in products and services; ii) identify sources of contamination; iii) selection of disinfection treatments; iv) identify microorganisms bioindicators of the ecosystem, v) identify microbial strains that promote plant growth and biological control agents; vi) study new microbial species; and vii) identify pathogenic or harmful strains, both for humans, plants and animals, among others (Emerson et al., 2008).
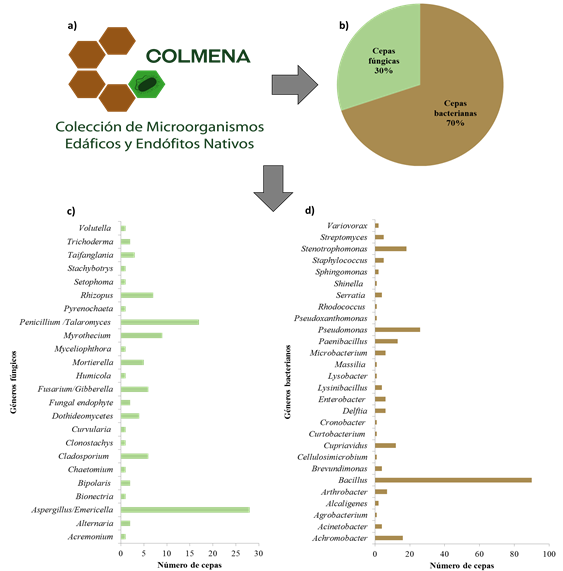
Figure 2 Composition of the strains sheltered in COLMENA. a) COLMENA logo; b) percentage of bacteria and fungi preserved in COLMENA; c) main fungal genera; and d) bacterials studied in COLMENA, obtained by the analysis of the 5.8S RNAr and 16S RNAr genes, respectively.
On the other hand, the identification of potentially pathogenic strains for human beings - through taxonomic studies and β-hemolytic activity - is carried out in COLMENA. Thus, up to the moment of a total of 258 evaluated microbial strains, 11% present β-hemolysis activity, which restricts its potential use as a microbial inoculant for the agricultural sector (Figure 3). COLMENA has specialized in identifying and characterizing microbial strains with metabolic capacities associated with the biological control of plant diseases and the promotion of plant growth, such as: the solubilization of phosphorus, biosynthesis of siderophores, production of indoles, production of lytic enzymes, among others (Figure 3).
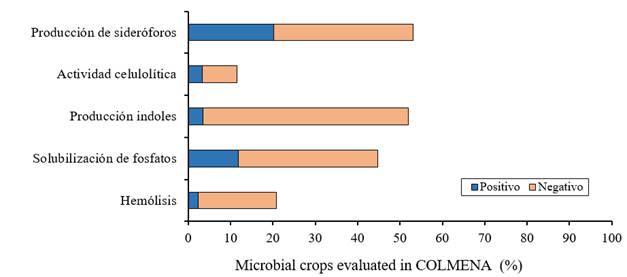
Figure 3 Percentage of promising strains for the promotion of plant growth and agents of biological control of phytopathogens conserved in COLMENA.
So far, 12% of the strains analyzed (660) have shown the ability to solubilize insoluble phosphorus, which is of great importance since in agro-systems, after nitrogen, phosphorus is the most important element in nutrition of plants, playing an important role in physiological processes such as photosynthesis, energy transfer, biosynthesis of macromolecules and respiration (Khan et al., 2010). However, between 95-99% of the phosphorus present in agricultural soils is found insoluble, limiting its use by plants, since they absorb the element in two soluble forms: monobasic (H2PO4) and dibasic ions (HPO42-) (Pandey and Maheshwari, 2007).
Thus, the microbiota associated with plants can contribute to the solubilization and use of insoluble phosphorus by these, by the release of complex compounds or mineral solvents (anions of organic acids, protons, hydroxyl ions, CO2), release of extracellular enzymes (mineralization phosphate biochemistry) and phosphate release during soil degradation (biological mineralization of phosphates) (Sharma et al., 2013).
Another important nutrient in the development of plants and microorganisms is iron, being the fourth most abundant element on the earth’s surface (1-6%), which is related in the transport of electrons, in the catalysis of enzymatic reactions involved in the metabolism of hydrogen, oxygen -synthesis of ATP- and nitrogen, as well as in the synthesis of DNA (Faraldo-Gómez and Sansom, 2003). However, under certain oxygen and pH conditions it is scarcely available, due to its rapid oxidation of Fe+2 to Fe+3, and to the subsequent formation of insoluble hydroxides (Faraldo-Gómez and Sansom, 2003).
Bacteria have developed various mechanisms for the acquisition of iron, one of the most used mechanisms is the synthesis of chelating compounds called siderophores (Crichton, 2001). The production and excretion of siderophores by microorganisms associated with agricultural crops stimulates the growth of these, improving nutrition or inhibiting the establishment of phytopathogens through the sequestration of iron from the environment, which limits their growth (de Souza et al., 2015). In this sense, 290 microbial strains capable of biosynthesizing different classes of siderophores have been identified.
Likewise, 50 microorganisms have been identified with the production capacity of indoles, a group to which the indole acetic acid belongs, the most important natural auxin in plants. This phytohormone plays a central role in cell division, elongation, fruit development and senescence, so it is directly involved in the regulation of plant growth, promotes the formation of apical domains, longitudinal increase of the root and development of organs (Camelo et al., 2011; Grover et al., 2011; Duca et al., 2014). Some microorganisms are able to synthesize indole acetic acid which, at ideal concentrations in the plant, stimulates the formation of radicular hair, increases the number and length of primary and lateral roots (Duca et al., 2014).
In COLMENA have been identified 60 microbial strains with the ability to produce cellulolytic enzymes, which may be involved in various biological control mechanisms by microbial genera, such as: Trichoderma, Fusarium, Penicillium, Alternaria, Cellolomonas, Bacillus, Pseudomonas, among others (Lynd et al., 2002; Ahmad et al., 2008). In COLMENA microbial species reported as pathogenic to plants are also conserved, such as: Sclerotinia sclerotiorum (causal agent of white mold in beans), Fusarium verticillioides (causal agent of ear rot of corn), and Bipolaris sorokiniana (causal agent of the spot blurred in wheat) (Pal and McSpadden-Gardener, 2006; Villa-Rodríguez et al., 2016). Thus, the study of these potentially phytopathogenic strains to various agricultural crops of national importance will allow us to know their mechanisms of action, leading to the development of more efficient and sustainable strategies for their control.
Conclusions
The progressive requirement of food as a consequence of the world population growth rate demands the increase of agricultural productivity, through the use of efficient and sustainable agricultural practices. An alternative is the use of native microbial diversity associated with agricultural crops, representing a potential agro-biotechnological resource for agriculture and the scientific community. In this way, the microbial collections have an important contribution in the conservation and sustainable use of the microbial resource, providing authentic biological material, stable, and useful to develop the proposed strategies.
The Collection of Native Endophysical and Microorganisms (COLMENA) preserves and characterizes microorganisms associated with agricultural crops of national importance, through the identification of plant growth promoting strains and biological control agents of phytopathogens. In addition, COLMENA is a dynamic project, which will include a greater number of agricultural areas nationwide, continuing with the metabolic and molecular characterization of the microorganisms protected in the collection, as well as implementing studies on additional metabolic characteristics such as the production of antibiotics, stress tolerance tests, formulation of bio-inoculants, field tests, to name a few.
Gratefulness
The authors thank the support received by the National Council of Science and Technology through the financing of the project 253663 “Strengthening the infrastructure of the Biotechnology Laboratory of the Microbial Resource of the ITSON for the creation of the Collection of Native Endogenic Microorganisms and Endophytes ( COLMENA), to contribute to regional and national food security" and to the National Institute of Forestry, Agriculture and Livestock Research (INIFAP), for the financing of the fiscal project 2315932912 “Isolation and characterization of promising microorganisms to strengthen the cultivation of corn in the south of Sonora and north of Sinaloa”. As well as the entire team working in the biotechnology laboratory of the microbial resource for their dedication and commitment to the creation of COLMENA.
REFERENCES
Adesemoye, A. O.; Torbert, H. A. and Kloepper, J. W. 2009. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Estados Unidos de América. Microbial Ecol. 58(4):921-929. [ Links ]
Ahmad, F.; Ahmad, I. and Khan, M. S. 2008. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Paises Bajos. Microbiol. Res. 163(2):173-181. [ Links ]
Alexander, D. B. and Zuberer, D. A. 1991. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Alemania. Biol Fert Soils. 12(1):39-45. [ Links ]
Bashan, Y.; de-Bashan, L. E.; Prabhu, S. R. and Hernandez, J. P. 2014. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998-2013). Paises Bajos. Plant Soil 378(1-2):1-33. [ Links ]
Brahmaprakash, G. P. and Sahu, P. K. 2012. Biofertilizers for sustainability. India. J. Ind. Institute Sci. 92(1):37-62. [ Links ]
Camelo, M.; Vera, S. y Bonilla, R. 2011. Mecanismos de acción de las rizobacterias promotoras del crecimiento vegetal. Colombia. Corpoica Ciencia Tecnología Agropecuaria. 12(2):159-166. [ Links ]
Centro Internacional de Mejoramiento de Maíz y Trigo (CIMMYT). 2017. Wheat Atlas by CIMMYT. http://wheatatlas.org/country/mex/?aspxautodetectcookiesupport=1. [ Links ]
Crichton, R. 2001. Inorganic biochemistry of iron metabolism: from molecular mechanisms to clinical consequences. 2nd (Ed.). John Wiley & Sons, Ltd., Chichester. 326 p. [ Links ]
de Souza, R.; Ambrosini, A. and Passaglia, L. M. P. 2015. Plant growth-promoting bacteria as inoculants in agricultural soils. Brasil. Gen. Mol. Biol. 38(4):401-419. [ Links ]
Dimkpa, C.; Weinand, T. and Asch, F. 2009. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Reino Unido. Plant Cell Environ. 32(12):1682-1694. [ Links ]
Dohrmann, A. B.; Ku, M.; Ju, S.; Jaenicke, S.; Schlu, A. and Tebbe, C. C. 2013. Importance of rare taxa for bacterial diversity in the rhizosphere of Bt - and conventional maize varieties. Reino Unido. ISME J. 7(1):37-49. [ Links ]
Dotterweich, M. 2013. The history of human-induced soil erosion: geomorphic legacies, early descriptions and research, and the development of soil conservation- a global synopsis. Países Bajos. Geomorphology. 201(1):1-34. [ Links ]
Duca, D.; Lorv, J.; Patten, C. L.; Rose, D. and Glick, B. R. 2014. Indole-3-acetic acid in plant - microbe interactions. Paises Bajos. Antonie Van Leeuwenhoek. 106(1):85-125. [ Links ]
Emerson, D.; Agulto, L.; Liu, H. and Liu, L. 2008. Identifying and characterizing bacteria in an era of genomics and proteomic. Estados Unidos. BioSci. 58(10):925-936. [ Links ]
FAO (Organización de las Naciones Unidas para la Agricultura y la Alimentación). 1996. Declaración de Roma sobre la Seguridad Alimentaria, en Cumbre Mundial sobre la Alimentación. 13-17 de noviembre, 1996. Roma, Italia. http://www.fao.org/docrep/003/ w3613s/w3613s00.htm. [ Links ]
FAO (Organización de las Naciones Unidas para la Alimentación y la Agrícultura). 2016. Resumen: El estado mundial de la agricultura y la alimentación. Cambio climático, agricultura y seguridad alimentaria. [ Links ]
Faraldo, G. J. D. and Sansom, M. S. 2003. Acquisition of siderophores in gram-negative bacteria. Reino Unido. Nat Rev Mol Cell Biol. 4(2):105-116. [ Links ]
Friedrich, T. 2014. La seguridad alimentaria: retos actuales. Cuba. Revista Cubana de Ciencia Agrícola. 48(4):319-322. [ Links ]
Galindo, E.; Serrano, C. L.; Gutiérrez, C. R.; Allende, R.; Balderas, K.; Patiño, M.; Trejo, M.; Wong, M.; Rayo, E.; Isauro, D. and Jurado C. 2013. The challenges of introducing a new biofungicide to the market: a case study. Chile. Electronic J. Biotechnol. 16(3):5-5. [ Links ]
Glick, B. 2012. Plant growth-promoting bacteria: Mechanisms and applications. Estados Unidos de América. Scientifica. 2012(1):1-15. [ Links ]
Godfray, H. C. J.; Beddington, J. R.; Crute, I. R.; Haddad, L.; Lawrence, D.; Muir, J. F.; Pretty, J.; Robison, S.; Thomas, S. M. and Toulmin, C. 2010. Food security: the challenge of feeding 9 billion people. Estados Unidos de América. Science. 327(5967):812-818. [ Links ]
Grover, M.; Ali, S. and Sandhya, V. 2011. Role of microorganisms in adaptation of agriculture crops to abiotic stress. Paises Bajos. World J. Microbiol. Biotechnol. 27(5):1231-1240. [ Links ]
Gupta, G.; Parihar, S. S.; Ahirwar, N. K.; Snehi, S. K. and Singh, V. 2015. Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. Estados Unidos. J. Microbial Biochem. Technol. 7(2):96-102. [ Links ]
Gyaneshwar, P.; Kumar, G. N.: Parekh, L. J. and Poole, P. S. 2002. Role of soil microorganisms in improving P nutrition of plants. In: Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities. Adu-Gyamfi J. J. (Ed). Springer Netherlands. Paises Bajos. 133-143 pp. [ Links ]
Hernández, M. A. C.; Herrera, E. L. and Kuri, H. W. 2016. Technology in society legislative environment and others factors that inhibit transfer of Mexican publicly funded research into commercial ventures. Estados Unidos de América. Technol. Soc. 46:100-108. [ Links ]
Kalia, A. and Gupta, R. P. 2005. Conservation and utilization of microbial diversity. India. NBA Scientific Bulletin. 9(7):307. [ Links ]
Kathiresan, G.; Manickam, G. and Parameswaran, P. 1995. Efficiency of phosphobacteria addition on cane yield and quality. India. Cooperative Sugar. 26:629-631. [ Links ]
Khan, M. S.; Zaidi, A.; Ahemad, M.; Oves, M. and Wani, P. A. 2010. Plant growth promotion by phosphate solubilizing fungi - current perspective. Reino Unido. Arch Agron Soil Sci. 56(1):73-98. [ Links ]
Köhl, J.; Postma, J.; Nicot, P.; Ruocco, M. and Blum, B. 2011. Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Estados Unidos de América. Biological Control. 57(1):1-12. [ Links ]
Lynd, L.; Weimer, P.; Zyl, H. and Pretorius, I. 2002. Microbial cellullose utilization: fundamentals and Biotechnology. Estados Unidos de América. Microbiol. Mol. Biol. Reviews. 66(3):506-577. [ Links ]
Malusà, E.; Pinzari, F. and Canfora, L. 2016. Efficacy of biofertilizers: challenges to improve crop production. In: Microbial Inoculants in Sustainable Agricultural Productivity. Singh, D. P.; Singh, H. B. and Prabha, R. (Eds). Springer. New Delhi, Heidelberg, New York, Estados Unidos de América. 17-40 pp. [ Links ]
McCauley, A.; Jones, C. and Jacobsen, J. 2005. Basic soil properties. Reino Unido soil and water management module. 1(1):1-12. [ Links ]
Naciones Unidas. 2015. Worls population prospects, key findings and advance tables. 28-02-2017. https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf . [ Links ]
Nannipieri, P.; Ascher J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G. and Renella G. 2003. Microbial diversity and soil functions. Reino Unido. Eur. J. Soil Sci. 68(1):12-26. [ Links ]
Nelson, E. J.; Kareiva, P.; Ruckelshaus, M.; Arkema, K.; Geller, G.; Girvetz, E.; Goodrich, D.; Matzek; V.; Pinsky; M.; Reid, W.; Saunders; M.; Semmens; D. and Tallis, H. 2013. Climate change’s impact on key ecosystem services and the human well-being they support in the US. Estados Unidos de América. Frontiers Ecol. Environ. 11(9):483-893. [ Links ]
Olafur, A. 2009. Soils and the living Earth. In: soils, society and global change: proceedings of the international forum: celebrating the centenary of conservation and restoration of soil vegetation in Iceland: 31 August-4 September 2007. Bigas, H.; Guðbrandsson, G. I. Montanarella, L. and Arnalds, A. (Eds.). European Communities. Selfoss, Islandia. 40-45 pp. [ Links ]
Pal, K. K. and McSpadden, G. B. 2006. Biological control of plant pathogens. Estados Unidos de América. The Plant Health Instructor. 2:1117-1142. [ Links ]
Pandey, P. and Maheshwari, D. K. 2007. Two sp. microbial consortium for growth promotion of Cajanus Cajan. Paises Bajos. Current Science. 92(8):1137-1142. [ Links ]
Pankhurst, C. E.; Doube, B. M. and Gupta, V. V. S. R. 1997. Biological indicators of soil health: Synthesis. In Biological Indicators of Soil Health. Pankhurst, C. E.; Doube, B. M. and Gupta, V. V. S. R. (Eds). CAB International. 419-435 pp. [ Links ]
Pankratova, E. M. 2006. Functioning of cyanobacteria in soil ecosystems. Federación Rusa. Eur. Soil Sci. 39(1):S118-S127. [ Links ]
Rao, A. N. 2013. Food, agriculture and education: science and technology education and future human needs. Elsevier. 6. New York, Estados Unidos de América. 288 p. [ Links ]
Ryan, R. P.; Germaine, K.; Franks, A.; Ryan, D. J. and Dowling, D. N. 2008. Bacterial endophytes: recent developments and applications. Reino Unido. FEMS Microbiol Lett. 278(1):1-9. [ Links ]
SAGARPA. 2011. Estudio de gran visión y factibilidad económica y financiera para el desarrollo de infraestructura de almacenamiento y distribución de granos y oleaginosas para el mediano y largo plazo a nivel nacional. http://www.sagarpa.gob.mx/agronegocios/documents/estudios_promercado/granos.pdf. [ Links ]
SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). 2017. Productores de trigo obtienen rendimiento de nueve toneladas. http://www.sicde.gob.mx/portal/bin/nota.php?accion=buscar¬aId=915414826576843306f86e. [ Links ]
Sharma A. and Shouche Y. 2014. Microbial Culture Collection (MCC) and International Depositary Authority (IDA) at National Centre for Cell Science, Pune. India. Ind. J. Microbiol. 54(2):129-133. [ Links ]
Sharma, S. B.; Sayyed, R. Z.; Trivedi, M. H. and Gobi, T. A. 2013. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Reino Unido. Springerplus. [ Links ]
SIAP-SAGARPA (Servicio de Información Agroalimentaria y Pesquera). 2016. http://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119?idiom=es. [ Links ]
Villa, R. E.; Lugo, E. C.; de los Santos, V. S.; Parra, C. F. I. and Figueroa, L. P. 2016. First report of Cochliobolus sativus causing spot blotch on durum wheat (Triticum durum) in The Yaqui Valley, Mexico. Estados Unidos de América. Plant Dis. 10:(11):2329. [ Links ]
World Bank. 2008. World Development Report 2008: agriculture for development. Washington, DC: World Bank. [ Links ]
World Federation for Culture Collections (2014). World directory of culture collections(sixth version, 2014). http://www.wfcc.info/ccinfo/index.php/home/content. [ Links ]
Received: December 00, 2017; Accepted: January 00, 2018











 texto em
texto em 


