Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.6 Texcoco ago./sep. 2017
Investigation note
Parasitoids of whitefly in three dates of sowing of cotton transgenic and conventional in Sinaloa
1Campo Experimental Valle del Fuerte-Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias. Carretera Internacional México-Nogales km 1619, Juan José Ríos, Sinaloa. CP. 81110. (cortez.edgardo@inifap.gob.mx.).
2Escuela Superior de Agricultura del Valle del Fuerte-UAS. Avenida Japaraqui y Calle 16, Juan José Ríos, Sinaloa CP. 81110.
The aim of the research was to define whitefly (MB) parasitoid species Bemisia tabaci Genn. Biotip “B” in varieties of transgenic cotton and conventional cotton in northern Sinaloa, in order to know if there are differences in parasitism between varieties modified with transgene and traditionally modified. Three cotton cultivars were evaluated: 1. DP 393 (conventional), 2. DP 167RF Flex Faena Solution® and 3. DP 0935B2RF Bollgard®II /Faena Flex Solution® in three plots located in the municipality of Guasave, Sinaloa, in three sowing dates. The research consisted of identifying the MB parasitoids and estimating the number of specimens obtained from large (third and fourth instar) MB nymphs present in cotton leaves, sampling at weekly intervals, and in January or February, May and June, adults of the pest insect were counted by direct inspections of leaves of the fifth apical knot in the upper stratum of the plant. A total of 771 MB parasitoid specimens were obtained in the three plots and in all cases the identified species was Eretmocerus eremicus (Rose and Zolnerowich). No significant differences between treatments (p> 0.05) regarding the number of parasitoids/ cotton leaf in MB nymphs, or abundance of adult MB were observed and its populations were significantly higher in the later planting date of autumn-winter.
Keywords: biological control; planting dates; population dynamics; breeding
El objetivo del estudio fue definir las especies parasitoides de mosca blanca (MB) Bemisia tabaci Genn. Biotipo “B” en variedades de algodonero transgénico y algodonero convencional en el norte de Sinaloa, con el propósito de saber si existen diferencias en parasitismo entre variedades modificadas con transgénes y modificadas tradicionalmente. Se evaluaron tres variedades de algodonero: 1. DP 393 (convencional), 2. DP 167RF Solución Faena Flex® y 3. DP 0935B2RF Bollgard®II/Solución Faena Flex®, en tres parcelas ubicadas en el municipio de Guasave, Sinaloa, en tres fechas de siembra. El estudio consistió en identificar los parasitoides de MB y estimar el número de ejemplares obtenidos de ninfas grandes (tercer y cuarto instar) de MB presentes en hojas de algodonero, en muestreos a intervalos semanales, además, en enero o febrero, mayo y junio, se contaron adultos del insecto plaga mediante inspecciones directas de hojas del quinto nudo apical, en el estrato superior de la planta. Se obtuvo un total de 771 especímenes parasitoides de MB en los tres lotes de evaluación y en todos los casos la especie identificada fue Eretmocerus eremicus (Rose y Zolnerowich). No se observó diferencia significativa entre tratamientos (p> 0.05) respecto al número de parasitoides/hoja de algodonero en ninfas de MB, ni respecto a la abundancia de adultos de MB y sus poblaciones fueron significativamente mayores en la fecha de siembra más tardía de otoño-invierno.
Palabras claves: control biológico; fechas de siembra; fluctuación poblacional; mejoramiento genético
Most commercial cotton varieties currently used for industry, are genetically altered by the inclusion of Bacillus thuringiensis (Bt) Berliner proteins Cry1Ab and Cry2Ab, for the control of larvae of different Lepidoptera species (Jain et al., 2006; Chaparro, 2011). Wich contributes to the adoption rate of technology, which is one of the highest among several countries (Chaparro, 2011). However, no positive or negative repercussions on different aspects are known (Orr, 2009), one of them is the possible secondary effect on other phytophagous species, entomophagous, pollinator or other insects. In this regard, the objective of this research was to identify the parasitoid species associated with whitefly and to determine its parasitism percentage and to know the effect of transgenic and conventional cotton varieties on parasitism in three plots, in three planting dates, in northern Sinaloa.
The research was carried out in northern Sinaloa, in three commercial agricultural lots located in the municipality of Guasave, in the lot La Maroma N= 25°.69110 O= 108°.51027, in the lot El Coyote N= 25°.78130 O= 108°.68712 and in the Miguel Leyson Experimental Field N= 25° 30 5.32 O= 108° 22 36.28, in cotton cultivation established on October 27th, November 10th and December 1st 2011, in that order. The agronomic management of the crop was in charge of the producers owning the plots and the work was recorded at the time.
Three cotton cultivars were evaluated: 1. DP 393 (conventional), 2. DP 167RF Flex Faena Solution® and 3. DP 0935B2RF Bollgard®II/Flex Faena Solution®; The study consisted of identifying the MB parasitoids and estimating the number of specimens obtained from large nymphs (third and fourth instar) in 10 fully developed cotton leaves collected from the middle stratum of the plant in three replicates; that is, 30 leaves per treatment (varieties), from the second week of April to the second, third week of June and first week of July, according to the plot, in samples at weekly intervals; at the Coyote lot the samples started from 50% fruiting to 50% open buds, in the Leyson Field from the beginning of acorns up to 50% of open buds and in La Maroma in the phenological stage of the beginning of the first buds (floral buds) (PC) until the beginning of open buds. Samples were collected in the morning in the cotton fields and in duly labeled paper bags they were transferred to the Entomology Laboratory of INIFAP- Valle del Fuerte Experimental Field, where each leaf was confined in a disposable plastic cup num. 12 and covered with polypropylene fabric (Agribon®) tied with a rubber band, in order to allow ventilation and to prevent the escape or introduction of insects and were kept for one week and then observed under a microscope the insects contained in the cup to separate and identify the parasitoids according to Werner taxonomic keys (s/f) (a sample of the specimens obtained is preserved in the ENIFAP-CEVAF Entomology laboratory) and to count the emerged parasitoids. In addition, three samplings were carried out in January or February, May and June, to count the adult population of the pests insects by direct inspections of leaves to the fifth apical node in the upper stratum of the plant, according to the modified binomial sampling (Ellsworth et al., 1994), reviewing 20 leaves per sample (one/plant), 60 leaves per treatment, in order to determine the population of the pest insect relative to the presence of parasitoids.
A completely random experimental design with three replicates in each experimental plot was used; the results data were statistically analyzed with the SAS V8 program (Statical Analyzes System, 2008), according to the experimental design used, an analysis of variance was carried out using each sowing date as replicate in the two measured variables and a Tukey mean comparison test of the treatments was conducted at p≤ 0.05.
Parasitoids identification and its abundance
A total of 771 MB parasitoid specimens were obtained in the three evaluation plots, 136 from El Coyote, 270 from Campo Leyson and the largest number of 365 specimens was obtained in La Maroma (Figures 1, 2 and 3). In all cases the identified parasitoid species was Eretmocerus eremicus (Rose and Zolnerowich) (=Eretmocerus sp. nr. californicus, Arizona strain) (Hymenoptera: Aphelinidae), the MB most important parasitoid in northern Sinaloa due to its abundance, another one present in the region but not obtained in this study is Encarsia formosa Gahan (Hymenoptera: Aphelinidae) (Avilés et al., 2004; Cortez et al., 2005). Unfortunately, the percentage of induced parasitism was not measured; however, it should have been interesting, as averages of almost 20 parasitoids were obtained on average/leaf/sampling date (Figures 2 and 3), despite insecticides applications against different pest insects and even against MB in the plot La Maroma; at El Coyote insecticides were sprayed against lepidoptera and cotton weevil, and at Campo Leyson only against lepidoptera.

Figure 1 Average number of parasitoids/MB leaf obtained from nymphs, in three cotton varieties at Coyote plot.
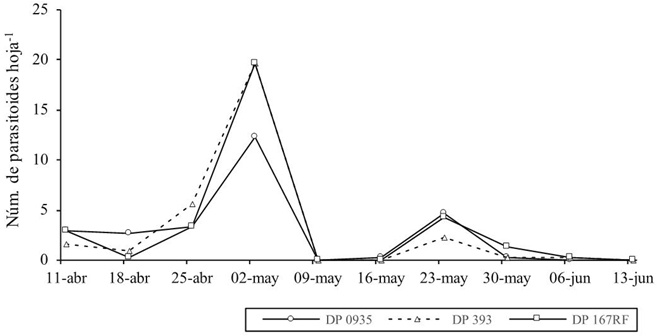
Figure 2 Average number of parasitoids/MB leaf obtained from nymphs, in three cotton varieties at Campo Leyson plot.

Figure 3 Average number of parasitoids/MB leaf obtained from nymphs, in three cotton varieties at La Maroma plot.
No significant difference between treatments (varieties) transgenic and conventional (p> 0.05) were observed regarding to the number of parasitoids/cotton leaf of MB, which coincides with Romeis et al. (2006) in the sense that transgenic cultivars do not affect the presence and behavior of non-target insects; at El Coyote, the highest mean of parasitoids per sample was for the DP 393 conventional treatment with 6.3 little wasps/leaf, 4.9 and 4.4 little wasps/leaves for the treatments DP 0935 and DP 167RF (Figure 1), at Campo Field the highest average corresponded to the transgenic variety DP 167RF with 9.7 little wasps/leaf, 9.3 and 8.0 little wasps/leaves for DP 393 and DP 0935 varieties (Figure 2), at La Maroma the highest mean was for the DP 0935 variety with 11 eretmocerus/leaf, 10.5 and 6.6 parasitoids/sample for DP 393 and DP 167RF (Figure 3).
Meaning that the nature of the evaluated treatments had no effect on MB parasitism. This is possibly due to the Bt technology having no direct effect on MB, in this regard, Bernal et al. (2010) found that transgenic maize negatively affects the search for Cotesia marginiventris (Cresson) parasitoid of larvae of the Spodoptera frugiperda spider worm (JE Smith) fed with GM maize tissue; besides the Bt endotoxins present in spider worm larvae affect the C. marginiventris parasitoid (Ramírez et al., 2007). On the other hand, although the real values of adult populations showed greater abundance in the conventional variety over transgenic in the three experimental plots (Figure 4), no significant difference between treatments (p> 0.05) was detected by the ANOVA.

Figure 4 Average number of MB adults leaf-1 of the fifth apical node in three cotton varieties, in three comercial plots.
It is not known the cause of the greater uniform increase of the parasitoid during the third sampling date of April and first week of May in the three plots and in the three varieties, it might be possibly related to the climatic conditions, especially temperature, in this sense Weeden et al. (2012) mention that the optimum temperatures for the rapid development and egg production of E. eremicus is 25-29 °C. In the municipality of Guasave, around the experimental plot, historical average temperature of April and May is 22.6 and 24.6 °C (Ruiz et al., 2005), the temperatures of the end of May and even June are still conducive to the parasitoid, but in this research host populations declined earlier or at least were more abundant at earlier dates.
Abundance and behavior of the whitefly
The E. eremicus was positively related to the abundance of the host [positive numerical response (Rodríguez, 2007)] increasing between the first and second experimental plot as the host increased; while in the third date the parasitoids abundance in the population peaks was similar to the second planting date, possibly due to the insecticides spray for the control of the same MB; however, the total specimens of eremicus wasp was the largest (365) because the period of parasitoid increase was for one more week and by June 30th they were no longer obtained. According to the above, the largest MB and parasitoid population was at the La Maroma plot, followed by the Campo Leyson and El Coyote showed the smallest population (Figure 4), corresponding to the population behavior of the insect pest to the effect of the planting date; as already indicated, cotton was planted on October 27th in El Coyote, November 11th in Campo Leyson and December 1st in La Maroma, that is, population density was influenced by the sowing date (Cortez et al., 2005), among other factors, which in turn was influenced by weather conditions, mainly temperature.
In the three evaluation plots the fluctuacional behavior of E. eremicus parasitoid was similar, with some exceptions, it was detected from the first sample and its population increased in the third week of April, showing the greatest increase in the last week of that month and in the first week of May, and by the third week of May the population decreased practically to the same population level observed in the first sampling. It should be noted that the population behavior of MB was different in each plot, in three samples made in the first stages (floral buds), 50% flowering and 50% buds, in El Coyote the largest number of adults were observed in the first sampling (PC) on January 27th, in Campo Leyson the highest adult abundance of MB occurred at the second sampling (50% flowering) on May 16th and in La Maroma the largest number of MB adults were recorded as in El Coyote, in the first sampling (PC).
On April 4th, in each evaluation plot the MB abundance was homogeneous; that is, the three cotton varieties showed the highest insect population in the same sampling date, indicating that the development stage of the crop was determinant to the MB’s population growth, which is documented (Cortez et al., 2005). However, the fluctuation behavior of the parasitoid was similar in the three plots (Figures 1, 2 and 3) and in the three evaluated cotton varieties, with different planting dates and different times of MB population increase.
Implications of results
The results allow to suggest biological control by conservation and use of E. eremicus on MB in cotton, establishing an early crop in the planting date authorized for northern Sinaloa by the Secretaría de Agricultura, Ganadería, Pesca y Alimentación (SAGARPA) (November 15 to December 15, CEVAF, 2003). The use of transgenic varieties, effective for the control of lepidopteran plague of cotton, as determined in different parts of the world, as well as in the north of the entity (Cortez-Mondaca and Martínez-Carrillo, 2007), is subject to authorization from the Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). This paper is the first formal report on parasitism of E. eremicus on MB in cotton, in northern Sinaloa.
Conclusions
The parasitoid species of whitefly on cotton was Eretmocerus eremicus (Rose and Zolnerowich) (= Eretmocerus sp. nr. californicus, Arizona strain) (Hymenoptera: Aphelinidae). No significant difference was observed in whitefly parasitism among transgenic and conventional traditionally modified cotton varieties. As well as among adult populations of whitefly in transgenic and conventional cotton varieties, and its populations were significantly higher at the later planting date (December 1) at La Maroma plot and the lowest at the earlier planting date (October 27) at El Coyote plot.
Literatura citada
Avilés, G. M. C.; Nava, U. C.; Garzón, J. A. T.; Wong, P. J. de J. y Pérez,V. J. J. 2004. Manejo integrado de la mosquita blanca Bemisiasp., en tomate para consumo fresco. INIFAP-CIRNO. Campo Experimental Valle de Culiacán. Culiacán, Sinaloa, México.Folleto técnico núm. 28. 76 p. [ Links ]
Bernal, J. R.; Ramírez, R. A. H.; Bokonon, G. y Desneux, N. El maíz transgénico entorpece la búsqueda de hospederos (Spodoptera frugiperda) por el parasitoide Cotesia marginiventris. Cruz, M.S. G.; Tello, J. F.; Mendoza, A. E. y Morales, A. M. (Editores).Entomología Mexicana. 9(1):322- 327. [ Links ]
CEVAF. 2003. Guía para la asistencia técnica agrícola para el área de influencia del Campo Experimental Valle del Fuerte. INIFAP-CIRNO.Agenda técnica. Sextaedición. Juan José Ríos, Sinaloa,México. 208 p. [ Links ]
Chaparro, G. A. 2011. Cultivos transgénicos: entre los riesgos biológicos y los beneficios ambientales y económicos. Acta Biológica Colombiana. 16(3):231- 251. [ Links ]
Cortez, M. E. F. G.; Rodríguez, C. J. L.; Martínez, C. y Macías, J. C.2005. Tecnología de producción y manejo de la mosca blanca de la hoja plateada en el cultivo de soya en el norte de Sinaloa.Campo Experimental Valle del Fuerte-INIFAP. Los Mochis,Sinaloa, México. Folleto técnico núm. 25. 52 p. [ Links ]
Cortez, M. E. y Martínez, C. J. L. 2007. Comparación de poblaciones de lepidópteros en algodonero convencional y transgénico en el norte de Sinaloa. In: memorias de reunión binacional México-Estados Unidos de América; International Cotton Pest Work Committee. Mazatlán, Sinaloa, México. [ Links ]
Ellsworth, P. J.; Diehl, T. D. and Naranjo, S. 1994. Sampling sweetpotato whiteflies in cotton. University of Arizona. IPM series number 2. Tucson, Arizona. [ Links ]
Jain, D. V.; Udayasuriyan, P. I. and Arulselvi, S. S. D. and Sangeetha,P. 2006. Cloning, characterization, and expression of a new cry2Ab gene from Bacillus thuringiensis strain 14-1. Appl Biochem Biotechnol. 128(1):185-94. [ Links ]
Orr, D. 2009. Biological control and integrated pest management. 207-239 pp. In: Peshin, R. and Dhawan, A. K. (Ed.). Integrated pest management: innovation-development process. Springer Science+Business Media B. V. [ Links ]
Ramírez, R. R.; Bernal, J. S.; Chaufaux, J. and Kaiser. L. 2007. Impact assessment of Bt-maize on a moth parasitoid, Cotesia marginiventris, via host exposure to purified Cry1 Ab protein or Bt-plants. Crop Protection. 26(1):953-962. [ Links ]
Rodríguez, B. L. A. 2007. Fundamentos ecológicos del control biológico.In: Rodríguez, B. L. A. y Arredondo, B. H. C. (Eds.). Teoría y aplicación del control biológico. Servicio nacional de sanidad,inocuidad y calidad agroalimentaria y sociedad mexicana de control biológico. México. 303 p. [ Links ]
Romeis, J.; Meissle, M. and Bigler, F. 2006. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nature Biotechnol. 24(1):63-71. [ Links ]
Ruíz, C. J. A.; Medina, G. G.; Macías, J. C.; Silva, M. M. S. y Díaz, G. P.2005. Estadísticas climatológicas básicas del estado de Sinaloa(periodo 1961-2003). Secretaría de Agricultura, Ganadería,Pesca y Alimentación (SAGARPA)-Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP).Prometeo Editores, S. A de C. V. Guadalajara, Jalisco. Libro técnico núm. 2. 151 p. [ Links ]
SAS Institute. 2008. SAS systems for information delivery for Windows. Release 9.2. Cary, North Caroline. USA. [ Links ]
Weeden, C. R.; Shelton, A. M. and Hoffman, M. P. 2012. Biological control: a guide to natural enemies in North America. http://www.biocontrol.entomology.cornell.edu/parasitoids/eretmocerus. [ Links ]
Werner, F. G. S. Keys for the identification of parasitic insects in Arizona agricultural areas. Technical bulletin 236. College of Agriculture-Agricultural Experiment Station-The University of Arizona. Tucson, Arizona. 38 p. [ Links ]
Received: August 2017; Accepted: September 2017











 texto en
texto en 


