Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.8 Texcoco Nov./Dez. 2017
https://doi.org/10.29312/remexca.v8i8.701
Articles
Species of Hemiptera-Heteroptera associated to Opuntia spp. and Nopalea spp. in the Mexican Chihuahuense Desert
1Universidad Autónoma Agraria Antonio Narro-Departamento de Parasitología Agrícola y Departamento de Botánica. Calzada Antonio Narro 1923, Buenavista, Saltillo, Coahuila, México. CP. 25315. Tel. 01 (844) 4110296. (rosagloria.rochaflores@gmail.com; mxjavq12@yahoo.com.mx).
The Chihuahuense Desert has an approximate extension of 507 000 km2 where a vast diversity of organisms is expressed, with a good number of Hemiptera-Heteroptera species present. In this study we determined species of the group in the Chihuahuense Desert, covering six states of the Mexican Republic of this important ecosystem. 10 families of Heteroptera were identified, among them Miridae and Coreidae, in the latter the first report is highlighted of Chelinidea vittiger aequoris Uhler, 1963 for this ecosystem and Cimicidae and Reduviidae, which have medical-veterinary importance.
Keywords: Cactaceae; Chelinidea vittiger; Coreidae; Mexico; nopales
El desierto chihuahuense tiene una extensión aproximada de 507 000 km2 donde se expresa una vasta diversidad de organismos, estando presentes buen número de especies de Hemíptera-Heteróptera. En este estudio se determinaron especies del grupo en el desierto chihuahuense, abarcando seis estados de la República Mexicana de éste importante ecosistema. Se determinaron 10 familias de Heteroptera, entre las cuales se encuentran Miridae y Coreidae, en esta última se destaca el primer reporte de Chelinidea vittiger aequoris Uhler, 1963 para este ecosistema y Cimicidae y Reduviidae, que tienen importancia médico-veterinaria.
Palabras clave: Cactaceae; Chelinidea vittiger; Coreidae; México; nopales
Introduction
The Chihuahuese desert (DC) is the largest in North America, considered as one of the world’s richest dry regions (Morafka, 1977; Sutton, 2000; Hoyt, 2002), has an approximate extension of 507 000 km2 ranging from center of Mexico (states of Guanajuato, Hidalgo and Queretaro) to the north, in South Texas, New Mexico and a small portion of Arizona. To the west and east is delimited by the Sierras Madre Occidental and Madre Oriental, respectively (Hernandez et al., 2008). Henrickson and Johnston (1986) consider eight primary subdivisions, namely chihuahuense scrub, lechuguilla scrub, izotal forest, Prosopis-Atriplex scrub, alkaline scrub, gypsum scrub, cactus scrub and riparian forest. Rzedowski (1978) named a specific region as a cactus-mesquite thicket, and Miranda and Hernandez (1963) to another as a nopalera. Opuntia spp. species are distributed in different types of vegetation, (Miranda and Hernandez X, 1993); is included in the DC to the arid zone of Tehuacan-Cuicatlan and to the Balsas River Basin (Rzedowskii, 1978).
In Mexico there are more than 100 species of nopales, most of them located in arid zones; these plants have morphological and physiological characteristics adapted to the scarce availability of water, extreme variations in temperature and the conditions of arid and semi-arid zones. In the states of Aguascalientes, Guanajuato, Hidalgo, San Luis Potosi, Zacatecas and part of Jalisco, Michoacan and Queretaro, there is the greatest richness of wild nopal species (35% of total Opuntia spp.) and cultivated varieties (Gallegos-Vazquez et al., 2003).
The national production of nopal vegetables is concentrated in the states of Morelos and Mexico; the most productive states of forage nopal are Coahuila, Aguascalientes and Oaxaca, which has great economic and social importance (SIAP, 2016). Some species of the genus Opuntiaspp. are considered threatened and endangered (Norma NOM-059-ECOL-2001), due to illegal trade, the extraction of their habitats, diseases and insect pests.
The nopales are a relevant resource for Mexico in the ecosystems of arid and semi-arid zones, present in more than 50% of their territory. They are distributed throughout the country, but the highest concentration is in the arid and semi-arid zones of the DC and in the semi-arid zone of Tehuacan-Cuicatlan (Scheinvar et al., 1982), 29 species are microendemic or of restricted distribution, occurring in very specific areas; The regions with the highest species richness are the central and northern Altiplano, the northwest, the Bajio, the Neovolcanic Axis and the Tehuacan-Cuicatlan Valley. In the tropical dry regions and deserts of the north there is less wealth, but endemic species of great importance are often found (CONABIO, 2017).
The endemism of Opuntia spp. In Mexico, which is 73% (Hérnandez and Godinez, 1994), there are many species of insects cohabiting in them, for Mexico, Soria (1993) reports to eight as main pests of the nopal and 22 as harmful, highlighting a species of Miridae, three of Coreidae and one of Lygaidae. Some of the important pests that attack both wild and cultivated nopales are: Narnia femorata Stal (Hemiptera: Coreidae) affecting Opuntia ficus-indica (Palomares et al., 2012), Cactophagus spinolae Gyllenhall (Coleoptera: Curculionidae), Cactoblastis cactorum Berc (Lepidoptera: Pyralidae) (Zimmermann et al., 2007), weevil weevil, Cylindrocoptorus biradiatus Champs, white caterpillar worm, Lanifera cyclades Druce, zebra worm, Olycella nephelepsa Dyar and cochineal, Dactylopius indica Green and D. opuntiae (Vanegas, 2010).
The Hemiptera order is one of the most abundant in the world. The objective of the present work was to know the diversity of Hemiptera of the suborder Heteroptera associated with Opuntia spp. in the Chihuahuense Desert.
Materials and methods
The insect collections were carried out during the months of July 2016 to May 2017 in different states located in the DC of the Mexican Republic, for which four sampling routes were considered:
Route 1. Highway 40, Saltillo-Chihuahua; Route 2. Highway 57, Saltillo-San Luis Potosí; Route 3. Highway 23, Saltillo-Durango, Route 4. Highway 57, Saltillo-Piedras Negras.
In each route, every 100 km, random insects were collected in the different species of wild nopales that were close to 200-300 m on either side of the road, spending at least two hours at each site. To collect the insects, five forms were used, namely, manteo, redeo, soil samples, fall traps with fruit and directly with the hands.
Manteo. Blanket cloth was used to be placed on the ground, under the drip area of nopales or nopal groups and then applied, with a pump 80% Cypermethrin diluted in water, is allowed to pass 30 min to collect clamp or brush was used, very carefully, to the insects fallen on the blanket, which were placed in clear plastic bottles of 100 ml, labeled (collector, date, route, road, kilometer, form of collection) containing 75% ethyl alcohol (v/v).
Redeo. With an entomological network of 30 cm in diameter, at each site, the largest possible number of rounds around nopales was given; the captured insects were placed in flasks labeled with 75% ethyl alcohol.
Direct collection. Nymphs, nymphs, larvae, pupae and insect adults present directly with the hands using tweezers, brushes or mini entomological net were carefully observed, the insects obtained were placed in ethyl alcohol as previously mentioned.
Falling traps. At each site, holes were drilled in the soil underneath nopals to place in the same labeled bottles of 100 mL containing rotten fruits as attractive and whose open mouth remained flush with the soil and remained 1-3 days in accordance with routes (they were placed on the way and picked up on the way back). The captured insects were preserved in 75% ethyl alcohol.
Floor. At each collection site, five soil samples of 1 kg taken 0 to 20 cm deep under nopales were randomly taken; then collected and stirred to take a 1 kg that was put in a bag of labeled paper that was placed in an empty ice maker.
All insects collected by the different methods were transferred to the Insects and Mites Laboratory (LIA) of the Department of Agricultural Parasitology of the Saltillo Headquarters (DPA-S) of the Autonomous University of Agraria Antonio Narro, Campus Buenavista (UAAAN-CB), where considering the routes, sites and forms of sampling were counted and separated first by family, and then to genus and species.
Results and discussion
With the procedures described above, 1 770 insect specimens were collected, of which 750 corresponded to Hemiptera and 719 to Hemiptera-Heteroptera.
The number of samples varied between routes, for example, in the one of Chihuahua 14 were realized, in the one of Durango nine and in the one of Piedras Negras and San Luis Potosí, five.
In the Chihuahua route (Saltillo, Coahuila;Torreón, Coahuila; Gómez Palacio, Durango; Jiménez, Chihuahua; Ciudad Juárez, Chihuahua), 51% insects were collected, followed by the Saltillo-Piedras Negras, Coahuila (Saltillo, Monclova, Sabinas, Piedras Negras), where it was obtained 23.7%, the San Luis Potosi route (Saltillo, San Rafael, Matehuala, San Luis Potosi) with 15% and the Durango route (Saltillo, Torreon, Gomez Palacios and Durango, Durango) 10.2% (Table 1).
Table 1 Hemiptera-Heteroptera families and number of specimens collected in the Mexican Chihuahuense Desert in four sampling routes, using five techniques, 2017.
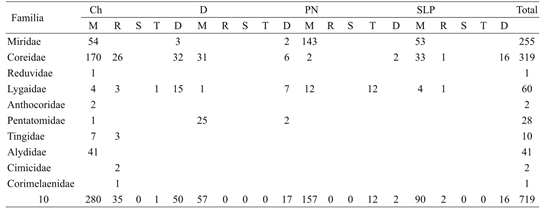
Ch= ruta Chihuahua; D= ruta Durango; PN= ruta Piedras Negras, Coahuila; SLP= ruta San Luis Potosí. M= manteo; R= redeo; S= suelo; T= trampas; D= directo.
In the route Chihuahua, ten families of Heteroptera were obtained; in the one of Durango four and in those of Piedras Negras and San Luis Potosi three.
Of the ten families identified seven are characterized by phytophagous habits (Miridae, Coreidae, Lygaidae, Pentatomidae, Tingidae, Alydidae and Corimelaenidae), while Reduviidae and Anthocoridae have predatory habits and Cimicidae is hematophagous. The families more numerically collected were Coreidae and Miridae (phytophagous). The abundance of specimens with phytophagous habit represented 99% (Table 1).
The maintenance and the direct collection were the best methods to obtain insects since with them 93% of the total registered was obtained.
The frequency of sampling, type of sampling, time of year, ecological situations, among other aspects, are variables that explain the difference in the number of specimens obtained and the diversity in each of the routes.
In the ten families of insects Hemiptera-Heteroptera associated to Opuntia spp. and Nopalea spp. The 14 genera and 17 species were determined. Ten genera include phytophagous species, two hematophagous species and one predator. At the species level, eleven are phytophagous, three hematophagous and two predators (Table 2).
Table 2 Families, genera and species of Hemiptera- Heteroptera collected in the Mexican Chihuahuan Desert.

Of the total specimens, 353 included four species of Coreidae and 252 to two species of Miridae, respectively, this reference is made because species of Coreidae and Miridae are reported as important pests of Opuntia spp.(Soria, 1993; Palomares et al., 2012).
Coreidae. A taxonomic group with 80 species reported in North America, have developed odorous glands; most are phytophagous, few are predatory (Borror and White, 2005).
Chelinidia tabulata (Burmeister, 1835). There are reports of Chelinidia tabulata in the area of Milpa Alta, Estado de México (Vargas-Mendoza et al., 2008). Brailovsky (1994) found this species in the Tehuacan Valley, Puebla and describes it as robust with jugum surpassing the tylus.
Chelinidea vittiger aequoris (Uhler, 1863). It was reported for the first time in Florida (McAtee, 1919; Hamlin, 1924 and Torre-Bueno, 1941). McAtee (1919) recognized two species of Chelinidea from the Nearathic Region and divided C. vittiger into two subspecies. He observed variations in color in each subspecies naming them C. v. aequoris and C. v. artuatra. For the Mexican Chihuahuan Desert there are no reports of C. vittiger aequoris so far. The adult measures from 10 mm to 13.5 mm, pronotum 4 to 5 mm wide, conspicuous back, yellowish orange bands on the head, pronotum yellowish, with yellow veins. The general appearance is very similar to that of the common chants.
Narnia femorata (Stål, 1862). Palomares et al. (2015) reported to N. femorata for the region of Milpa Alta. De Lestang and Miller (2009), mention that these choruses feed mainly on fruits of some opuntia such as O. humifusa, although they also comment that they can feed on cladodes. For O. ficus-indica, there is no precise information indicating the presence of the insect on this vegetable or the damage it causes (Mann, 1969; Brailovsky and Sánchez, 1983; Lestang and Miller, 2009). Palomares et al. (2015) state that it feeds mainly on tender brambles and feeding points cause a small scar that prevents the growth of the cladodium, taking a heart aspect.
Anasa tristis (De Geer, 1773). Hunter et al. (1912) report it as a pest of the main cacti of the United States of America. Brailovsky (1985) found it on stems of O. streptacantha in Honduras.
Miridae (Hahn, 1831). It is the largest family of the order Hemiptera with about 1 750 species in North America. They have phytophagous and predatory habits; soft body 4-10 mm long marked with red, orange, green or white colors; presence of cuneo and one or two closed cells at the base of the membrane (Borror and White, 2005).
Hesperolabops gelastops (Kirkaldy, 1902). Ruíz et al. (2010) made the first report of H. gelastops in O. ficus-indica in the eastern part of the State of Mexico; the nymphs and adults of the red bug eat sap of the cladodes (Mann, 1969; Badii and Flores, 2001). The feeding of this insect is associated with brown pustules that interfere with the photosynthesis of the plant, which is known as a symptom of the “nopal cactus” (Palomares et al., 2010).
Taylorlygus pallidulus (Blanchard, 1852). Lo Verde and La Mantia (2011) found it in bloom of O. ficus-indica. Richmond (1968) reported it in cacti species.
Pentatomidae (Leach, 1815). More than 200 species are known in North America; are recognized by their rounded body to ovoid and antennas of five segments, is divided into the subfamilies, Asopinae, Discocephalinae, Edessinae, Podopinae and Pentatominae. The last four are phytophagous (Borror and White, 2005).
Euchistus variolarius (Uhler 1857). Walker (2012) reported this insect present in Opuntia spp. Rider (2012) reported on different species of Arkansas flora, including Opuntia spp. The annual report (2014) of the University of Cornell, mentions to O. humifusa like host of E. variolarius causing damages in stems.
Chinavia hilaris (Say, 1832). Also known as Acrosternum hilare (Schwertner and Grazia, 2007; Rider 2009) is the main cotton pest Gossypium hirsutum L. (Barbour et al., 1990). It has also been found in maize and peanuts (Panizzi et al., 1980; Velasco and Walter, 1992; Bundy and McPherson, 2000; Ehler, 2000; Tillman et al., 2009; Toews and Shurley, 2009; Tillman, 2011), so far there are no reports in Opuntia spp. in Mexico.
Cimicidae (Latreille, 1802). They are nocturnal insects, flattened, oval, 6 mm in length, hematophagous of birds and mammals; some species are urban pests and attack bats and birds.
Hesperocimex coloradiensis (List, 1925). Hematophagous; Scudder and Smith (2011) describe the genus Hesperocimex List, 1925 which includes a rare species found in various species of wildlife in the state of Dakota.
Hesperocimex cochimiensis (Ryckman, 1958). Bird hematophagous. Ryckman and Ueshima (1973) found it parasitizing birds that are commonly known as Kingfisher.
Alydidae (Amyot and Serville, 1843). Similar to coreidae, the head is as large as the pronotum and the body is long and narrow. They emit more odors than Pentatomidae (Triplehorn and Johnson, 2005).
Stenocoris tipuloides (De Geer, 1773). Offman (2000) reported on sorghum and Opuntia spp.
Anthocoridae (Fieber, 1837). Known as pirate bugs; are flattened small insects 2-5 mm long, elongated. Many species are black with white markings; have predatory habits (Triplehorn and Johnson, 2005).
Orius laevigatus (Fieber 1860). Laxmi et al. (2015) consider this species as useful in the biological control of insects that affect species of flowers, generally considered to protect different crops, including Opuntia spp.
Orius niger (Wolff, 1811). Lundgren (2009) in his study on natural enemies includes different species of Orius present in Opuntia spp. Figueroa et al. (2014) found O. niger in Cactaceae species.
Corimelaenidae (Uhler, 1872). Insect’s oval, convex, bright black 3-6 mm in length. They have a long scutellum covering most of the abdomen and wings (Triplehorn and Johnson, 2005).
Corimelaena lateralis (White, 1839). Heads et al. (2015) reported the diversity of Heteroptera in Illinois, USA listing C. laterallis. Rider (2012) reports it associated with Opuntia spp. in Dakota.
Tingidae (Laporte, 1807). Commonly called lace bedbugs, they are a group with about 140 species in North America, small, less than 5 mm in length, phytophagous (Triplehorn and Johnson, 2005).
Telemonemia scrupulosa (Stål, 1873). Phytophagous species that Julien et al. (2008) consider it as harmful as Dactylopius ceylonicus in Opuntia spp. and Cactoblastis cactorum (Berg, 1885; Srivastava and Singh, 1997).
Lygaeidae (Schilling, 1829). The species present bright colors, orange and black or red and black. They have dorsal abdominal spiracles. They feed on seeds or plants toxic to other organisms. 75 species are known in North America (Triplehorn and Johnson, 2005).
Nysius vinitor (Dallas, 1852). The genus Nyzius is cosmopolitan and includes N. californicus Stål and N. raphanus (Howard, 1872) is a major cotton pest. For Mexico other species of this genus are reported as potential pests in various crops such as peanuts, cotton, wheat and malvae (Coronado and Marquez, 1981). Cartron et al. report to Nysius spp. in Opuntia spp.
Reduviidae (Latreille, 1807). Known as killer bugs. It is a group with more than 160 species in North America, they have predatory habits, many species are blackish to cafesuscas or brightly colored, elongated head (Triplehorn and Johnson, 2005).
Triatoma guasayana (Wygodzinsky and Abalos, 1949). It is a peridomiciliary vector of Chagas’ disease, of epidemiological importance. In the wild, abundant populations of this species occur mainly in biotypes that include Cactaceae (Opuntia quimilo), chaguares (Bromelia sp.) And trunks, feeding on rodents, marsupials and birds (Carcavallo et al., 1988; Vezzani et al., 2001).
Conclusions
The diversity of Hemiptera-Heteroptera associated to Opuntia spp. in the Mexican Chihuahuense Desert is diverse and important, since it includes species that are playing different ecological roles in the ecosystem, including phytophagous pests, including nopals, and haematophages of veterinary medical interest.
The Coreidae family is present in abundance with respect to the other families in the Chihuahuense desert. The largest species was C. vittiger aequoris. Abiotic factors as well as number of samples can explain such diversity between sites.
It is reported for the first time in the Chihuahuense Desert to Chelinidea vittiger aequoris (Uhler, 1863) and to Chinavia hilaris (Say, 1832).
Literatura citada
Badii, M. H. y Flores, A. E. 2001. Prickly pear cacti pests and their control in Mexico. Florida Entomol. 84:503-505. [ Links ]
Barbour, K. S.; J. R. Bradley, Jr. and J. Bachelor, S. 1990. Reduction in yield and quality of cotton damaged by green stink bug (Hemiptera: Pentatomidae). J. Econ. Entomol. 83:842-845. [ Links ]
Brailovsky, H.; Barrera, E.; Mayorga, C. y Ortega, G. 1994. Estadios ninfales de los coreidos del valle de Tehucán-Puebla (Hemiptera-Heteroptera) C. stafilessi, C. tabulata y N. femorata. Anales. Instituto de Biología. Universidad Nacional Autónoma de México (UNAM). Ser. Zool. 65(2):241-264. [ Links ]
Brailovsky, H. 1985. Revisión del género Anasa Amyot-Serville (Hemiptera-Heteroptera-Coreidae-Coreinae-Coreini). Monografías. Instituto de Biología. Universidad Nacional Autónoma de México (UNAM). 2:1-266. [ Links ]
Bravo, A. D.; Rendón, A. B.; Zavala, H. J. A. y Fornoni, J. 2014. Primer registro de Cactophagus spinolae (Coleoptera: Curculionidae) sobre dos especies de Stenocereus (Cactaceae) en el centro de México. Rev. Mex. Biod. 85:972-974. DOI: 10.7550/rmb.43764. [ Links ]
Borror, D. and White, R. 2005. A field guide to insects: America North of Mexico 2nd edition. [ Links ]
Bundy, C. S. and McPherson, R. M. 2000. Dynamicsand seasonal abundance of stink bugs (Heteroptera: Pentatomidae) in a cotton-soybean ecosystem. J. Econ. Entomol. 93:697-706. [ Links ]
Coronado, R. y Márquez, A. 1981. Introducción a la entomología, morfología y taxonomía de los insectos. Décima reimpresión. Limusa. México, D. F. 282 p. [ Links ]
De Lestang, F. y Miller, C. 2009. Effects of diet on development and survivorship of Narnia femorata Nymphs (Hemiptera: Coreidae) Florida Entomologist. 92(3):511-512. [ Links ]
Ehler, L. E. 2000. Farmscape ecology of stink bugs in northern California. Memorial Thomas Say Publications of Entomology, Entomological Society of America Press, Lanham, MD. 65 p. [ Links ]
Figueroa, D.; Valverde, P.; Vite, F. and Carrillo, H. 2014. Spatial variation in the community of insects associated with the flowers of Pachycereus weberi (Caryophyllales: Cactaceae). Environ. Entomol. 43(4):889-895. [ Links ]
Gallegos- Vázquez, C. J.; Cervantes, H. y Medina, G. G. 2003. La cadena productiva del nopal en Zacatecas: bases para un desarrollo sostenido. Fundación Produce. Zacatecas, Zacatecas. 167-173 pp. [ Links ]
Hamlin, J. C. 1924. A review of the genus Chelinidea (Hemiptera-Heteroptera) with biological data. Ann. Entomol. Soc. Ame. 25:89-20. [ Links ]
Heads, S.; Taylor, S.; Swanson, D. and Thomas, M. 2014. Regional biodiversity of terrestrial Heteroptera and Orthoptera in southwestern Illinois: Illinois Natural History Survey Technical Report 2015. 32 p. [ Links ]
Hernández, M. H.; Goettsch, B.; Gómez, C. and Arita, T. H. 2008. Cactus species turnover and diversity along a latitudinal transect in the Chihuahuan Desert Region. Biodivers Conserv. 17:703-720. [ Links ]
Hernández, H. y Godinez, H. 1994. Contribución al conocimiento de las cactáceas mexicanas amenazadas. Acta Bot. Mex. 26:33-52. [ Links ]
Henrickson, J. Y. and Johnston, M. C. 1986. Vegetation and community types of the Chihuahuan Desert. In: Barlow, J. C.; Powell, A. M. and Timmermann, B. N. 2:20-39. [ Links ]
Hoffman, R. 1994. Additions and Emendations to the Virginia Fauna of “True Bugs” (Heteroptera: Cydnidae, Scutelleridae, Pentatomidae, Alydidae). Banisrmo. Virginia Natural History Society. 3:5. [ Links ]
Hoyt, A. C. 2002. The Chihuahuan Desert: diversity at risk. Endangered Species Bulletin. 27(2):16-17. [ Links ]
Hunter, W. D.; Pratt, E. C. and Mitchel, J. D.1912. Principal cactus insects of the United States. Bull. USDA. Div. Ent. (N, S).113: 1-71. [ Links ]
Laxmi, R. V.; Sharma, P. and Kushwaha, R. 2015. Beneficial Insects and their value to agriculture getanjal. Res. J. Agric. Forestry Sci. 3(5):25-30. [ Links ]
Lo Verde, G. and La Mantia. 2011. The role of native flower visitors in pollinating Opuntia ficus-indica (L.) Mill. naturalized in Sicily. Acta Oecologica. 37(5):413-417. [ Links ]
Lundgren, J. G.; Kris, A.; Wyckhusys, G. and Desneux, N. 2009. Population responses by Orius insidiosus to vegetational diversity. BioControl 54:135-142. [ Links ]
Mann J. 1969. Cactus-feeding insects and mites. U.S. National Museum Bulletin. 256:1-158. [ Links ]
McAtee, W. L. 1919. Notes on nearctic Heteroptera. Bulletin of the Brooklyn Entomological Society 14:8-15. [ Links ]
Miranda, F. y Hernández, X. E. 1963. Los tipos de vegetación de México y su clasificación. Boletín de la Sociedad Botánica de México. 28:29-179 [ Links ]
Morafka, D. J. 1977. A biogeographical analysis of the Chihuahua desert through its herpetofauna. Junk, B. V. W. Publishers, The Hague. 5 p. [ Links ]
Palomares-Pérez, M.; Galeana-de la Cruz, M.; Carrillo-Fonseca, C. y Sancén-Plaza, A. 2012. Reporte de Narnia femorata Stål (Hemiptera: Coreidae) Sobre Opuntia ficus indica L. (Miller) en Milpa Alta, Ciudad de México. Southwestern Entomologist. 37:3. [ Links ]
Panizzi, A. R.; Galileo, M. H. M.; Gastal, H. A. O.; Toledo, J. F. F. and Wild, C. H. 1980. Dispersal of Nezara viridula and Piezodorus guildinii nymphs in soybeans. Environ. Entomol. 9:293-297. [ Links ]
Rzedoswki, J. 1978. Vegetación de México. Ed. Limusa, México. Reporte Anual Cornell University. 432 p. https://cuaes.cals.cornell.edu/sites/cuaes.cals.cornell.edu/files/shared/2014AnnualReport_comp.pdf. [ Links ]
Richmond, E. 1968. A supplement to the fauna and flora of Horn Island, Mississippi. Gulf Research Reports. 2(3):213-254. [ Links ]
Rickman, R. and Ueshima, N. 1963. Hesperocimex cochimensis, new species from Baja California, México. Proc. Ent.Soc. Wash. 65(3):247-251. [ Links ]
Rider, D. 2009. Chinavia hilaris (Say, 1831). (http://www.ndsu.nodak.edu/ ndsu/rider/Pentatomoidea/Species_Nezarini/Chinavia_hilaris.htm). [ Links ]
Rider, D. 2012. The Heteroptera (Hemiptera) of North Dakota I: Pentatomomorpha: Pentatomoidea. The Great Lakes Entomologist 45:3-4. [ Links ]
Shannon, W. J: 2003. Indice de Pielou. Total abundance (individuals/m2) and diversity of macroinvertebrates of the Oja River A: abundance, H: Shannon-Weaver index, J : Pielou index. 15 p. [ Links ]
Scheinvar, L. 1982. La familia de las cáctaceas en el Valle de México. Tesis doctoral. Facultad de Ciencias. Universidad Nacional Autónoma de México. [ Links ]
Schwertner, C. F. e Grazia, J. 2007. O geˆnero Chinavia Orian (Hemiptera, Pentatomidae, Pentatominae) no Brasil, corn chave picto´rica para os adultos. Rev. Bras. Entomol. 51:416-435. [ Links ]
Scudder, G. G. E. and Smith, I. M. 2011. Introduction and summary of the Montane Cordillera Ecozone. In: assessment of species diversity in the montane Cordillera Ecozone. Edited Scudder, G. G. E. and Smith, I. M. 1-26 pp. [ Links ]
Sutton, A. 2000. El desierto chihuahuense, nuestro desierto. Fondo Mundial para la Naturaleza. http://www.pronatura.org. [ Links ]
Toews, M. D. and Shurley, W. D. 2009. Crop juxtaposition affects cotton fiber quality in Georgia farmscapes. J. Econ. Entomol. 102:1515-1522. [ Links ]
Torre-Bueno, de la J. R. 1941. A synopsis of the Hemiptera-Heteroptera of America north of Mexico. Part II. Families Coreidae, Alydidae, Corizidae, Neididae, Pyrrhocoridae and Thaumastotheriidae. Entomol. Am. 21:41-122. [ Links ]
Tillman, P. G. 2011. Influence of corn on stink bugs (Heteroptera: Pentatomidae) in subsequent crops. Environ. Entomol. 40:1159-1176. [ Links ]
Tillman, P. G.; Northfield, T. D.; Mizell, R. F. and Riddle, T. C. 2009. Spatiotemporal patterns and dispersal of stink bugs (Heteroptera: Pentatomidae) in peanut-cotton farmscapes. Environ. Entomol. 38:1038-1052. [ Links ]
Triplehorn, C. A. and Johnson, N. F. 2005. Borror and delong’s lntroduction to the study of lnsects. Thomson Brooks/Cole, USA, Seventh Edition. 864 p. [ Links ]
Trujano, O. M.; García, V. U. O. y Nieto, M. O. A. 2016. Diversidad de grupos selectos de vertebrados (Reptilia, Amphibia) e insectos (Lepidoptera: Papilionoidea y Hesperioidea; Odonata; Diptera: Bombyliidae) en el Valle de Cuatrociénegas y Sierra de la Madera, Coahuila, México. Facultad de Ciencias. Informe final SNIB-CONABIO proyecto No. JF065. México D. F. 38 p. [ Links ]
Vanegas-Rico, J. M.; Lomeli-Flores, J. R.; Rodríguez-Leyva, E.; Mora-Aguilera, G. y Valdez, J. M. 2010. Enemigos naturales de Dactylopius opuntiae (Cockerell) en Opuntia ficus-indica L. (Miller) en el centro de México. Acta Zool. Mex. 26(2):415-433. [ Links ]
Vargas, A.; Flores, A. y Bazaldúa. J. 2008. Dinámica poblacional de las principales plagas de Opuntia spp. en la zona semiárida de Querétaro. Rev. Chapingo Ser. Zonas Aridas.7:21-27. [ Links ]
Velasco, L. R. I. and Walter, G. H. 1992. Availability of different host plant species and changing abundance of the polyphagous bug Nezara viridula. Environ. Entomol. 21:751-759. [ Links ]
Vezzani, D.; Schweigmann, N.; Pietrokovsky, S. M. and Wisnivesky-Colli C. 2001. Characterization of Triatoma guasayana Biotopes in a Hardwood Forest of Santiago del Estero, Argentina. 7 p. [ Links ]
Zimmermann, H.; Bloem, S. and Klein, H. 2007. Cactoblastis cactorum the biology, history, threat, surveillance and control of the cactus Moth. OIEA Viena, OIEA/FAO IAEA Vienna, IAEA/FAO-BSC/CM. 47 p. [ Links ]
Received: October 2017; Accepted: December 2017











 texto em
texto em 


