Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 spe 19 Texcoco nov./dic. 2017
https://doi.org/10.29312/remexca.v0i19.674
Essays
Postharvest fruits: maturation and biochemical changes
1Universidad Autónoma de Nayarit-Posgrado en Ciencias Biológico Agropecuarias. Ciudad de la cultura “Amado Nervo”, Tepic, Nayarit, México. CP. 63155. (mc.monica.martínez@gmail.com).
2Universidad Autónoma de Nayarit-Unidad de Tecnología de Alimentos. Ciudad de la cultura “Amado Nervo” s/n. Tepic, Nayarit, México. CP. 63155. (pasingan@gmail.com; lguzman2303@hotmail.com).
3Universidad Autónoma del Estado de Morelos-Posgrado en Ciencias Agropecuarias y Desarrollo Rural. Av. Universidad núm. 1001, Col. Chamilpa, Cuernavaca, Morelos, México. CP. 62209. (iran.alia@uaem.mx).
4Centro Nacional de Recursos Genéticos, INIFAP. México.
The formation of fleshy fruits involves three stages: growth, development and maturation. The objective of this review is to compile information on relevant research related to fruit maturation and biochemical changes occurring during post-harvest management. In order to carry out this study search we used multiple databases (Scielo, Redalyc, Elsevier, Scopus, Wiley online library, Sciencedirect, Springer). The ripening of the fruit is an important process that activates a whole set of biochemical routes that make it attractive and desirable for consumers. The study of the maturation of fruits has been of great interest in the research since the biochemical and physicochemical changes that occur during this stage cause great economic losses. It is important to have knowledge of the processes, which allows biotechnologists and plant breeders to generate more knowledge or propose outstanding horticultural materials, application of postharvest management techniques more effective and useable to reduce postharvest losses.
Keywords: climacteric fruits; ethylene; maturation; post-harvest
La formación de frutos carnosos involucra tres etapas: crecimiento, desarrollo y maduración. El objetivo de esta revisión se enfoca en la recopilación de la información sobre investigaciones relevantes relacionadas con la maduración de los frutos y los cambios bioquímicos que ocurren durante el manejo postcosecha de éstos. Para realizar esta búsqueda de estudios se realizó uso de múltiples bases de datos (Scielo, Redalyc, Elsevier, Scopus, Wiley online library, Sciencedirect, Springer). La maduración del fruto es un importante proceso que activa a todo un conjunto de rutas bioquímicas que hacen que éste sea atractivo y deseable para los consumidores. El estudio de la maduración de los frutos ha sido de gran interés en la investigación ya que los cambios bioquímicos y fisicoquímicos que ocurren durante esta etapa ocasionan grandes pérdidas económicas. Es importante tener conocimiento de los procesos, lo que permite que los investigadores biotecnologos y fitomejoradores generan mas conocimiento o propongan materiales hortofrutícolas sobresalientes, aplicación de técnicas de manejo poscosecha mas efectivas y aprovechables para disminuir las pérdidas postcosecha.
Palabras clave: etileno; frutos climatéricos; maduración; poscosecha
Introduction
The softening of fruits is a series of genetically programmed events, characterized by biochemical and physiological processes that alter its firmness, color, taste and texture (Nishiyama et al., 2007). Since most of the quality attributes are the result of the maturation process, it has been considered essential to understand the regulatory mechanisms involved in this stage of fruit development (Bouzayen et al., 2010).
Fruits are highly perishable products due to their cellular architecture and intense metabolic activity (Dos Santos et al., 2015). Some deterioration processes cause farmers to lose up to 40% of the value of the crop before they reach the consumer (Kitinoja et al., 2011). The application of appropriate technologies to maintain quality depends on the knowledge of fruit structure, physiology and metabolic transformations (Pech et al., 2013). Therefore, studies have been carried out in order to better understand the floral organ and the development of the fruit (Bao et al., 2010; Seymour et al., 2013), the role of hormones and genes related to development and maturation (Alexander and Grierson, 2002; Cara and Giovannoni, 2008; Kumar et al., 2014), and physiological disorders (Pegoraro et al., 2010) and epigenetic alterations associated with maturation (Manning et al., 2006; Zhong et al., 2013) (Dos Santos et al., 2015).
The objective of this research is to compile the most relevant information published concerning the changes that occur in the fruits during the post-harvest stage.
Physiological maturation of fruits
The development of the fruit occurs in three stages: growth, development and ripening, followed by softening and senescence (Alba et al., 2005).
The fruit begins to develop shortly after pollination and fertilization (O’Neill, 1997) through cell division, a phenomenon that occurs in the early stages of development (Dos Santos et al., 2015). After this period, the growth occurs due to the increase of size of the cell when the vacuoles appear. This stage is characterized by the growth and elongation of the fruit, followed by a maturation stage, where the number of cells remains relatively constant, being observed an increase in the size of the same ones (Dos Santos et al., 2015). This expansion increases in maturation, stage where the fruit is able to ripen still attached to the plant.
Within the mentioned stages, several steps occur between the beginning of fruit development and its senescence (Figure 1). What is called physiological maturity occurs before the complete development of the fruit that after harvesting must survive with its own accumulated substrates (Dos Santos et al., 2015). This is an intermediate step between the end of growth and the onset of senescence (Dos Santos et al., 2015). The biochemical and physiological activities involved in softening, such as changes in firmness and respiration rate, among others; are irreversible once initiated (Omboki et al., 2015). They can only delay or slow down with the external application of certain procedures (Omboki et al., 2015).
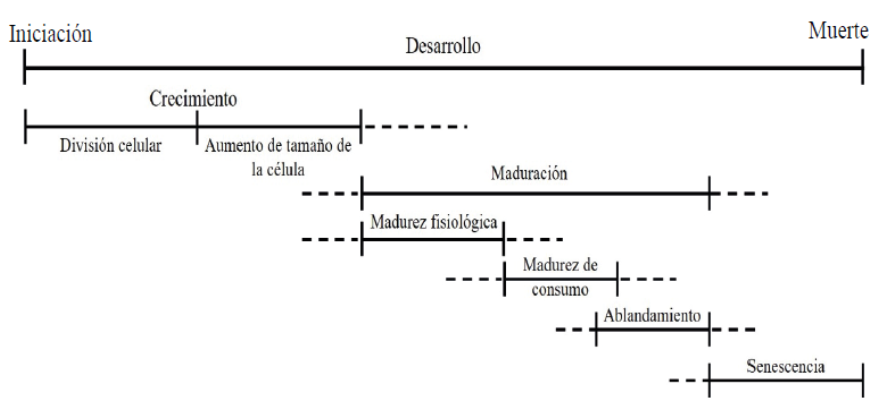
Figure 1 Stages between fruit formation and senescence (Watada et al., 1984; Dos Santos et al., 2015).
In addition, from development to maturation, several genes are involved and among these are transcription factors (TFs) that are of great importance in modulating the expression of various genes and metabolic processes (O’Neill 1997; Giovannoni, 2001).
The process of maturation, biochemical and sensory changes
In the final stages of growth and development, the maturation process takes place in two steps: physiological maturity, when the fruit reaches its maximum size and the greatest vigor of the seeds; and second, the maturity of consumption, here the changes of the fruit include 1) the color modification through the alteration in the content of chlorophylls, carotenoids and the accumulation of flavonoids; 2) modification of the texture via alteration of the cellular turgor and the structure of the cell wall and by the metabolism; 3) modification of sugars, organic acids and volatile compounds that affect the nutritional quality, flavor and aroma of the fruit; and 4) increased susceptibility to attack by opportunistic pathogens that are associated with loss of cell wall integrity (Giovannoni, 2004; Seymour et al., 2013; Dos Santos et al., 2015). These phenomena are described in more detail below:
Color modification. The pigments are essential for attractive fruits, they accumulate commonly in the cuticle during the maturation process, although many climacteric fruits also accumulate pigments in the pulp tissue during post-harvest maturation, unlike non-climacteric fruits (Bouzayen et al., 2010). The most important pigments are carotenoids and anthocyanins (Bartley and Scolnik, 1995). In addition to their role in pigmentation, they are important for human health as sources of vitamin A and antioxidant compounds, respectively (Bartley and Scolnik, 1995).
Carotenoids include carotenes, such as lycopene, β-carotene, and xanthophylls including lutein (Bouzayen et al., 2010). The anthocyanins belong to the flavonoid subclass of phenolic compounds (He and Giusti, 2010). In grape (Vitis vinifera), where anthocyanins are crucial for wine quality, it has been shown that ethylene stimulates berry coloration, so it is concluded that this hormone is involved in the regulation of the genes of the biosynthesis of anthocyanins (El-Kereamy et al., 2003; Bouzayen et al., 2010). It is known that environmental conditions and orchard management, including irrigation, pruning and fertilization, strongly impact the coloring of fruits (Bouzayen et al., 2010).
Studies have shown that there is a positive correlation between anthocyanin synthesis and sunlight intensity in various fruits such as apple (Malus domestica) (Ju et al., 1999), grape (Vitis vinifera) (Dokoozlian and Kliewer, 1996; Bergqvist et al., 2001; Spayd et al., 2002), peach (Prunus persica) (Jia et al., 2005), strawberry (Fragaria ananassa) (Da Silva et al., 2007) and litchi (Litchi chinensis) (Tyas et al., 1998). In these cases, sunlight increases the synthesis of anthocyanins (de Pascual-Teresa and Sánchez-Ballesta, 2008). The temperature changes also play an important role, it has been observed that environments with low temperatures favor the accumulation of anthocyanins, whereas warm climates decrease the synthesis of these compounds (Leng et al., 2000; Li et al., 2004; Mori et al., 2005).
Nutrient deficiencies, especially phosphorus (P) and nitrogen (N) induce the accumulation of anthocyanins in different species (Hodges and Niozillo, 1995; de Pascual-Teresa and Sánchez-Ballesta, 2008). In tomato (Solanum lycopersicum), in addition to increasing the flavonoid content, N stress also produces effects on the differential expression of genes encoding enzymes for anthocyanin biosynthesis (Bongue-Bartelsman and Phillips, 1995). In contrast, high levels of N applied to peach (Prunus persica) and nectarine (Prunus persica var. nucipersica) trees have been reported to adversely affect fruit quality, as maturity is delayed, the percentage of red coloration decreases and fruit size does not increase compared to fruits treated with optimal N levels (Daane et al., 1995). Similar results were observed in black aronia (Aronia melanocarpa cv. Viking) when using a fertilizer with combination of N, P and potassium (K) (Jeppsson, 2000).
Modification of texture. One of the main factors associated with fruit post-harvest deterioration is softening velocity, which results in shorter shelf life, reducing transport and distribution times and increasing post-harvest losses (Bapat et al., 2010).
The softening of the fruits is caused by the cumulative effect of a series of modifications occurring in the polymer networks constituting the primary cell wall. The softening of the fruit is a complex process involving three subsequent steps: 1) relaxation of the cell wall mediated by expansins; 2) depilimerization of hemicelluloses; and 3) depolymerization of polyuronides by polygalacturonase or other hydrolytic enzymes (Brummell et al., 1999; Payasi et al., 2009); which contributes to a loss of firmness and changes in texture quality (Brummell and Harpster, 2001). Modifications in cell wall polymers during softening are complicated and are considered to involve coordinated and interdependent action of a range of cell wall modifying enzymes and proteins such as polygalacturonase (PG, EC 3.2.1.15), pectinmethylesterase (PME, EC 3.1.1.11), β-galactosidase (EC 3.2.1.23) xyloglucan endotransglicosilase (XET, EC 2.4.1.207) and expansins (Brummell and Harpster, 2001; Payasi et al., 2009).
Modification of aroma. Aroma is a complex blend of a wide range of compounds. Volatile aroma compounds contribute decisively to the sensory quality of fruits (Bouzayen et al., 2010). In recent years, research efforts have focused on the isolation of genes related to volatile compounds in fruits (Aharoni et al., 2000; Yahyaoui et al., 2002; Beekwilder et al., 2004; El-Sharkawy et al., 2004; Pech et al., 2008). The most important classes of odor-conferring compounds are monoterpenes, sesquiterpenes and compounds derived from lipids, sugars and amino acids.
It is known that ethylene controls ripening velocity, shelf life and most maturation events in climacteric fruits (Bouzayen et al., 2010). It has also been shown that ethylene plays a key role in regulating genes involved in the production of volatile compounds in multiple fruit species through the use of mutants in tomato, transgenic lines of antisense RNA in apple and other inhibitors of ethylene receptors (in apples, Schaffer et al., 2007, in tomato, Kovacs et al., 2009, DeFillipi et al., 2009 and Gapper et al., 2013). Therefore, it has been observed that genotypes generated to have an extended shelf life have resulted in a severe loss of taste and odor, as many aroma biosynthesis genes are regulated by ethylene (El-Sharkawy et al., 2004; Manríquez et al., 2006).
Dandekar et al. (2004), reported a differential regulation of ethylene with respect to the components of fruit quality in apple. It has been reported that there is a direct correlation between ethylene and aroma production during apple fruit maturation (Wang et al., 2007). Also, Schaffer et al. (2007), identified 17 candidate genes that were likely to be ethylene control points with respect to apple aroma production, although only certain points on the aroma biosynthesis pathways were regulated by ethylene. That is, the first step in some routes and the last steps of all the biosynthetic routes contained enzymes regulated by ethylene.
These findings concluded that the initial and final steps of biosynthetic pathways are important points of transcriptional regulation for apple aroma production. A major challenge for the future will be to disentangle ethylene depletion regulation from the inhibition of the production of volatile compounds (Bouzayen et al., 2010).
At the end of the consumer maturation stage, some physiological changes related to senescence leading to membrane deterioration and cell death occur. In this respect, consumer maturity can be considered as the first step in a programmed cell death process (Bouzayen et al., 2010). During senescence the synthesis of carbohydrates ceases and degradation of proteins, chlorophylls, lipids and nucleic acids takes place, which requires the synthesis of hydrolytic enzymes, as well as the synthesis of carotenoids and antioxidant compounds (Gapper et al., 2013).
These enzymes are synthesized from the activation of genes encoding them; as well as all the biochemical and physiological changes that take place during this stage are promoted by the coordinated expression of genes related to fruit ripening (Bouzayen et al., 2010). They also encode regulatory proteins that participate in signaling pathways and in the transcriptional machinery that regulates gene expression and generates the maturation development program (Bouzayen et al., 2010). The set of genes controlling the firmness, taste, color and aroma of the fruit are regulated by a different set of specific genes which in turn can be regulated either by a single or by a set of transcription factors (Nath et al., 2007).
Respiratory activity
The fruits are defined physiologically based on the presence (climacterics) or absence (non-climacterics) of an increase in respiration and in the synthesis of ethylene at the beginning of the maturity of consumption (Lelièvre et al., 1997).
The climacteric fruits are those that can ripen not only to the plant, but also after harvest, when cut in the pre-climacteric stage, such as tomato (Solanum lycopersicum), apple (Malus domestica) and banana Musa spp.) this type of fruit reaches senescence sooner (Fernandez-Trujillo et al., 2007; Obando-Ulloa et al., 2008) in view of the fact that respiration is accompanied by a similar increase in ethylene levels, which coordinates and synchronizes the maturation process (Omboki et al., 2015).
On the other hand, non-climacteric fruits such as strawberry (Fragaria spp.), grape (Vitis vinifera L.) and citrus fruits, only reach ripeness when still attached to the plant, since they do not present an increase in respiration and in the production of ethylene after harvest (Biale, 1964; Given et al., 1988; Chervin et al., 2004). Non-climacteric fruits do not develop climacteric patterns that include increased respiration, ethylene biosynthesis and autocatalytic response to ethylene, but show some typical responses to ethylene as degreed (changes in green to yellow or orange coloration and softening (synthesis of enzymes that degrade the cell wall), among others (Dos Santos et al., 2015) , that is, the same biochemical changes are carried out in the color, texture, taste and smell of the fruit. This suggests that the genes involved are the same ones that are differentially expressed due to the evolution that their regulators have been conserved via evolutionary processes (Omboki et al., 2015).
The climacteric fruits undergo a massive deterioration during post-harvest handling, which translates into significant economic losses (Bapat et al., 2010). The process of maturation involves aspects such as regulation of metabolic control, communication between organelles, growth regulators and gene expression (Alexander and Grierson, 2002). Several genetic studies have suggested that the maturation process is programmed in the cell and requires differential expression of genes, resulting in the transcription of specific mRNAs and the synthesis of de novo proteins (Lincoln and Fischer, 1988; Darley et al., 2001).
In this sense, we have used molecular biology techniques aimed at isolating, recognition and expression of the genes of the major enzymes involved during softening that occurs in fruit ripening (Brummell and Harpster, 2001); however, the molecular differences between climatic and non-climacteric maturation are still poorly understood (Giovannoni, 2004).
Although the specific role of climacteric respiration in fruit maturation is not yet clear, the incorporation of ethylene as a coordinator of the maturation of climacteric species is likely to facilitate rapid and coordinated maturation (Giovannoni, 2004).
Ethylene
The growth and development of the fucus are controlled by the production of hormones, which are susceptible to environmental changes (McClellan and Chang, 2008). Among these hormones is ethylene, which controls many processes in higher plants, such as organ senescence, stress response, seed germination (Owino et al., 2006; Zhu and Zhou; 2007; Jiang et al., 2011; Oms-Oliu et al., 2011; Zheng et al., 2013), wound healing (Capitani et al., 1999), in addition to interactions with other hormones and metal ions (Cervantes, 2002). It has also been identified as the main hormone that initiates and controls the ripening process of the fruit (Abeles et al., 1992; Lara and Vendrell, 2003; Owino et al., 2006; McClellan and Chang, 2008; Pech et al., 2008; Asif et al., 2009; Wang et al., 2009; Bapat et al., 2010; Iguaran and Alzate, 2014). In summary, the presence of ethylene initiates maturation and completes it in several steps (Omboki et al., 2015).
In fleshy fruits, attempts have been made to decrease ethylene biosynthesis during maturation to retard post-harvest deterioration (Bapat et al., 2010) since once maturation has been initiated, the process is uncontrollable (Jiang et al., 2011). Most of the procedures used to limit ethylene biosynthesis focus on increasing or decreasing the temperature and changing the atmosphere in which the fruits (Lara and Vendrell, 2003; Zhu and Zhou, 2007; Asif et al., 2009).
Biosynthesis of ethylene
Ethylene is produced in most plant tissues (Oms-Oliu et al., 2011). In the fruits there are two distinct systems of biosynthesis.
System 1 corresponds to a low ethylene production in the pre-climacteric period of the climacteric fruits and is present throughout the development of non-climacteric fruits. System 2 refers to a production of self -regulating ethylene called “autocatalytic synthesis”, and is specific for climacteric fruits (Bapat et al., 2010) . That is, at the beginning of maturation, the climacteric fruits present a maximum point of respiration, followed by an explosion in the production of ethylene, whereas, in non-climacteric fruits, maturation is independent of ethylene, which is present only at a basal level (Asif et al., 2009). In addition, the climatic explosion of ethylene production stimulates the genes responsible for ethylene biosynthesis (Lara and Vendrell, 2003).
The ethylene biosynthetic pathway (Figure 2) is well established (Yang and Hoffman, 1984). This maturation hormone begins with the conversion of methionine to S-adenosyl-L-methionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC) (Kende, 1993). Two key enzymes are involved in the biosynthetic pathway, ACC synthase (ACS), which converts SAM into ACC, and ACC oxidase (ACO) which converts ACC into ethylene (Kende, 1993) and identified and characterized genes which encode them (Sato and Theologis, 1989; Hamilton et al., 1990, 1991). The expression profiles and regulatory mechanisms of ACS and ACO genes in fruits have been investigated in plants (Liu et al., 2015).
In tomato it has been reported that both ACS and ACO are encoded by a family of multigenes of five and nine members, respectively, with differentially regulated expression during the development and maturity of the fruit (Bapat et al., 2010; Bouzayen et al., 2010). In addition, ACS and ACO genes have been used as targets to suppress ethylene production and retard fruit ripening and senescence in other species such as melon (Cucumis melo var. cantalupensis; Ayub et al., 1996), apple (Wang et al., 2009) and on mulberry fruit (Morus atropurpurea cv. Jialing; Liu et al., 2015).
Chemical control of the ethylene response
An ethylene inhibitor, a compound called 1-methylcyclopropene (1-MCP), has proven to be a potent antagonist of the action of ethylene and is now used as a research tool to reach a better understanding of the ethylene regulatory processes and for the extension of the shelf life of fruits and vegetables (Blankenship and Dole, 2003). A wide range of effects have been observed that vary between species and even between cultivars (Watkins, 2006). It appears that this compound has limitations in many species, but its greatest success has been to prolong the shelf life of apple fruits, which led to it being widely used in the industry.
The 1-MCP is best applied after maturation has begun. Preclimeric application results in severe inhibition of maturation that may be problematic for recovery (Omboki et al., 2015). It was determined that 1 -MCP activity is influenced by internal ethylene levels (Zhengke et al., 2009). This is an important discovery that can be exploited for horticultural cultivation on an industrial or commercial scale (Omboki et al., 2015).
Conclusions
There is remarkable progress in studying the mechanisms of fruit ripening, but a large number of questions remain unanswered.
Ethylene plays a decisive role in the maturation process and its relation to the different processes that occur in this stage in the climacteric fruits, but the role of other hormones and the way in which they act together with ethylene still remains to be addressed. Also, another topic on which more information is required is the mechanism by which ethylene selects specific genes for regulation of maturation.
On the other hand, although in non-climacteric fruits there is information about the mechanisms that regulate the ripening process, there is interest in the subject and studies are being carried out that are generating valuable information.
As a result of this exhaustive search for information related to the biochemical changes in fruit maturation during post-harvest management, it is possible to update what is done in research on the subject, the information of which will be used by biotechnologists and plant breeders knowledge or propose outstanding plant materials with a more effective and applicable postharvest management technique, which would impact the economy of countries whose main activity is agriculture.
Literatura citada
Abeles, F.; Morgan, P. and Saltveit, M. 1992. Ethylene in plant biology.Academic Press. 2a (Ed.). New York, EE.UU. 414 p. [ Links ]
Aharoni, A.; Keizer, L. C.; Bouwmeester, H. J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H. A.; Blass, J.; van Houwelingen, A. M. M. L.; de Vos, R. C. H., Van der Voet; Jansen, R. C.; Guis, M.; Mol, J.; Davis, R. W.; Schena, M.; van Tunen, A. J. y O’Connell, A. P. 2000. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. The Plant Cell. 12(5):647-661. [ Links ]
Alba, R.; Payton, P.; Fei, Z.; McQuinn, R.; Debbie, P.; Martin, G. B. and Giovannoni, J. J. 2005. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. The Plant Cell. 17(11):2954-2965. [ Links ]
Alexander, L. and Grierson, D. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 53: 2039-2055. [ Links ]
Asif, M.; Pathak, N.; Solomos, T. and Trivedi, P. 2009. Effect of low oxygen, temperature and 1-methylcyclopropene on the expression of genes regulating ethylene biosynthesis and perception during ripening in apple. South Afr. J. Bot. 75(1):137-144. [ Links ]
Bao, F.; Azhakanandam, S. and Franks, R. G. 2010. SEUSS and SEUSS-LIKE transcriptional adaptors regulate floral and embryonic development in Arabidopsis. Plant Physiol. 152: 821-836. [ Links ]
Bapat, V. A.; Trivedi, P. K.; Ghosh, A.; Sane, V. A.; Ganapathi, T. R. and Nath, P. 2010. Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnol. Adv. 28(1):94-107. [ Links ]
Bartley, G. E. and Scolnik, P. A. 1995. Plant carotenoids: pigments for photoprotection, visual attraction, and human health.The Plant Cell. 7(7):1027-1038. [ Links ]
Beekwilder, J.; Alvarez-Huerta, M.; Neef, E.; Verstappen, F. W.; Bouwmeester, H. J. and Aharoni, A. 2004. Functional characterization of enzymes forming volatile esters from strawberry and banana. Plant Physiology. 135: 1865-1878. [ Links ]
Bergqvist, J.; Dokoozlian, N. and Ebisuda, N. 2001. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Viticulture. 52(1):1-7. [ Links ]
Biale, J. B. 1964. Growth, maturation, and senescence in fruits. Science. 146: 880-888. [ Links ]
Blankenship, S. M. and Dole, J. M. 2003. 1-Methylcyclopropene: a review. Postharvest Biol. Technol. 28(1):1-25. [ Links ]
Bongue-Bartelsman, M. and Phillips, D. A. 1995. Nitrogen stress regulates gene expression of enzymes in the flavonoid biosynthetic pathway of tomato. Plant Physiol. Biochem. 33(5):539-546. [ Links ]
Bouzayen, M.; Latché, A.; Nath, P. and Pech, J. C. 2010. Mechanism of fruit ripening. In: plant developmental biology-biotechnological perspectives. Pua, E. C. y Davey, M. R. (Eds.). Springer-Verlag. Berlin, Germany. Vol. 1. 319-339 pp. [ Links ]
Brummell, D. A.; Harpster, M. H.; Civello, P. M.: Palys, J. M.; Bennett, A. B. and Dunsmuir, P. 1999. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening.The Plant Cell. 11(11):2203-2216. [ Links ]
Brummell, D. A. and Harpster, M. H. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. In: plant cell walls. Carpita, N.C.; Campbell, M. y Tierney, M. (Eds.). 1a ed. Springer Science. Netherlands. 311-340 pp. [ Links ]
Capitani, G.; Hohenester, E.; Feng, L.; Storici, P.; Kirsch, J. F. and Jansonius, J. N. 1999. Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene. J. Mol. Biol. 294(3):745-756. [ Links ]
Cara, B. and Giovannoni, J. J. 2008 Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci. 175: 106-113. [ Links ]
Cervantes, E. 2002. Ethylene: new interactions, still ripening. Trends in plant science. 7(8):334-335. [ Links ]
Chervin, C.; El-Kereamy, A.; Roustan, J. P.; Latché, A.; Lamon, J. and Bouzayen, M. 2004. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 167(6):1301-1305. [ Links ]
Da Silva, F. L.; Escribano-Bailón, M. T.; Alonso, J. J. P.; Rivas-Gonzalo, J. C. and Santos-Buelga, C. 2007. Anthocyanin pigments in strawberry. LWT-Food Sci. Technol. 40(2):374-382. [ Links ]
Daane, K. M.; Johnson, R. S.; Michailides, T. J.; Crisosto, C. H.; Dlott, J. W.; Ramirez, H. T. Y. and Morgan, D. P. 1995. Nitrogen fertilization affects nectarine fruit yield, storage qualities, and susceptibility to brown rot and insect damage. California Agric. 49(4). [ Links ]
Dandekar, A. M.; Teo, G.; Defilippi, B. G.; Uratsu, S. L.; Passey, A. J.; Kader, A. A.; Stow, J. R.; Colgan, R. J. and James, D. J. 2004. Effect of down -regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 13(4):373-384. [ Links ]
Darley, C. P.; Forrester, A. M. and McQueen-Mason, S. J. 2001. The molecular basis of plant cell wall extension. In: plant cell walls. Carpita, N.C.; Campbell, M. y Tierney, M. (Eds.). 1a ed. Springer Science. Netherlands. 179-195 pp. [ Links ]
de Pascual-Teresa, S. and Sánchez-Ballesta, M. T. 2008. Anthocyanins: from plant to health. Phytochemistry reviews. 7(2):281-299. [ Links ]
Dokoozlian, N. K. and Kliewer, W. M. 1996. Influence of light on grape berry growth and composition varies during fruit development. J. Am. Soc. Hortic. Sci. 121(5):869-874. [ Links ]
Dos Santos, R. S.; Arge, L. W. P.; Costa, S. I.; Machado, N. D.; de Mello-Farias, P. C.; Rombaldi, C. V. and de Oliveira, A. C. 2015. Genetic regulation and the impact of omics in fruit ripening. Plant Omics. 8(2):78-88. [ Links ]
El-Kereamy, A.; Chervin, C.; Roustan, J. P.; Cheynier, V.; Souquet, J. M.; Moutounet, M.; Raynal, J.; Ford, C.; Latché, A.; Pech, J. C. and Bouzayen, M. 2003. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum. 119: 175-182. [ Links ]
El-Sharkawy, I.; Jones, B.; Gentzbittel, L.; Lelièvre, J. M.; Pech, J. C. and Latché, A. 2004 Differential regulation of ACC synthase genes in cold-dependent and -independent ripening in pear fruit. Plant Cell Environ. 27: 1197-1210. [ Links ]
Fernández-Trujillo, J. P.; Obando, J.; Martínez, J. A.; Alarcón, A. L.; Eduardo, I.; Arús, P. and Monforte, A. J. 2007. Mapping fruit susceptibility to postharvest physiological disorders and decay using a collection of near-isogenic lines of melon. J. Am. Soc. Hortic. Sci. 132(5):739-748. [ Links ]
Gapper, N. E.; McQuinn, R. P. and Giovannoni, J. J. 2013. Molecular and genetic regulation of fruit ripening. Plant Mol. Biol. 82(6):575-591. [ Links ]
Giovannoni J. J. 2001. Molecular biology of fruit maturation and ripening. Annual Rev. Plant Physiol. 52: 725-749. [ Links ]
Giovannoni, J. J. 2004. Genetic regulation of fruit development and ripening. The plant cell. 16(1):S170-S180. [ Links ]
Given, N. K.; Veis, M. A. and Gierson, D. 1988. Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta. 174(3):402-406. [ Links ]
Hamilton, A. J.; Bouzayen, M. and Grierson, D. 1991. Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proceedings of the National Academy of Sciences. 88(16):7434-7437. [ Links ]
Hamilton, A. J.; Lycett, G. W. and Grierson, D. 1990. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 346(6281):284-287. [ Links ]
He, J. and Giusti, M. M. 2010. Anthocyanins: natural colorants with health-promoting properties. Annual review of food science and technology. 1: 163-187. [ Links ]
Hodges, D. M. and Nozzolillo, C. 1995. Anthocyanin and anthocyanoplast content of cruciferous seedlings subjected to mineral nutrient deficiencies. J. Plant Physiol. 147(6):749-754. [ Links ]
Iguarán, E. J. C. and Alzate, O. A. T. 2014. Hallazgos de la biosíntesis del etileno en frutas climatéricas y de los factores que afectan la ruta metabólica. Alimentos Hoy. 22(31):46-63. [ Links ]
Jeppsson, N. 2000. The effects of fertilizer rate on vegetative growth, yield and fruit quality, with special respect to pigments, in black chokeberry (Aronia melanocarpa) cv. Viking. Sci. Hortic. 83(2):127-137. [ Links ]
Jia, H. J.; Araki, A. and Okamoto, G. 2005. Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’peach (Prunus persica Batsch). Postharvest Biol. Technol. 35(1):61-68. [ Links ]
Jiang, T.; Wang, P.; Yin, X.; Zhang, B.; Xu, C.; Li, X. and Chen K. 2011. Ethylene biosynthesis and expression of related genes in loquat fruit at different developmental and ripening stages. Sci. Hortic. 130(2):452-458. [ Links ]
Ju, Z.; Duan, Y. and Ju, Z. 1999. Effects of covering the orchard floor with reflecting films on pigment accumulation and fruit coloration in Fuji apples. Sci. Hortic . 82(1):47-56. [ Links ]
Kende, H. 1993. Ethylene biosynthesis. Annual Rev. Plant Biol. 44(1):283-307. [ Links ]
Kitinoja, L.; Saran, S.; Roy, S. K. and Kader, A. A. 2011. Postharvest technology for developing countries: challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 91: 597-603. [ Links ]
Kumar, R.; Khurana, A. and Sharma, A. K. 2014 Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 65: 4561-4575. [ Links ]
Lara, I. and Vendrell, M. 2003. Cold -induced ethylene biosynthesis is differentially regulated in peel and pulp tissues of ‘Granny Smith’apple fruit. Postharvest Biol. Technol. 29(2):109-119. [ Links ]
Lelièvre, J. M.; Latché, A. ; Jones, B.; Bouzayen, M. and Pech, J. C. 1997. Ethylene and fruit ripening. Physiol. Plantarum. 101: 727-739. [ Links ]
Leng, P.; Itamura, H.; Yamamura, H. and Deng, X. M. 2000. Anthocyanin accumulation in apple and peach shoots during cold acclimation. Sci. Hortic . 83(1):43-50. [ Links ]
Li, X. J.; Hou, J. H.; Zhang, G. L.; Liu, R. S.; Yang, Y. G.; Hu, Y. X. and Lin, J. X. 2004. Comparison of anthocyanin accumulation and morpho -anatomical features in apple skin during color formation at two habitats. Sci. Hortic . 99(1):41-53. [ Links ]
Lincoln, J. E. and Fischer, R. L. 1988. Diverse mechanisms for the regulation of ethylene-inducible gene expression. Mol. General Genetics. 212(1):71-75. [ Links ]
Liu, C.; Zhao, A.; Zhu, P.; Li, J.; Han, L.; Wang, X.; Fan, W.; Lü, R.; Wang, C.; Li, Z.; Lu, C. and Lu, C. 2015. Characterization and expression of genes involved in the ethylene biosynthesis and signal transduction during ripening of mulberry fruit. PloS one. 10(3):e0122081. [ Links ]
Manning, K.; Tor, M.; Poole, M.; Hong, Y.; Thompson, A. J.; King, G. J.; Giovannoni, J. J. and Seymour, G. B. 2006. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nature Genetics. 38(8):948-952. [ Links ]
Manríquez, D.; El-Sharkawy, I.; Flores. F. B.; Regad, F.; Bouzayen, M. ; Latché, A. and Pech, J. C. 2006. Fruit -specific gene expression and biochemical characteristics of two highly divergent alcohol dehydrogenases of melon. Plant Mol. Biol. 61: 675-685. [ Links ]
McClellan, C. and Chang, C. 2008. The role of protein turnover in ethylene biosynthesis and response. Plant Science. 175(1-2):24-31. [ Links ]
Mori, K.; Sugaya, S. and Gemma, H. 2005. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic . 105(3):319-330. [ Links ]
Nath, P.; Sane, A. P.; Trivedi, P. K.; Sane, V. A. and Asif, M. H. 2007. Role of transcription factors in regulating ripening, senescence and organ abscission in plants. Stewart Postharvest Review. 3(2):1-14. [ Links ]
Nishiyama, K.; Guis, M.; Rose, J. K.; Kubo, Y.; Bennett, K. A.; Wangjin, L.; Kato, K.; Koichiro, U.; Nakano, R.; Inaba, A.; Bouzayen, M. ; Latché, A. ; Pech, J. C. and Bennett, A. B. 2007. Ethylene regulation of fruit softening and cell wall disassembly in Charentais melon. J. Exp. Bot. 58(6):1281-1290. [ Links ]
O’Neill, S. D. 1997. Pollination regulation of flower development. Ann. Rev. Plant Phys. 48: 547-574. [ Links ]
Obando-Ulloa, J. M.; Moreno, E.; García-Mas, J.; Nicolai, B.; Lammertyn, J.; Monforte, A. J. and Fernández-Trujillo, J. P. 2008. Climacteric or non-climacteric behavior in melon fruit: Aroma volatiles. Postharvest Biol. Technol. 49(1):27-37. [ Links ]
Omboki, R. B.; Wu, W.; Xie, X. and Mamadou, G. 2015. Ripening genetics of the tomato fruit. Inter. J. Agric. Crop Sci. 8(4):567-572. [ Links ]
Oms-Oliu, G.; Hertog, M.; Poel, B. V. d.; Ampofo-Asiama J.; Geeraerd A. and Nicolaï, B. 2011. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol . 62(1):7-16. [ Links ]
Owino, W.; Manabe, Y.; Mathooko, F.; Kubo, Y. and Inaba, A. 2006. Regulatory mechanisms of ethylene biosynthesis in response to various stimuli during maturation and ripening in fig fruit (Ficus carica L.). Plant Physiol. Biochem. 44(5-6):335-342. [ Links ]
Payasi, A.; Mishra, N. N.; Chaves, A. L. S. and Singh, R. 2009. Biochemistry of fruit softening: an overview. Physiol. Mol. Biol. Plants. 15(2):103-113. [ Links ]
Pech, J. C. ; Latché, A. and van der Rest, B. 2008. Genes involved in the biosynthesis of aroma volatiles in fruit and vegetables and biotechnological applications. In: fruit and vegetable flavour: recent advances and future prospects. Brückner, B. and Wyllie, S. G. (Eds). 1a ed. Woodhead Publishing. Cambridge, England. 254- 271 pp. [ Links ]
Pech, J. C.; Purgatto, E.; Girardi, C. L.; Rombaldi, C. V. and Latché, A. 2013. Current challenges in postharvest biology of fruit ripening. Current Agric. Sci. Technol. 19(1-18). [ Links ]
Pegoraro, C.; Zanuzo, M. R.; Chaves, F. C.; Brackmann, A.; Girardi, C. L.; Lucchetta, L.; Nora, L.; Silva, J. A. and Rombaldi, C. V. 2010. Physiological and molecular changes associated with prevention of woolliness in peach following pre - harvest application of gibberellic acid. Postharvest Biol. Technol . 57(1):19-26 [ Links ]
Sato, T. and Theologis, A. 1989. Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proceedings of the National Academy of Sciences. 86(17):6621-6625. [ Links ]
Schaffer, R. J.; Friel, E. N.; Souleyre, E. J.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J. H.; Ma, J. H.; Nain, B.; Cohen, D.; Gleave, A. P.; Crowhurst, R. N.; Janssen, B. J.; Yao, J. L. and Newcomb, R. D. 2007. A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 144(4):1899-1912. [ Links ]
Seymour, G. B.; Ostergaard, L.; Chapman, N. H.; Knapp, S. and Martin, C. 2013. Fruit development and ripening. Ann. Rev. Plant Biol. 64: 219-241. [ Links ]
Spayd, S. E.; Tarara, J. M.; Mee, D. L. and Ferguson, J. C. 2002. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Viticulture. 53(3):171-182. [ Links ]
Tyas, J. A.; Hofman, P. J.; Underhill, S. J. and Bell, K. L. 1998. Fruit canopy position and panicle bagging affects yield and quality ofTai So’lychee. Sci. Hortic . 72(3):203-213. [ Links ]
Wang, A.; Tan, D.; Takahashi, A.; Zhong Li, T. and Harada, T. 2007. MdERFs, two ethylene-response factors involved in apple fruit ripening. J. Exp. Bot. 58(13):3743-3748. [ Links ]
Wang, H.; Schauer, N.; Usadel, B.; Frasse, P.; Zouine, M.; Hernould, M.; Latché, A.; Pech, J. C.; Fernie, A. R. and Bouzayen, M. 2009. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 21: 1428-1452. [ Links ]
Watada, A. E.; Herner, R. C.; Kader, A. A.; Romani, R. J. and Staby, G. L. 1984. Terminology for the description of developmental stages of horticultural crops. HortSci. 19(1):20-21. [ Links ]
Watkins, C. B. 2006. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 24(4):389-409. [ Links ]
Yahyaoui, E. F.; Wongs-Aree, C.; Latché, A.; Hackett, R.; Grierson, D. and Pech, J. C. 2002. Molecular and biochemical characteristics of a gene encoding an alcohol acyl-transferase involved in the generation of aroma volatile esters during melon ripening. Eur. J. Biochem. 269: 2359-2366. [ Links ]
Yang, S. F. and Hoffman, N. E. 1984. Ethylene biosynthesis and its regulation in higher plants. Ann. Rev. Plant Physiol. 35(1):155-189. [ Links ]
Zheng, Q.; Song, J.; Campbell-Palmer, L.; Thompson, K.; Li, L.; Walker, B.; Cui, Y. and Li, X. 2013. A proteomic investigation of apple fruit during ripening and in response to ethylene treatment. J. Proteomics. 93(0):276-294. [ Links ]
Zhengke, Z.; Donald, J. H.; Brandon, M. H. and Jing, P. R. 2009. Delay of tomato fruit ripening in response to 1-methylcyclopropene is influenced by internal ethylene levels. Postharvest Biol. Technol . 54(1):1-8. [ Links ]
Zhong, S.; Fei, Z.; Chen, Y. R.; Zheng, Y.; Huang, M.; Vrebalov, J.; Mcquinn, R.; Gapper, N.; Liu, B.; Xiang, J. S. and Giovannoni, J. J. 2013. Sinngle-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nature Biotechnol. 31(2):154-159. [ Links ]
Zhu, S. and Zhou, J. 2007. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chem. 100(4):1517-1522. [ Links ]
Received: August 00, 2017; Accepted: October 00, 2017











 texto en
texto en 



