Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.8 Texcoco nov./dic. 2017
https://doi.org/10.29312/remexca.v8i8.702
Articles
Mycobiota and Metarhizium isolated of borer worm larvae from the ruezno (Cydia caryana)
1Instituto Tecnológico Superior de Mulege. Loma Los Frailes SN. Col. Centro, Santa Rosalía, BCS. (diana-rosales@live.com.mx). Tel. 01 (615) 1521673.
2Campo Experimental La Laguna-INIFAP. Blvd. José Santos Valdez 1200 Pte., Col. Centro, Matamoros, Coahuila, México. CP. 27440. Tel. 01 (800) 0882222, ext. 82402. (gaytan.arturo@inifap.gob.mx; chew.yasmin@inifap.gob.mx).
3Unidad Regional Universitaria de Zonas Áridas-Universidad Autónoma Chapingo. Carretera Gómez Palacio-Chihuahua-Bermejillo km 40, Durango, México. CP. 35230. Tel. 01 (872) 7760190. (selenne.marquez@hotmail.com).
4Instituto Tecnológico de Torreón. Carretera Torreón -San Pedro km 7.5, Ejido Ana. CP. 27170. Tel. 01 (871) 3153771. (crispindelrio@yahoo.com.mx).
The necessity of having native organisms with potential for the biological control of pests for walnut pecan, motivated the registration of the mycobiota and search for entomopathogens in larvae of the worm borer of the rinds (GBR) Cydia caryana. To do so, in the year 2013, in a walnut orchard in The Laguna Mexico, 5 000 rindss were collected and processed to extract GBR larvae, which were incubated in a humid chamber or soil. Of larvae obtained before September 12 and placed in soil recovered 9% of Fusarium spp. + Gliocladium spp., In contrast, both fungi appeared 40% in larvae obtained after that date. In larvae obtained in December and placed in a humid chamber, Fusarium + Gliocladium spp. appeared 80%. It was only evidently Metarhizium anisopliae as entomopathogens of 5% of GBR larvae placed in soil after September 12, this fungus was also entomopathogenic of the giant red mite Trombidium sp., Black aphid of the walnut Melanocallis caryaefoliae, Tick Rhipicephalus sanguineus, and ant red Solenopsis sp. The GBR larvae can be used as a trap to recover Metarhizium and probably other entomopathogenic fungi on the soil of pecan walnut orchards.
Keywords: entomopathogenic fungi; GBR; walnut
La necesidad de contar con organismos nativos con potencial para el control biológico de plagas para el nogal pecanero, motivaron el registro de la micobiota y búsqueda de entomopatógenos en larvas del gusano barrenador del ruezno (GBR) Cydia caryana. Para ello, en el año 2013, en una huerta de nogal en La Laguna México, se recolectaron y procesaron 5 000 rueznos para extraer larvas de GBR, que fueron incubadas en cámara húmeda o suelo. De larvas obtenidas antes del 12 de septiembre y colocadas en suelo se recobró 9% de Fusarium spp. + Gliocladium spp., en contraste, ambos hongos aparecieron 40% en larvas obtenidas después de esa fecha. En larvas obtenidas en diciembre y se colocaron en cámara húmeda, Fusarium + Gliocladium spp. aparecieron 80%. Únicamente fue evidentemente a Metarhizium anisopliae como entomopatógenos de 5% de larvas del GBR colocadas en suelo después del 12 de septiembre, éste hongo también fue entomopatógenos del ácaro rojo gigante Trombidium sp., pulgón negro del nogal Melanocallis caryaefoliae, Garrapata Rhipicephalus sanguineus, y hormiga roja Solenopsis sp. Larvas de GBR pueden usarse como trampa para recobrar Metarhizium y probablemente otros hongos entomopatógenos en suelo de huertas de nogal pecanero.
Palabras clave: GBR; hongos entomopatógenos; nogal
Introduction
Mexico, is considered a mega diverse country, including a source of natural enemies for pests and plant diseases, which are part of biological control programs in the world, in fact our country, brings to the world more organisms for the biological control of pests than those he receives; however, only four genera of fungi and one of bacteria are widely used as biological control of pests. In addition to the above, there is a need to protect against new pests in Mexico and other countries, so it is important to have native organisms with potential for biological pest control (Williams et al., 2013).
In addition, the need to maintain a sustainable agriculture has led to the research, development and adoption of biological control methods, particularly in our country, has occurred in a marked way in the last 20 years. Among the fungi with the greatest potential for biological control of insects are the Beauveria, Metarhizium and Paecilomyces species, which are cosmopolitan and some of their species attack a wide range of insect orders (Garcia and Mier, 2010).
On the other hand, pests of importance to the walnut nut Carya illinoinensis (Wangenh.) K.Koch, such as nut borers (GBR) and rinds (GBN) Acrobasis nuxvorella (Neunzig) and Cydia caryana (Fitch) respectively (Nava and Ramírez, 2002), although little is known of the fungi as natural enemies of both pests. In contrast, in agricultural soils cultivated with sugarcane Saccharum officinarum L., bean Phaseolus vulgaris L. and maize Zea mays L., entomopathogenic fungi have been isolated (Barajas et al., 2009; Bautista et al., 2012; Hernández et al., 2011).
Commonly, entomopathogenic fungi are isolated from agricultural or forest soils using larvae from the pilary of apiaria Galleria mellonella L. (Hernández et al., 2011; Rodrígues et al., 2005; Sookar et al., 2008). The GBR larvae usually damage the rinds (outer bark that covers the shell of the walnut) from where they feed and move to pupa and finally adult, in the process, some nuts fall to the ground where the larvae could be infected by entomopathogenic fungi. Therefore, the objectives of the work were: i) to record the mycobiota and to find entomopathogenic fungi in GBR larvae inside the rinds and in larvae used as a trap in walnut cultivated soil; and ii) to evaluate the pathogenicity of entomopathogens on GBR larvae, the black aphid of the walnut Melanocallis caryaefoliae Davis and other insects.
Materials and methods
Field work was carried out during the summer of 2013, in the “Hormiguero” orchard, located on the Road Torreon-San Pedro km 16, municipality of Matamoros, Coahuila, whose coordinates at the entrance of the orchard are +25° 41’ 23.98”, -103° 20’ 5.59”. The laboratory study was carried out in the Phytopathology Laboratory of the National Institute of Forestry, Agriculture and Livestock Research (INIFAP) in Matamoros, Coahuila.
Rueznos and GBR. Three dates of harvesting of rinds were made, in the first two were collected nuts to obtain their rinds with signs of entry by the GBR, in the third date were collected 100 kg of rinds newly separated from the nut by means of industrial machinery (separator of walnut ring). All the rinds obtained were stored in a cold room at temperature 5-8 °C until extracted their larvae and pupae of GBR (rinds processed). The dates of collection and processing of rinds to extract larvae and pupae from the GBR and storage time before processing are indicated in Table 1.
Table 1 Collection, storage and processing of pecan nut rinds, to obtain larvae and pupae of the worm borer of rinds, during 2013 in the “Hormiguero” orchard in Matamoros, Coahuila, Mexico.

†= Los rueznos se almacenaron de 5-8 °C antes de procesarlos. ¥= El procesamiento consistió en extraer las larvas y pupas del barrenador del ruezno. £= Larvas y pupas expresadas como porcentaje con relación al número de rueznos procesados.
Colonization of larvae and pupae. In the anthill garden, samples of 20 g were collected from the surface of the soil under sterile plastic petri dishes. To the floor of each box was added sterile distilled water at 80% saturation. Then, five larvae and pupae (live or dead) obtained on 30-08-2013 and 06-09-2013 were placed per box and incubated for seven days at 28 °C. Live larvae obtained on dates 09-09-2013, 10-10-2013, 17-10-2013, 01-11-2013 and 11-11-2013 were also placed in Petri dishes with soil and incubated for seven days at 28 °C . The larvae that were obtained on 11-12-2013 were placed in groups of five in each humid chamber (Petri dish with wet sterile blotting paper) for ten days at 28 °C and incubated seven days at 28 °C.
Isolation and purification of fungi that invaded larvae and pupae. After incubating the larvae and pupae under aseptic conditions with a sterile hypodermic needle, the fungi were harvested, then the fungi were transferred to two culture media: water agar (AA) and potato dextrose agar (PDA). The two culture media were added 50 mg L-1 of the antibiotic amoxicillin after sterilization. The AA and the PDA were prepared by adding per liter of distilled water 17 g of agar for both media and only for the PDA, 20 g of food grade fructose (Gold Bell®) and the liquid from 200 g of potato cooked in one liter of distilled water.
When the fungi were able to grow in single colonies and visibly uncontaminated with bacteria, they were transferred to tubes with sloping medium (PDA + 2 g L-1 of CaCO3) and incubated until they observed that they colonized the surface of the medium at 28 °C. The tubes with the fungi were stored at 5 to 10 °C, for subsequent resurfacing in PDA and identification at the genus level (Domsch et al., 1980) except for Metarhizium that was identified to species (Tulloch, 1976).
Pathogenicity test of Metarhizium anisopliae. The GBR larvae used in this test were obtained directly from the rue of nuts attached to the shoots. Directly from the ground larvae were obtained, covered by spores M. anisopliae, these larvae were taken with dissecting forceps and placed in direct contact with freshly obtained larvae. They were then incubated in a humid chamber for ten days at 28 °C or in a petri dish that had the dry sterile secant paper (dry chamber). In both wet and dry chambers five larvae were placed and five boxes (replicates) were made. As a control treatment, uninoculated larvae were placed in both types of chambers. The entire test was repeated seven times, which were taken as treatments. On the third day of incubation live larvae, dead and sporulation of the fungus were recorded on the larvae, the latter repeated on the tenth day.
A second way of inoculating each GBR larva was using M. anisopliae spores with 100 uL of a solution of 108 mL-1, in which case the same larvae were used per box, boxes and replicates of the previous test. The spore solution was obtained by scraping the surface of the Sabouraud culture medium where the fungus grew 30 d at 28 °C, the spores were placed in sterile distilled water with 0.01% Tween 80, and with the aid of a Neubauer camera a concentration of 108 mL-1. In order to individually inoculate the giant red mite Trombidium sp., The black aphid Melanocallis caryaefoliae Davis, the tick Rhipicephalus sanguineus Latreille, and the red ant Solenopsis sp., cylinders of 0.8 cm were cut with spores of the colony of M. anisopliae that grew 30 to 28 °C in Sabouraud medium and were put in direct contact with each specimen. For this, a humid chamber was used with five insects per box and five boxes (replicates), the test was repeated three times.
Analysis of data. The analyze were performed using the SAS (2010) program. Data from GBR larvae killed or colonized by M. anisopliae were compared using the chi-square test (ꭓ2). The analyze were performed separately for i) the third or tenth day; and (ii) contact or spore inoculation.
Results and discussion
Rueznos and GBR. The GBR larvae recovered in a greater percentage (13-30%) from walnuts with symptoms (barren), compared to the rinds collected from the clean mechanics of the nut (7-9%), also, only were able to recover pupae of barren walnuts Table 1.
Colonization of larvae and pupae. The first attempt to isolate entomopathogenic fungi was made by inoculating living and dead larvae and pupae in the soil, in which case mixtures of fungi or fungi-bacteria appeared. On the bodies of the live larvae appeared the highest percentages of fungi and bacteria (5-9%), predominantly mixtures of Fusarium spp. with other fungi and bacteria, as well as the genus Gliocladium and the family Dermateaceae that did not sporulate or identify, Figure 1. In contrast, larvae placed in a humid chamber predominated the appearance of the Fusarium + Gliocladium 88% mixture, Figure 2. Larvae of the (GBR) were inoculated on moist soil, where Fusarium, Metarhizium, Gliocladium and Aspergillus were present in 40, 5, 4 and 4%, respectively Figure 3.

Figure 1 Microorganisms found of live larvae (57%) and dead (17%), live (20%) and dead (9%) pupae of Cydia Caryana, after being placed in soil and incubated for seven days at 28 °C. The dates of obtaining larvae and pupae were 30-08-2013 and 06-09-2013. A= Aspergillus spp. G= Gliocladium sp. D= Dermateaceae; T= Trichoderma sp.; F= Fusarium spp. B= Bacterias; V= Verticillium sp. AF= Aspergillus flavus.

Figure 2 Microorganisms found (%) in 125 live larvae of the genus Cydia Caryana, after placing in a humid chamber and incubating for ten days at 28 °C. The date of obtaining of larva was 12-11-2013. A= actinomycetes; G= Gliocladium sp. F= Fusarium spp. M= Mucoraceos; N= nothing.
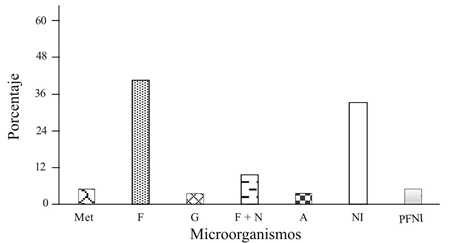
Figure 3 Microorganisms found (%) in 292 larvae of the borer of Cydia Caryana, after being placed in moist soil and incubated for seven days at 28 °C. The dates of obtaining larvae were 12-09- 2013, 10-10-2013, 17-10- 2013, 01-11-2013 and 11-11-2013. Met = Metarhizium anisopliae; A = actinomycetes; G = Gliocladium sp. F = Fusarium spp. N = nematodes NI = not invaded; PFNI= formed pupae not invaded.
Pathogenicity test of M. anisopliae. GBR larvae survived 100 and 92% after 3 and 10 d of incubation, respectively; in contrast, died from 65 to 100% when inoculated by contact or spores of M. anisopliae and incubated in a humid chamber; in contrast, larvae inoculated with both methods and incubated 3 d survived 100% and incubated 10 d survived 87 and 73% when inoculated by contact or spores, respectively (Table 2).
Table 2 Survival of larvae of the worm borer of the rinds Cydia Caryana after three days of having been inoculated or not with Metarhizium anisopliae.

£= larvas sin inocular, promedio de tres repeticiones, con cinco larvas por repetición; §= las larvas se inocularon al ponerlas en contacto directo con larvas micosadas; ¥= las larvas se colocaron en cajas petri con papel secante estéril seco; valores promedio de siete tratamientos, cada uno con tres repeticiones y cada repetición con cinco larvas. †= las larvas se colocaron en cajas petri con papel secante estéril húmedo (saturado), valores promedio siete tratamientos, cada uno con tres repeticiones y cada repetición con cinco larvas; ¶= se inocularon las larvas con 100 uL de una solución de esporas de 108 mL-1.
The treatments (control), wet or dry chamber were significant for the inoculation form, so for inoculated larvae in contact or spores at 3 d at (X2= 230, gl=14, p≤ 0.001) y (X2= 149, gl= 14, p< 0.001), respectively; Similarly, inoculation treatments for inoculated larvae on contact or spores at 10 d with X2= 290, gl= 14, p≤ 0.001 y X2= 198, gl= 14, p< 0.001, respectively, were significant. M. anisopliae sporulated on GBR larvae of 63 and 100% after inoculation with the fungus and incubated in humid chamber 3 and 10 d, respectively; but the fungus did not spore on the dry chamber larvae Table 3.
Table 3 Sporulation of Metarhizium sp. on larvae of the ruezno borer, the larvae were inoculated by direct contact with the fungus and incubated at 28 ° C for up to 10 days.

†= las larvas inoculadas se colocaron en cajas petri con papel secante estéril húmedo (cámara húmeda) o papel secante seco (cámara seca); valores promedio siete tratamientos, cada uno con tres repeticiones y cada repetición con cinco larvas.
At 24 h after inoculation of the GBR larva with M. anisopliae the fungus generates mycelium on the insect, at 36 h it forms a cottony mycelium and on the third day an aerial mycelium is formed in the form of a dome with the first spores, finally, after the fourth day the mycelium in dome form generates the spores Figure 4.

Figure 4 Mitosis of M. anisopliae on GBR larvae. A= invasión at 24 h; B= invasion at 36 h; C= production of spores at three days on the surface of cotton wool mycelium; and D= abundant sporulation after the fourth day.
The giant red mite, the black aphid of the walnut, the female tick as its eggs and the red ant allowed the sporulation of M. anisopliae, in all cases the fungus sporulated in 100%. The dome-shaped mycelium was also formed in the giant mite and a layer of mycelium not very profuse on the eggs of the tick and ant (Figure 5).

Figure 5 Insects that attacked M. anisopliae. A and D= tick egg and adult, respectively; B= giant mite; C= red ant: and E= black aphid of walnut.
The nematodes, Fusarium spp. and Gliocladium sp. by themselves or associates could be entomopathogens of the GBR, although it was not possible to asylum nematodes, nor was it confirmed that the fungi were entomopathogenic; however, it is shown in Figure 6 how the larvae were invaded by the mentioned microorganisms.
Discussion
A maximum of 30% of larvae of rinds obtained from the walnut was recovered, that low percentage could be due to the effect of the insecticide applied against the GBR and the emergence of some adults who leave the rinds no drill; the percentage of larvae of 9% of the rinds separated from the nut by means of machinery, could be explained to the mechanical effects and of decomposition of the rinds. For practical purposes, it was facilitated to obtain judgments from this second way. Time (13-46) and storage temperature (5-8 °C) most likely prevented the formation and production of pupae (Table 1).
When the larvae were obtained from rinds detached from the walnut or rinds separated by machinery they were not colonized by microorganisms in 60 and 37%, respectively (Figure 1 and 3), which could be due to the larvae acquiring some microorganisms during their storage or acquire susceptibility to be invaded by soil fungi. The lowest percentage (6%) of non-colonized larvae was obtained in a humid chamber (Figure 2) where Fusarium spp. predominated (88%); that is, the fungus seems to be associated with the larvae during storage; however, it was not possible to asylum Metarhizium anisopliae, which was only possible to do so from the soil of larvae obtained from rinds separated by machinery.
Some larvae were parasitized and killed by nematodes and associated with Fusarium spp., However, we could not isolate nematodes, although Fusarium. In 2014, in the Hormiguero garden, GBR larvae were very scarce, which is why it was not possible to test Fusarium asilies and other fungi as GBR entomopathogens. However, the isolates of M. anisopliae and the fungi mentioned are safeguarded in the plant laboratory of Phytopathology.
The larvae were only harvested in a humid chamber (Table 3) although some fungi inoculated with the fungus died after 10 days of incubation (Table 2). M. anisopliae is capable of killing insects through toxins and enzymes, but it does not always sporulate on the dead insect (Téllez et al., 2009), we consider it improbable that the fungus killed the GBR larvae in dry conditions (dry chamber). The relative humidity (HR) is determinant for the sporulation of M. anisopliae on the insects it attacks, previous work indicates that the fungus sporulates when the RH oscillates between 50-100% (Magalhães et al., 2000), the temperature also is an important factor for the virulence and adaptation of this fungus, particularly, strains obtained heat tolerant (growth> 25 °C) are virulent and warm regions are adapted (García et al., 2010; Rodrígues et al., 2005; Toriello et al., 2008). The M. anisopliae isolate obtained had maximum growth at 28 °C coupled with the virulence shown in an applied range of insects, it confers potential use characteristics for the biological control of pests in the soil.
Previously, different isolates of M. anisopliae have been reported to attack and kill up to 95% Rhipicephalus microplus Canestrini ticks (Ruvalcaba et al., 2011), the isolate evaluated in this work had a similar behavior. In addition, the species of ant Atta cephalotes L. and the black aphid of M. caryaefoliae were micasados by M. anisopliae (Lemus et al., 2008; Shapiro et al., 2008), both works indicate the capacity of the fungus to attack species or genera similar to those recorded in our work. Additionally, it is known that Metarhizium species have specialized in attacking or a very restricted or very broad range of insects (Hu et al., 2014), in our work, for M. anisopliae, all indicate that it attacks an applied range of insects.
Practically, Metarhizium species have usually been used for pest control by incorporating their spores into the soil, such as pest control of maize and sugarcane Saccharum officinarum L, among others (Lezama et al., 2005; Matabanchoy et al., 2012). The use of the isolate obtained in this work could be used by adding spores in the soil to infect RBM larvae within the rinds that are found in the soil.
Finally, it is known that different species of Metarhizium possess at the same time three ways of surviving, one as saprobes in soil, another associated with roots and a third as entomopathogens (Fisher et al., 2011; Barelli, 2013), which increases the perspectives of the use of these fungi to control pests in the soil, including C. caryana.
Conclusions
The GBR larvae can be used as a trap to isolate Metarhizium and probably other soil entomopathogens.
The isolate of M. anisopliae obtained in this work, attacks and kills different insects, reason why it is considered non-specialized.
Other recovered fungi and nematodes (unrecovered) showed the ability to macerate GBR larvae, although this effect was not confirmed.
Literatura citada
Barelli, L. 2013. Starvation induces expression of the plant-adhesin gene, Mad2, of the Entomopathogenic Fungus Metarhizium robertsii. Thesis Master of Science. Faculty of Graduate Studies, Brock University St. Catharines, Ontario, Canada. 108 p. [ Links ]
Bautista, G. A.; Barrera, J. F.; Payró, de la C. E.; Salgado, G. S.; Gómez, R. J. and Gomez, L. J. F. 2012. Genetic characterization of Metarhizium anisopliae (Metchnikoff) Sorokin isolates from sugarcane fields and their pathogenicity against Aeneolamia postica (Walker) (Hemiptera: Cercopidae). Universidad y Ciencia. 28(3):217-229. [ Links ]
Barajas, O. C. G.; Morales, R. M. D.; del Pozo, N. E. M.; Rodríguez, A. M. L. y Núñez, L. J. J. 2009. Condiciones para el desarrollo de Beauveria bassiana y Metarhizium anisopliae para el control biológico de Chapulín frijolero. Tecnociencia Chihuahua. 3(1):33-39. [ Links ]
Domsch, K. H.; Gams, W. and Anderson, T. H. 1980. Compendium of soil fungi. Volume 1. Academic Press (London) Ltd. 860 p. [ Links ]
Fisher, J. J.; Rehner, S. A. and Bruck, D. J. 2011. Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J. Invertebrate Pathol. 106(2):289-295. [ Links ]
García, G. C.; Cháirez, H, I. y Medrano, R, H. 2010. Efecto de la temperatura en la viabilidad de esporas y toxicidad de Beauveria bassiana y Metarhizium anisopliae sobre Pieris rapae (L.) (Lepidoptera: Pieridae). Folia Entomol. Mex. 49(1):1-7. [ Links ]
García, L. S. y Mier, T. 2010. Visión general de la producción y aplicación de bioplaguicidas en México. Sociedades Rurales, Producción y Medio Ambiente. 10:37-63. [ Links ]
Hernández, V. V. M.; Cervantes, E. Z.; Villalobos, F. J.; García, L. L. y Peña, C. G. 2011. Aislamiento de hongos entomopatógenos en suelo y sobre gallinas ciegas (Coleoptera: Melolonthidae) en agroecosistemas de maíz. Acta Zoo. Mex. 27(3):591-599. [ Links ]
Hu, X.; Xiao, G.; Zheng, P.; Shang, Y.; Su, Y.; Zhang, X.; Liu X.; Zhan, S.; Leger, S. J. and Wang, C. 2014. Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proceedings of the National Academy of Sciences. 111(47):16796-16801. [ Links ]
Lezama, R.; Molina, J.; López, M.; Pescador, A.; Galindo, E.; Ángel, C. A. y Michel, A. C. 2005. Efecto del hongo entomopatógeno Metarhizium anisopliae sobre el control del gusano cogollero del maíz en campo. Avances en Investigación Agropecuaria. 9(1):1-5. [ Links ]
Lemus, Y.; Rodríguez, G.; Cuervo, R.; Vanegas, J. A. D. y Zuluaga, C. L. 2008. Determinación de la factibilidad del hongo Metarhizium anisopliae para ser usado como control biológico de la hormiga arriera (Atta cephalotes). Revista Guillermo de Ockham. 6(1):91-98. [ Links ]
Magalhães, B. P.; Goettel, M. S. and Frazão, H. D. S. 2000. Sporulation of Metarhizium anisopliae var. acridum and Beauveria bassiana on Rhammatocerus schistocercoides under humid and dry conditions. Braz. J. Microbiol. 31(3):161-163. [ Links ]
Matabanchoy, S. J. A.; Bustillo, P. A. E.; Castro, V. U.; Mesa, C. N. C. y Moreno, G. C. A. 2012. Efficacy of Metarhizium anisopliae to control Aeneolamia varia (Hemiptera: Cercopidae), in a sugar cane. Rev. Colomb. Entomol. 38(2):177-181. [ Links ]
Nava, C. U. y Ramírez, D. M. 2002. Manejo integrado de plagas del nogal. In: Arreola, Á. J. y Reyes, J. I. (Eds.). Tecnología de producción del nogal Pecanero. Campo Experimental La Laguna. INIFAP. Matamoros, Coahuila, México. 220 p. [ Links ]
Rodrígues, S; Peveling, R.; Nagel, P. and Keller, S. 2005. The natural distribution of the entomopathogenic soil fungus Metarhizium anisopliae in different regions and habitat types in Switzerland. Insect Pathogens Insect Parasit Nematodes Melolontha. 28(2):185-188. [ Links ]
Ruvalcaba, M. F.; Padilla, A. M. B.; Vázquez, C. C. y Velázquez, V. M. H. 2011. Evaluación de cepas de Beauveria bassiana y Metarhizium anisopliae sobre la inhibición de oviposición, eclosión y potencial reproductivo en una cepa triple resistente de garrapata Rhipicephalus (Boophilus) microplus (Canestrini) (Acari: Ixodidae). Entomotropica. 25(3):109-115. [ Links ]
SAS, Institute. 2010. Statistical Analysis Systems for Windows. Cary N.C. [ Links ]
Shapiro, I. D. I.; Cottrell, T. E.; Jackson, M. A. and Wood, B. W. 2008. Virulence of hypocreales fungi to pecan aphids (Hemiptera: Aphididae) in the laboratory. J. Invertebrate Pathol. 99(3):312-317. [ Links ]
Sookar, P.; Bhagwant, S. and Awuor, O. E. 2008. Isolation of entomopathogenic fungi from the soil and their pathogenicity to two fruit fly species (Diptera: Tephritidae). J. Appl. Entomol. 132(9-10):778-788. [ Links ]
Téllez, J. A.; Cruz, R. M. G.; Mercado, F. Y.; Asaff, T. A. y Arana, C. A. 2009. Mecanismos de acción y respuesta en la relación de hongos entomopatógenos e insectos. Rev. Mex. Micol. 30:73-80. [ Links ]
Toriello, C.; Montoya, S. E.; Zavala, R. M.; Navarro, B. H.; Basilio, H. D.; Hernández, V. V. y Mier, T. 2008. Virulencia y termotolerancia de cultivos monospóricos de Metarhizium anisopliae var. anisopliae de la mosca pinta (Hemiptera: Cercopidae). Rev. Mex. Micol. 28(SPE):57-66. [ Links ]
Tulloch, M. 1976. The genus Metarhizium. Transactions of the British Mycological Society. 66(3):407-411. [ Links ]
Williams, T.; Arredondo, B. H. C. and Rodríguez, del B. L. A. 2013. Biological pest control in Mexico. Ann. Rev. Entomol. 58:119-140. [ Links ]
Received: June 2017; Accepted: November 2017











 texto en
texto en 



