Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 spe 19 Texcoco nov./dic. 2017
https://doi.org/10.29312/remexca.v0i19.675
Essays
Postharvest fruits: maturation, softening and transcriptional control
1Universidad Autónoma de Nayarit-Posgrado en Ciencias Biológico Agropecuarias. Ciudad de la cultura “Amado Nervo”, Tepic, Nayarit, México. CP. 63155. (mc.monica.martínez@gmail.com).
2Universidad Autónoma de Nayarit-Unidad de Tecnología de Alimentos. Ciudad de la cultura “Amado Nervo” s/n. Tepic, Nayarit, México. CP. 63155. (pasingan@gmail.com; lguzman2303@hotmail.com).
3Universidad Autónoma del estado de Morelos-Posgrado en Ciencias Agropecuarias y Desarrollo Rural. Av. Universidad núm. 1001, Col. Chamilpa, Cuernavaca, Morelos, México. CP. 62209. (iran.alia@uaem.mx).
4Centro Nacional de Recursos Genéticos, INIFAP. México.
The process of fruit formation requires a complex series of gene interaction and signaling pathways for the conversion of a flower ovary into a fruit. In fleshy fruits it involves the growth, development and maturation of the fruit. The objective of this review is to compile the information on relevant research related to the ripening, softening and transcriptional regulation of fruits during post - harvest management. In order to carry out this study search, multiple databases were used (Scielo, Redalyc, Elsevier, Scopus, Wiley Online Library, ScienceDirect, Springer). The importance of fruit maturation and its analysis at the molecular level has been of great interest in research, as it is a complex physiological and biochemical process leading to changes in appearance, texture, taste and aroma. Transcription factors have been shown to be of great importance, not only during early development, but also in the regulatory control of maturation and senescence; although there is progress in identifying these regulators, much remains to be investigated. In the near future, it will be possible to control the maturation of fruits and extend their shelf life by manipulating ethylene production using a transgenic approach.
Keywords: ethylene; maturation; postharvest; regulation; softening; transcriptional control
El proceso de formación de un fruto requiere una serie compleja de interacción de genes y rutas de señalización para la conversión de un ovario de la flor en un fruto. En frutos carnosos involucra el crecimiento, el desarrollo y la maduración del fruto. El objetivo de esta revisión se enfoca en la recopilación de la información sobre investigaciones relevantes relacionadas con la maduración, ablandamiento y regulación transcripcional de los frutos durante el manejo postcosecha de éstos. Para realizar esta búsqueda se hizo uso de múltiples bases de datos (Scielo, Redalyc, Elsevier, Scopus, Wiley Online Library, ScienceDirect, Springer). La importancia de la maduración de los frutos y su análisis a nivel molecular ha sido de gran interés en la investigación, ya que es un proceso fisiológico y bioquímico complejo que conduce a cambios en la apariencia, la textura, el sabor y el aroma. Los factores de transcripción han mostrado tener una gran importancia, no sólo durante el desarrollo temprano, sino también en el control regulatorio de la maduración y la senescencia; aunque hay avances en la identificación de estos reguladores, queda mucho por investigar. En un futuro próximo, será posible controlar la maduración de los frutos y alargar su vida de anaquel manipulando la producción de etileno utilizando un enfoque transgénico.
Palabras clave: ablandamiento; control transcripcional; etileno; maduración; poscosecha; regulación
Introduction
Seeds represent the germplasm of the plant, so the strategy for dispersal is critical to ensure the survival of the next generation. The fruits have developed complex mechanisms to maximize the efficiency of this process (Karlova et al., 2014) . From an ecological point of view, the immature fruits represent an organ that must be protected, so it should not be attractive and its green color allows it to camouflage itself with the leaves (Iqbal et al., 2017). The maturation is a coordinated event of different biochemical pathways regulated by ethylene that produces changes in the tissue surrounding the seeds, making the fruit more attractive and thus helping the dispersion of the same. These changes affect the color, smell, texture and sugar content, which has been exploited by humans for the domestication of crops (Klee and Giovannoni, 2011; Seymour et al., 2013).
Therefore, studies have been carried out for a better understanding of the floral organ development of the fruit (Bao et al., 2010; Seymour et al., 2013) the role of hormones and genes related to the development and the maturation of fruits (Alexander and Grierson, 2002; Cara and Giovannoni, 2008; Kumar et al., 2014), as well as physiological disorders (Pegoraro et al., 2010) and the maturation-associated epigenetic alterations (Manning et al., 2006; Zhong et al., 2013).
In this sense, molecular biology has contributed significantly to elucidating and occur growth and fruit development (Gapper et al., 2013; McAtee et al., 2013; Pech et al., 2013; Seymour et al., 2013; Gapper et al., 2014; Kumar et al., 2014; Dos Santos et al., 2015).
Studies by Bouzayen et al. (2010) revealed through advanced genomic and post-genomic tools the mechanisms by which nutritional and sensorial quality are developed during the stages of physiological maturity and consumption of the fruit using.
One of the most studied phenomena is the softening of the fruit, which is a genetically programmed series of events, characterized by biochemical and physiological processes that alter its firmness, color, taste and texture (Nishiyama et al., 2007). Since most of the quality attributes are generated during the maturation process, it has been considered essential to better understand the mechanisms involved in this last stage of development (Bouzayen et al., 2010). The objective of this review is to compile the information on relevant research related to the ripening, softening and transcriptional regulation of fruits during post-harvest management.
Ethylene biosynthesis and fruit classification
The fruits can be classified as climacteric and non-climacteric according to their pattern of increase in the production of ethylene and carbon dioxide during maturation, although there are important variations in the synchrony and rate of increase, technically represents a very useful categorization for the management of the harvest and post-harvest handling of the fruits (Lelièvre et al., 1997). Those that present a climacteric respiratory and an increase in the production of ethylene, are known as climacteric fruits and include fruits such as tomato, banana, apple, pear, mango and papaya (Omboki et al., 2015). In contrast, in non-climacteric fruits such as strawberry, grape and citrus, increased respiration does not show a climax and ethylene production is low or absent (Dos Santos et al., 2015).
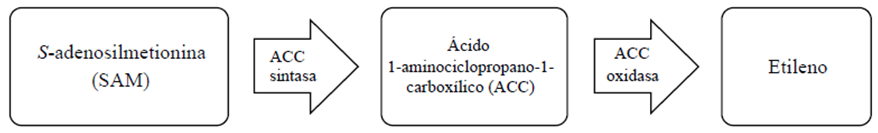
The major steps in ethylene biosynthesis (Figure 1) involve the conversion of S-adenosylmethionine (SAM) to 1-aminocyclopropane-1 -carboxylic acid (ACC) by ACC synthase (ACS) and then by ACC oxidase (ACO) to ethylene (Alexander and Grierson, 2002). In the climacteric fruit tissue, ethylene biosynthesis begins at a low level during development (system 1), but at the beginning of maturation it becomes autoclaved (system 2) (Karlova et al., 2014), because presence of ethylene activates the action of the gene encoding the ACO enzyme that converts ACC to ethylene (Liu et al., 2015).
Several genetic studies have suggested that the maturation process is programmed into the cell and requires differential expression of genes, resulting in the transcription of specific mRNAs and the synthesis of de novo proteins (Lincoln and Fischer, 1988; Darley et al., 2001).
In this sense, a trend in research has been the use of molecular biology techniques aimed at the isolation, recognition and expression of the genes of the main enzymes that act during the softening occurred in the maturation of the fruits (Brummell and Harpster, 2001). However, the molecular differences between climatic and non-climacteric maturation are still poorly known, and it has been proposed that ethylene plays a role as a maturation coordinator in climacteric species to facilitate rapid and coordinated maturation (Giovannoni, 2004).
Molecular regulation of ethylene biosynthesis
Understanding the mode of action of ethylene has progressed with the identification and use of transcription factors capable of binding to the promoter region of genes related to biosynthesis and ethylene action, such as the transcriptional regulators belonging to the type Apetala2 (AP2)/ethylene response factor (ERF) (Klee and Giovannoni, 2011; Pech et al., 2012; Grierson, 2013; Pech et al., 2013).
Previously, the molecular bases of ethylene synthesis and regulation were a focal point of the maturation analysis. However, more recent studies have emphasized the characterization of ethylene signal transduction (Giovannoni, 2004). However, ethylene by itself is not sufficient for maturation and for there to be a response to ethylene there must be adequate development (Lelièvre et al., 1997; Giovannoni, 2001). Consequently, immature fruits do not usually mature in response to the application of exogenous ethylene. Bauchot et al. (1998) observed that in transgenic melons with silenced ethylene synthesis some aspects of climatic maturation, such as the production of volatile aroma compounds, are regulated by development factors that must be adequately coordinated with ethylene synthesis.
At least six different putative ethylene receptors in the tomato genome, LeETR1, LeETR2 (Zhou et al., 1996; Lashbrook et al., 1998), NR (Wilkinson et al., 1995; Payton et al., 1996), LeERT4, LeETR5 (Tieman y Klee, 1999), and LeETR6 (Ciardi y Klee, 2001), differentially expressed in several tissues such as leaves, seeds and flower (Alexander y Grierson, 2002).
Transcripts encoding two ethylene receptors (NR and LeTR4) accumulate at high levels in maturing tomato fruits, suggesting that these two receptors may function in ethylene-induced maturation (Wilkinson et al., 1995; Yen et al., 1995; Alba et al., 2005).
Alba et al. (2005) demonstrated that ethylene regulates biochemistry, morphology, and transcriptomic activity throughout fruit development. Comparison of the expression patterns of genes encoding enzymes in fruit development pathways to products measured from these routes (ethylene, carotenoids and ascorbate) suggest new regulatory points of fruit development influenced by ethylene.
The activity of the enzyme ACC synthase is affected by protein phosphorylation. This evidence reinforces the idea that ethylene synthesis is regulated from transcription to its activity (Argueso et al., 2007); for example, the contrasting profiles of the E4 and E8 genes. Transcription of the E4 gene is stimulated by both system 1 and system 2 of ethylene biosynthesis. Whereas transcription of the E8 gene is induced only in mature fruits; that is, by system 2, a strong indicator that both are developmentally regulated and are specific to certain tissues (Omboki et al., 2015).
Many genes are involved in ethylene biosynthesis, but the genes encoding the ACO and ACS enzymes are the most characterized (Liu et al., 2015). Barry et al. (2000) reported that nine genes encode the enzyme ACS, LeACS1A, LeACS1AB and LeACS2 to 8, of which 4 are expressed during fruit maturation: LeACS1A, LeACS2, LeACS4 and LeACS6. LeACS6 is the main ACS gene involved in the synthesis of ethylene in green fruits, although LeACS1A was also expressed in the same tissues (10-day tomato seedlings). Expression of LeACS1 and LeACS4 is induced during the maturation step depending on the RIN-MADS transcription factor (Vrebalov et al., 2002).
On the other hand, the ACO enzyme is encoded by five genes in tomato where three of them, LeACO1, 3 and 4 are differentially expressed (Van-der-Hoeven et al ., 2002). The levels of LeACO1 and 4 increase massively in the immature green stage of a fruit and are highly expressed during climatic maturation, while their levels were maintained during the maturation period (Omboki et al., 2015), while expression of LeACO3 is highly dependent on ethylene (Llop-Tous et al., 2000). The genes encoding ACO and ACS have also been characterized in peach (Tatsuki et al., 2006), melon (Yamamoto et al., 1995) and apple (Dandekar et al., 2004; Omboki et al., 2015).
In strawberry, a non-climacteric modeling fruit, it has been found that the ethylene concentration is relatively high in green fruits, decreases in white fruits and finally increases again in the red stage of ripening (Perkins -Veazie et al., 1996; Iannetta et al., 2006). In addition, this last increase is accompanied by a higher rate of respiration that resembles that produced in climacteric fruits at the beginning of maturation (Iannetta et al., 2006).
Recent evidence of regulation of MADS-box maturation, both tomato (climacteric) and strawberry (non-climacteric) genes, suggests common regulatory mechanisms operating in both species, climacteric and non-climacteric (Vrebalov et al., 2002).
Transcriptional control of maturation
The study of the transcriptional regulation of the early development of fleshy fruits in species other than tomatoes is hampered by the lack of or difficulty of transformation protocols for functional studies and the lack of available mutants. Therefore, information on the gene function of these species is often incomplete and is derived from studies of expression or heterologous expression in other species (Karlova et al., 2014).
Recently, a significant advance has been made in the understanding of transcriptional control of maturation using tomato (Solanum lycopersicum) as a model system. The transcription factors ripening-inhibitor (RIN) (Vrebalov et al., 2002), nonripening (NOR) (Giovannoni, 2007) and colorless nonripening (CNR) (Manning et al., 2006) function as global regulators of maturation and act anti-ethylene, whereas additional transcription factors that are required for normal maturation include TOMATO agamous-like1 (TAGL1) (Itkin et al., 2009; Vrebalov et al., 2009), hd-zip homeobox protein-1 (HB-1) (Lin et al., 2008), apetala2a (AP2a) (Chung et al., 2010; Karlova et al., 2011), ethylene response factor 6 (ERF6) (Lee et al., 2012), arabidopsis pseudo response regulator2-like (APRR2-Like) (Pan et al., 2013) and fruitfull (TDR4/FUL1 and MBP7/FUL2) (Bemer et al., 2012).
Most positive transcriptional regulators, such as agamous, shatterproof, mads-box, fruitfull affecting maturation pathways have been described (Omboki et al., 2015). Many of these MADS-box transcription factors influence the synthesis of ethylene by direct binding of the ACS2 and ACS4 gene promoters as in the case of RIN and TAGL1 (Cherian et al., 2014). TAGL1 is involved in the normal maturation of the tomato and contributes to its “meatiness” (Itkin et al., 2009; Vrebalov et al., 2009).
The precise molecular mechanism by which the gene products encoded by RIN, NOR, CNR, TAGL1 and HB-1 operate in the regulatory network is still unknown (Seymour et al., 2013); however, it is likely that MADS-box proteins act as heterodimers or multimers (Giovannoni, 2007).
In addition, other components of the regulatory network, including transcription factors acting as negative regulators, such as AP2 and SIAP2a (belonging to the apetala2/erf family of transcription factors and acting in opposition to RIN, NOR and CNR ), have been reported as major regulators of ripening (Chung et al., 2010; Karlova et al., 2011). In addition, the negative effector SIAP2a is induced during maturation, but its repression leads to an acceleration in maturation, high levels of ethylene production and large accumulation of carotenoids (Chung et al., 2010; Karlova et al., 2011).
Loss of firmness
Softening is a maturation process programmed in many fruits, which provides them with different characteristics such as juicy, crunchy and firmness (Seymour et al., 2002). During this process enzymes are produced that modify the cell wall causing changes of texture in the fruit; making it more palatable to the animals that disperse the seeds, and/ or more susceptible to pathogens that release the seeds (Gapper et al., 2013). The chemistry of the cell wall is the main contributor, along with the changes in cellular turgor, the texture of the fruit and the genes that encode the enzymes responsible for these changes.
The latter have been subjected to genetic manipulation in the fruit, with the aim of extending shelf life (Vicente et al., 2007; Goulao and Oliveira, 2007; Matas et al., 2009). Cell wall metabolism during fruit maturation is a very complex process, including more than 50 genes related to cell wall structure (Tomato Genome Consortium, 2012).
Fruit softening is a significant change in the texture of climacteric fruits due to cell wall hemicellulose and the solubilization and depolymerization of pectin by several hydrolases (Rose et al., 2004). The polygalacturonase (PG) enzyme is only expressed in mature fruit tissues and its transcription is activated only during maturation (Omboki et al., 2015). Different regions of the PG promoter region interact positively and negatively with elements that mediate the expression of the gene encoding it (Omboki et al., 2015). The PG is believed to play a key role in softening the fruit, although transgenic studies have indicated that PG is not the only enzyme responsible (Giovannoni et al., 1989; Smith et al., 1990).
In addition to PG, which removes galacturosil residues from pectin (Atkinson et al., 1998), a set of cell wall modifying enzymes also participate in maturation and the genes encoding them have been isolated from some species, but mainly tomato: aL-arabinofuranosidase removes arabinosil and some other pectin residues (Sozzi et al., 2002; Itai et al., 2003); the rhamnogalacturonase (RG) A hydrolyzes α-1,2 bonds between the galacturonosyl and ramnosil residues in pectin (Wong, 2008), xyloglucan/endotransglucosylase/ hydrolase hydrolyzes or xyloglucan transglicosolate (Saladie et al., 2007); glycosidases (β-galactosidase) remove galactosyl residues from pectin and xyloglucan (Smith et al., 2002).
The mannanases hydrolyse the β bond of the mannan backbones that are part of the hemicellulose glycans at random (Rodríguez-Gacio et al., 2012); pectin lyases (PL) catalyze cleavage of deesterified pectin (Marin-Rodriguez et al., 2002); pseudomethylesterases (PME) degrade methyl esterified polyuronides (Wakabayashi et al., 2003) and expansins disrupt hydrogen bonds between cellulose microfibrils and crosslinked glycans (Rose et al., 1997; Brummell et al., 1999b). The exact contribution that each enzyme provides for the softening of the fruit remains unclear due to the fact that the mechanisms under the cell wall mediated by texture changes are complex.
Taking tomato as a model, some genes potentially related to cell wall degradation have been isolated (Bouzayen et al., 2010). However, suppression of candidate genes, such as those coding for PG, PME and β-glucanase, has been shown to have no major impact on the fruit’s firmness evolution (Giovannoni et al., 1989; Tieman et al., 1992; Brummell et al., 1999a).
Within the family of genes that degrade the cell wall of climacteric fruits, some members are regulated by ethylene, while others are not, confirming the coexistence of dependent and ethylene-independent processes (Flores et al., 2001; Nishiyama et al., 2007). In general, it appears that softening of the fruit involves many genes encoding a variety of enzymes that degrade the cell wall and non-enzymatic proteins. Each protein could play a specific role in softening and texture changes (Bouzayen et al., 2010).
Disassembly of the cell wall polysaccharide matrix related to maturation is generally the only factor mentioned in describing the structural basis of fruit softening and loss associated with shelf life and fruit quality, although there are others (Matas et al., 2009). However, recent studies have begun to examine the potential implication and relative importance of other physiological structures and processes. For example, it has been proposed that differences in the structure and composition of the cuticle of the tomato fruit may be associated with the substantial variation in shelf life of the fruit that has been reported in different genotypes (Saladie et al., 2007).
The cuticle fulfills biological functions of impact on the quality and shelf life of fruits, including the ability to maintain skin integrity (Hovav et al., 2007), control cuticular transpiration (Leide et al., 2007) and limit microbial infection. In addition, it plays a role in the plant-insect interaction as a component of signal translation for the activation of specific genes, controlling temperature changes and providing mechanical support (Tafolla-Arellano et al., 2013). Other reports have highlighted maturation processes that probably contribute to fruit firmness, such as turgor pressure (Saladie et al., 2007; Thomas et al., 2008).
Conclusions
Despite the progress in the study of the mechanisms of fruit maturation, a large number of questions remain, such as the relationship between ethylene synthesis and the differential expression of genes related to the production of volatile compounds, with the color change, but more so, with those that encode the enzymes related to softening.
Transcription factors have been shown to be of great importance, not only during early development but also in the regulatory control of maturation and senescence, although there is progress in identifying these regulators, much remains to be investigated.
The softening of the fruit is the process that occurs as a result of the hydrolysis of the various components of the cell wall including cellulose, hemicellulose, pectin and proteins. The hydrolysis of these components is produced by the action of PG, PME, PL, RG, endo-1,4-β-D-glucanase (EGase) and β-galactosidase. The role of these enzymes has been widely confirmed by the application of molecular techniques that involve overexpressing and diminishing the known enzymatic activity; that is, alteration in the hydrolysis process.
In the near future, it will be possible to control the maturation of fruits and extend their shelf life by manipulating ethylene production using a transgenic approach.
Literatura citada
Alba, R.; Payton, P.; Fei, Z.; McQuinn, R.; Debbie, P.; Martin, G. B. and Giovannoni, J. J. 2005. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. The Plant Cell. 17(11):2954-2965. [ Links ]
Alexander, L. and Grierson, D. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 53:2039-2055. [ Links ]
Argueso, C. T.; Hansen, M. and Kieber, J. J. 2007. Regulation of ethylene biosynthesis. J. Plant Growth Reg. 2(26):92-105. [ Links ]
Atkinson, R. G.; Bolitho, K. M.; Wright, M. A.; Iturriagagoitia-Bueno, T.; Reid, S. J. and Ross, G. S. 1998. Apple ACC-oxidase and polygalacturonase: ripening-specific gene expression and promoter analysis in transgenic tomato. Plant Molecular Biology. 38: 449-460. [ Links ]
Bao, F.; Azhakanandam, S. and Franks, R. G. 2010. SEUSS and SEUSS-LIKE transcriptional adaptors regulate floral and embryonic development in Arabidopsis. Plant Physiol. 152: 821-836. [ Links ]
Barry, C. S.; Llop-Tous, M. I. and Grierson, D. 2000. The regulation of 1- aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol . 123: 979-986. [ Links ]
Bauchot, A. D.; Mottram, D. S.; Dodson, A. T. and John, P. 1998. Effect of aminocyclopropane-1-carboxylic acid oxidase antisense gene on the formation of volatile esters in cantaloupe Charentais melon (cv. Vedrandais). J. Agric. Food Chem. 46(11):4787-4792. [ Links ]
Bemer, M.; Karlova, R.; Ballester, A. R.; Tikunov, Y. M.; Bovy, A. G.; Wolters-Arts, M.; Rossetto, Pde B.; Angenent, G. C. and de Maagd, R. A. 2012. The tomato fruitfull homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. The Plant Cell. 24(11):4437-4451. [ Links ]
Bouzayen, M.; Latché, A.; Nath, P. and Pech, J. C. 2010. Mechanism of fruit ripening. In: plant developmental biology- biotechnological perspectives, Pua, E. C. and Davey, M. R. (Eds.). Springer-Verlag. Berlin, Germany. Vol. 1. 319-339 pp. [ Links ]
Brummell, D. A.; Hall, B. D. and Bennett, A. B. 1999a. Antisense suppression of tomato endo- 1, 4-β -glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol. Biol. 40(4):615-622. [ Links ]
Brummell, D. A.; Harpster, M. H.; Civello, P. M.: Palys, J. M.; Bennett, A. B. and Dunsmuir, P. 1999b. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. The Plant Cell . 11(11):2203-2216. [ Links ]
Brummell, D. A. and Harpster, M. H. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. In: Plant Cell Walls. Carpita, N. C.; Campbell, M. and Tierney, M. (Eds.). 1a (Ed.). Springer Science. 311-340 pp. [ Links ]
Cara, B. and Giovannoni, J. J. 2008 Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci. 175:106-113. [ Links ]
Cherian, S.; Figueroa, C. R. and Nair, H. 2014. ‘Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. eru280. [ Links ]
Chung, M. Y.; Vrebalov, J.; Alba, R. ; Lee, J.; McQuinn, R. ; Chung, J. D. and Giovannoni, J. 2010. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. The Plant J. 64(6):936-947. [ Links ]
Ciardi, J. and Klee, H. 2001. Regulation of ethylene-mediated responses at the level of the receptor. Ann. Bot. 88(5):813-822. [ Links ]
Dandekar, A. M.; Teo, G.; Defilippi, B. G.; Uratsu, S. L.; Passey, A. J.; Kader, A. A.; Stow, J. R.; Colgan, R. J. and James, D. J. 2004. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 13(4):373-384. [ Links ]
Darley, C. P.; Forrester, A. M. and McQueen-Mason, S. J. 2001. The molecular basis of plant cell wall extension. In: Plant Cell Walls. Carpita, N. C.; Campbell, M. y Tierney, M. (Eds.). 1a (Ed.). Springer Science. 179-195 pp. [ Links ]
Dos Santos, R. S.; Arge, L. W. P.; Costa, S. I.; Machado, N. D.; de Mello-Farias, P.C.; Rombaldi, C. V. and de Oliveira, A. C. 2015. Genetic regulation and the impact of omics in fruit ripening. Plant Omics. 8(2):78-88. [ Links ]
Flores, F.; Ben Amor, M.; Jones, B.; Pech, J.C.; Bouzayen, M.; Latché, and Romojaro, F. 2001. The use of ethylene-suppressed lines to assess differential sensitivity to ethylene of the various ripening pathways in Cantaloupe melons. Physiol. Plantarum. 113:128-133. [ Links ]
Gapper, N. E.; Giovannoni, J. J. and Watkins, C. B. 2014. Understanding development and ripening of fruit crops in an ‘omics’ era. Hortic. Research. 1. [ Links ]
Gapper, N. E.; McQuinn, R. P. and Giovannoni, J. J. 2013. Molecular and genetic regulation of fruit ripening. Plant Mol. Biol . 82(6):575-591. [ Links ]
Giovannoni, J. J. 2001. Molecular biology of fruit maturation and ripening. Ann. Rev. Plant Physiol . 52: 725-749. [ Links ]
Giovannoni, J. J. 2004. Genetic regulation of fruit development and ripening. The Plant Cell . 16(1):S170-S180. [ Links ]
Giovannoni, J. J. 2007. Fruit ripening mutants yield insights into ripening control. Current Opinion Plant Biol. 10(3):283-289. [ Links ]
Giovannoni, J. J.; DellaPenna, D.; Bennett, A. B. and Fischer, R. L. 1989. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. The Plant Cell . 1(1):53-63. [ Links ]
Goulao, L. F.; Santos, J.; de Sousa, I. and Oliveira, C. M. 2007. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biol. Technol. 43(3):307-318. [ Links ]
Grierson, D. 2013. Ethylene and the control of fruit ripening. In: Seymour, G. B.; Poole, M.; Giovannoni, J. J. and Tucker, G. A. (Eds.). 1a (Ed.). The Molecular Biology and Biochemistry of Fruit Ripening. Blackwell Publishing Ltd. Ames, IA, USA. 43-73 pp. [ Links ]
Hovav, R.; Chehanovsky, N.; Moy, M.; Jetter, R. and Schaffer, A. A. 2007. The identification of a gene (Cwp1), silenced during Solanum evolution, which causes cuticle microfissuring and dehydration when expressed in tomato fruit. The Plant J. 52(4):627-639. [ Links ]
Iannetta, P. P.; Laarhoven, L. J.; Medina-Escobar, N.; James, E. K., McManus, M. T.; Davies, H. V. and Harren, F. J. 2006. Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiol. Plantarum. 127(2):247-259. [ Links ]
Iqbal, N.; Khan, N. A.; Ferrante, A.; Trivellini, A.; Francini, A. and Khan, M. I. R. 2017. Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Frontiers Plant Sci. 8. [ Links ]
Itai, A.; Ishihara, K. and Bewley, J. D. 2003. Characterization of expression, and cloning, of beta-D-xylosidase and alpha-L-arabinofuranosidase in developing and ripening tomato (Lycopersicon esculentum Mill.) fruit. J. Exp. Bot. 54: 2615-2622. [ Links ]
Itkin, M.; Seybold, H.; Breitel, D.; Rogachev, I.; Meir, S. and Aharoni, A. 2009. Tomato agamous-like 1 is a component of the fruit ripening regulatory network. The Plant J . 60(6):1081-1095. [ Links ]
Jiménez-Bermúdez, S.; Redondo-Nevado, J.; Muñoz-Blanco, J.; Caballero, J. L.; López-Aranda, J. M.; Valpuesta, V.; Pliego-Alfaro, F.; Quesada, M. A. and Mercado, J. A. 2002. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol . 128: 751-759. [ Links ]
Karlova, R.; Chapman, N.; David, K.; Angenent, G. C.; Seymour, G. B. and de Maagd, R. A. 2014. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot . 65(16):4527-4541. [ Links ]
Karlova, R. ; Rosin, F. M.; Busscher-Lange, J.; Parapunova, V.; Do, P. T.; Fernie, A. R.; Fraser, P. D.; Baxter, C.; Angenent, G. C. and de Maagd, R. A. 2011. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. The Plant Cell Online. 23(3):923-941. [ Links ]
Klee, H. J. and Giovannoni, J. J. 2011. Genetics and control of tomato fruit ripening and quality attributes. Ann. Rev. Gen. 45: 41-59. [ Links ]
Kumar, R.; Khurana, A. and Sharma, A. K. 2014 Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot . 65: 4561-4575. [ Links ]
Lashbrook, C.; Tieman, D. and Klee, H. 1998. Differential regulation of the tomato ETR gene family throughout plant development. Plant J. 15: 243-252. [ Links ]
Lee, J. M.; Joung, J. G.; McQuinn, R. ; Chung, M. Y.; Fei, Z. ; Tieman, D.; Klee, H. and Giovannoni, J. 2012. Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. The Plant J . 70(2):191-204. [ Links ]
Leide, J.; Hildebrandt, U.; Reussing, K.; Riederer, M. and Vogg, G. 2007. The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a β-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol . 144(3):1667-1679. [ Links ]
Lelièvre, J. M.; Latché, A.; Jones, B. ; Bouzayen, M. and Pech, J. C. 1997. Ethylene and fruit ripening. Physiol. Plantarum. 101:727-739. [ Links ]
Lin, Z.; Hong, Y.; Yin, M.; Li, C.; Zhang, K. and Grierson, D. 2008. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. The Plant J . 55(2):301-310. [ Links ]
Lincoln, J. E. and Fischer, R. L. 1988. Diverse mechanisms for the regulation of ethylene-inducible gene expression. Molecular and General Genetics MGG. 212(1):71-75. [ Links ]
Liu, C.; Zhao, A.; Zhu, P.; Li, J.; Han, L.; Wang, X.; Fan, W.; Lü, R.; Wang, C.; Li, Z.; Lu, C. and Lu, C. 2015. Characterization and expression of genes involved in the ethylene biosynthesis and signal transduction during ripening of mulberry fruit. PloS one. 10(3):e0122081. [ Links ]
Llop-Tous, I.; Barry, C. S. and Grierson, D. 2000. Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol . 123(3):971-978. [ Links ]
Manning, K.; Tor, M.; Poole, M.; Hong, Y. ; Thompson, A. J.; King, G. J.; Giovannoni, J. J. and Seymour, G. B. 2006. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nature Gen. 38(8):948-952. [ Links ]
Marín-Rodríguez, M. C.; Orchard, J. and Seymour, G. B. 2002. Pectate lyases, cell wall degradation and fruit softening. J. Exp. Bot . 53:2115-2119. [ Links ]
Matas, A. J.; Gapper, N. E.; Chung, M. Y.; Giovannoni, J. J. and Rose, J. K. 2009. Biology and genetic engineering of fruit maturation for enhanced quality and shelf-life. Current Opinion Biotechnol. 20(2):197-203. [ Links ]
McAtee, P.; Karim, S.; Schaffer, R. and David, K. 2013. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Frontiers in Plant Science. 4:79. [ Links ]
Nishiyama, K.; Guis, M., Rose, J. K. ; Kubo, Y.; Bennett, K. A.; Wangjin, L.; Kato, K.; Koichiro, U., Nakano, R.; Inaba, A.; Bouzayen, M. ; Latché, A. ; Pech, J. C. and Bennett, A. B. 2007. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. J. Exp. Bot . 58(6):1281-1290. [ Links ]
Omboki, R. B.; Wu, W.; Xie, X. and Mamadou, G. 2015. Ripening genetics of the tomato fruit. Inter. J. Agric. Crop Sci. 8(4):567-572. [ Links ]
Pan, Y.; Bradley, G.; Pyke, K.; Ball, G.; Lu, C.; Fray, R.; Marshall, A.; Jayasuta, S.; Baxter, C.; van Wijk, R.; Boyden, L.; Cade, R.; Chapman, N. H.; Fraser, P. D.; Hodgman, C. and Seymour, G. B. 2013. Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol . 161(3):1476-1485. [ Links ]
Payton, S.; Fray, R. G.; Brown, S. and Grierson, D. 1996. Ethylene receptor expression is regulated during fruit ripening, flower senescence and abscission. Plant Mol. Biol . 31(6):1227-1231. [ Links ]
Pech, J. C.; Purgatto, E.; Girardi, C. L.; Rombaldi, C. V. and Latché, A. 2013. Current challenges in postharvest biology of fruit ripening. Current Agric. Sci. Technol. 19(1-18). [ Links ]
Pech, J. C.; Purgatto, E.; Latché, A. and Bouzayen, M. 2012. Ethylene and fruit ripening. In: the plant hormone ethylene, annual plant reviews. McManus, M.T. (Ed.). 1a (Ed.). Blackwell Publishing. Oxford, UK. 44: 275-304. [ Links ]
Pegoraro, C.; Zanuzo, M. R.; Chaves, F. C.; Brackmann, A.; Girardi, C. L.; Lucchetta, L.; Nora, L.; Silva, J. A. and Rombaldi, C. V. 2010. Physiological and molecular changes associated with prevention of woolliness in peach following pre - harvest application of gibberellic acid. Postharvest Biol. Technol . 57(1):19-26. [ Links ]
Perkins-Veazie, P. M.; Huber, D. J. and Brecht, J. K. 1996. In vitro growth and ripening of strawberry fruit in the presence of ACC, STS or propylene. Ann. App. Biol. 128(1):105-116. [ Links ]
Rodríguez-Gacio, M. C.; Iglesias-Fernández, R.; Carbonero, P. and Matilla, A. J. 2012. Softening-upmannan-rich cellwalls. J. Exp. Bot . 63: 3976-3988. [ Links ]
Rose, J. K.; Lee, H. H. and Bennett, A. B. 1997. Expression of a divergent expansin gene is fruit-specific and ripening -regulated. Proceedings of the National Academy of Sciences of the USA. 94: 5955-5960. [ Links ]
Saladié, M.; Matas, A. J.; Isaacson, T.; Jenks, M. A.; Goodwin, S. M.; Niklas, K. J.; Xiaolin, R.; Labavitch, J. M.; Shackel, K. A.; Fernie, A. R.; Lytovchenko, A.; O’Neill, M. A.; Watkins, C. B. and Rose, J. K. 2007. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol . 144: 1012-1028. [ Links ]
Seymour, G. B.; Manning, K.; Eriksson, E. M.; Popovich, A. H. and King, G. J. 2002. Genetic identification and genomic organization of factors affecting fruit texture. J. Exp. Bot . 53: 2065-2071. [ Links ]
Seymour, G. B.; Ostergaard, L.; Chapman, N. H.; Knapp, S. and Martin, C. 2013. Fruit development and ripening. Ann. Rev. Plant Biol. 64: 219-241. [ Links ]
Smith, C. J. S.; Watson, C. F.; Bird, C. R.; Ray, J.; Schuch, W. and Grierson, D. 1990. Expression of a truncated tomato polygalacturonase gene inhibits expression of the endogenous gene in transgenic plants. Molecular and General Genetics MGG. 224(3):477-481. [ Links ]
Smith, D. L.; Abbott, J. A. and Gross, K. C. 2002. Down-regulation of tomato beta-galactosidase 4 results in decreased fruit softening. Plant Physiol . 129: 1755-1762. [ Links ]
Sozzi, G. O.; Greve, L. C.; Prody, G. A. and Labavitch, J. M. 2002. Gibberellic acid, synthetic auxins, and ethylene differentially modulate alpha-L-arabinofuranosidase activities in antisense 1-aminocyclopropane - 1-carboxylic acid synthase tomato pericarp discs. Plant Physiol . 129: 1330-1340. [ Links ]
Tafolla-Arellano, J. C.; González-León, A.; Tiznado-Hernández, M. E.; Zacarías García, L. and Báez-Sañudo, R. 2013. Composición, fisiología y biosíntesis de la cutícula en plantas. Rev. Fitotec. Mex. 36(1):3-12. [ Links ]
Tatsuki, M.; Haji, T. and Yamaguchi, M. 2006. The involvement of 1-aminocyclopropane-1-carboxylic acid synthase isogene, Pp-ACS1, in peach fruit softening. J. Exp. Bot . 57:1281-1289. [ Links ]
Tieman, D. M.; Harriman, R. W.; Ramamohan, G. and Handa, A. K. 1992. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. Plant Cell. 4: 667-669. [ Links ]
Tieman, D. M. and Klee, H. J. 1999. Differential expression of two novel members of the tomato ethylene-receptor family. Plant Physiol . 120(1):165-172. [ Links ]
Thomas, T. R.; Shackel, K. A. and Matthews, M. A. 2008. Mesocarp cell turgor in Vitis vinifera L. berries throughout development and its relation to firmness, growth, and the onset of ripening. Planta. 228(6):1067-1076. [ Links ]
Van-der-Hoeven, R.; Ronning, C.; Giovannoni, J.; Martin, G. and Tanksley, S. 2002. Deductions about the number, organization, and evolution of genes in the tomato genome based on analysis of a large expressed sequence tag collection and selective genomic sequencing. Plant Cell . 14: 1441-1456. [ Links ]
Vicente, A. R.; Saladié, M.; Rose, J.; Labavitch, K. C. and John, M. 2007. The linkage between cell wall metabolism and fruit softening: looking to the future. J. Sci. Food Agric. 87:1435-1448. [ Links ]
Vrebalov, J.; Ruezinsky, D.; Padmanabhan, V.; White, R.; Medrano, D.; Drake, R.; Schuch, W. and Giovannoni, J. 2002. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 296: 343-346. [ Links ]
Wakabayashi, K.; Hoson, T. and Huber, D. J. 2003. Methyl de-esterification as a major factor regulating the extent of pectin depolymerization during fruit ripening: a comparison of the action of avocado (Persea americana) and tomato (Lycopersicon esculentum) polygalacturonases. J. Plant Physiol . 160(6):667-673. [ Links ]
Wilkinson, J.; Lanahan, M.; Yen, H.- C.; Giovannoni, J. and Klee, H. 1995. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 270:1807-1809. [ Links ]
Wong, D. 2008. Enzymatic deconstruction of backbone structures of the ramified regions in pectins. Protein J. 27:30-42. [ Links ]
Yamamoto, M.; Miki, T.; Ishiki, Y.; Fujinami, K.; Yanagisawa, Y.; Nakagawa, H.; Ogura, N.; Hirabayashi, T. y Sato, T. 1995. The synthesis of ethylene in melon fruit during the early stage of ripening. Plant Cell Physiol. 36(4):591-596. [ Links ]
Yen, H. C.; Lee, S.; Tanksley, S.; Lanahan, M.; Klee, H. and Giovannoni, J. 1995. The tomato never-ripe locus regulates ethylene-inducible gene expression and is linked to a homolog of the Arabidopsis ETR1 gene. Plant Physiol . 107: 1343-1353. [ Links ]
Zhong, S.; Fei, Z.; Chen, Y.R.; Zheng, Y.; Huang, M.; Vrebalov, J.; Mcquinn, R.; Gapper, N.; Liu, B.; Xiang, J.; Shao and Giovannoni, J. J. 2013. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nature Biotechnol. 31(2): 154-159. [ Links ]
Zhou, D.; Kalaitzis, P.; Mattoo, A. K. and Tucker, M. L. 1996. The mRNA for an ETR1 homologue in tomato is constitutively expressed in vegetative and reproductive tissues. Plant Mol. Biol . 30(6):1331-1338. [ Links ]
Received: March 00, 2017; Accepted: May 00, 2017











 texto en
texto en