Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.6 Texcoco ago./sep. 2017
Articles
Cystatin expression and its relation with resistance to Tilletia indica in wheat
1Instituto Tecnológico de Sonora. Calle 5 de febrero 818 Sur, Colonia Centro, Cd. Obregón, Sonora. CP. 85130. (bramchaparro@outlook.com).
2Campo Experimental Norman E. Bourlaug-INIFAP. Norman Borlaug km 12, Cd. Obregón, Sonora. CP. 85000. (parra.fannie@inifap.gob.mx; camacho.miguel@inifap.gob.mx; fuentes.guillermo@inifap.gob.mx).
From the economic point of view, genetic improvement is one of the best options to fight karnal bunt caused by Tilletia indica fungus. Cystatin is a family of protease inhibitory proteins whose expression patterns may explain an effective resistance mechanism to karnal bunt. The objectives of this research were to evaluate the resistance/susceptibility of bread wheat lines (Triticum aestivum) and durum (T. turgidum ssp. durum) to T. indica and to determine the relative expression levels of cystatin genes; as well as to determine the correlation of that expression with phenotypic resistance. A trial with 9 lines of wheat flour and 9 lines of durum wheat with different degrees of resistance was established. Thirty spikes per line with allantoic sporidia were inoculated and the percentage of infection determined. The cystatin genes (Wheat Cystatin) WC1, WC3 and WC5 were amplified by real-time PCR to quantify the normalized expression (ΔCt) of cystatin. The correlation coefficient was then obtained for normalized expression and percentage of infection. The percentages of infection were within the range of 15.85% to 36.78% in wheat flour and from 0.19% to 54.87% in durum wheat. Furthermore, normalized levels of cystatin expression were comparatively higher in resistant lines (p> 0.05). Significant correlation coefficients (p< 0.05) of WC3 and WC5 genes during all stages of sampling were shown, highlighting in vegetative (0.969), boot stage (0.841) and post-anthesis (-0.789) stages.
Keywords: Triticum aestivum; Triticum turgidum; karnal bunt
Desde el punto de vista económico, el mejoramiento genético es una de las mejores opciones para combatir al carbón parcial causado por el hongo Tilletia indica. Las cistatinas son una familia de proteínas inhibidoras de proteasas cuyos patrones de expresión podrían explicar un mecanismo eficaz de resistencia a carbón parcial. Los objetivos fueron evaluar la resistencia/susceptibilidad de líneas de trigo harinero (Triticum aestivum) y cristalino (T. turgidum ssp. durum) a T. indica y determinar los niveles de expresión relativa de genes de cistatinas; así como, determinar la correlación de dicha expresión con la resistencia fenotípica. Se estableció un ensayo con nueve líneas de trigo harinero y nueve líneas de trigo cristalino con diferentes grados de resistencia. Se inocularon 30 espigas por línea con esporidios alantoides y se determinó el porcentaje de infección. Los genes de cistatinas (Wheat Cystatin) WC1, WC3 y WC5 fueron amplificados mediante PCR en tiempo real para cuantificar la expresión normalizada (ΔCt) de cistatinas. Posteriormente se obtuvo el coeficiente de correlación para la expresión normalizada y el porcentaje de infección. Los porcentajes estuvieron dentro de los rangos de 15.85% a 36.78% en trigo harinero y de 0.19% a 54.87% en trigo cristalino. Además, los niveles de expresión normalizada de cistatinas fueron comparativamente mayores en líneas resistentes (p> 0.05). Se presentaron coeficientes de correlación significativos (p< 0.05) de los genes WC3 y WC5 durante todas las etapas de muestreo, destacándose en estado vegetativo (0.969), embuche (0.841) y post-antesis (-0.789).
Palabras clave: Triticum aestivum; Triticum turgidum; carbón parcial
Introduction
Wheat is the main crop in Sonora, accounting for approximately 48% of the national production (Servicio de Información Agroalimentaria y Pesquera, 2015); however, different biotic and abiotic factors in the region favor the incidence of some phytopathogenic organisms, which, through different mechanisms and infection pathways, can cause diseases in different stages of plant development. One of the most important diseases affecting the wheat crop in the region is karnal bunt, caused by Tilletia indica fungus (Mitra), which is characterized by infecting from the embryo to the rest of the grain, showing coffee to black stains and affecting the organoleptic properties from 3% of infection (Bhat et al., 1980). Despite not having a significant impact on crop yield, it has been an international significance quarantine disease that has hindered exports from regions where the disease occurs, influencing the preference of farmers for the cultivation of durum wheat (Triticum turgidum subsp. durum). This because it has been found a remarkably high resistance of durum wheat varieties over bread wheat (Triticum aestivum), generally susceptible to the disease (Camacho-Casas et al., 2010).
Genetic improvement is one of the best options to combat the disease, since it does not increase the production cost and decreases the use of potentially harmful substances. Although no immunity has been detected in current commercial varieties, genetic differences in susceptibility to disease have been observed, in recent years wheat varieties obtained by breeding programs have been released, considering not only yield and quality factors, but also the resistance degree to karnal bunt (Fuentes-Dávila and Singh, 2007; Figueroa-López et al., 2012).
Cystatin is a family of proteins with protease inhibitory activity; there is evidence that cystatins play a role in activating the defense mechanisms of plants, having been identified in several cultures (Margis et al., 1998). The expression of cystatin differs in certain stages of plant development and in response to stress conditions due to the interaction with the pathogen. Some studies have linked the expression of cystatins with inhibition of pests (Santamaría et al., 2012) and diseases in different crops such as soybeans, rice and potato (Azzouz et al., 2005; Munger et al., 2012). In wheat, several authors point to cystatin as inhibitors of pathogens such as Microdochium nivale, causing the ptarmigan mold (Christova et al., 2006), Fusarium graminearum, which causes Fusarium head blight (Gottwald et al., 2012) and Tilletia indica, causal agent of karnal blunt (Gupta et al., 2010; Purwar et al., 2010).
The defense mechanism in which cystatin involves the inhibition of the cysteine proteases of T. indica, which are lytic enzymes whose function is to facilitate the nutrition of the fungus from the grain; this interaction affects the viability of infection development (Valdés-Rodríguez et al., 2010). Because of the above, the expression patterns of cystatin in wheat can elucidate an effective mechanism to explain the resistance to partial carbon. Finding evidence of the relationship between cistatin gene expression levels with partial carbon resistance may confirm their ability to be integrated as genetic markers in breeding programs. The aim of this research was to determine the expression patterns of three genes of the cystatine family and their correlation with the percentage of T. indica infection during three stages of wheat development.
Materials and methods
Field test
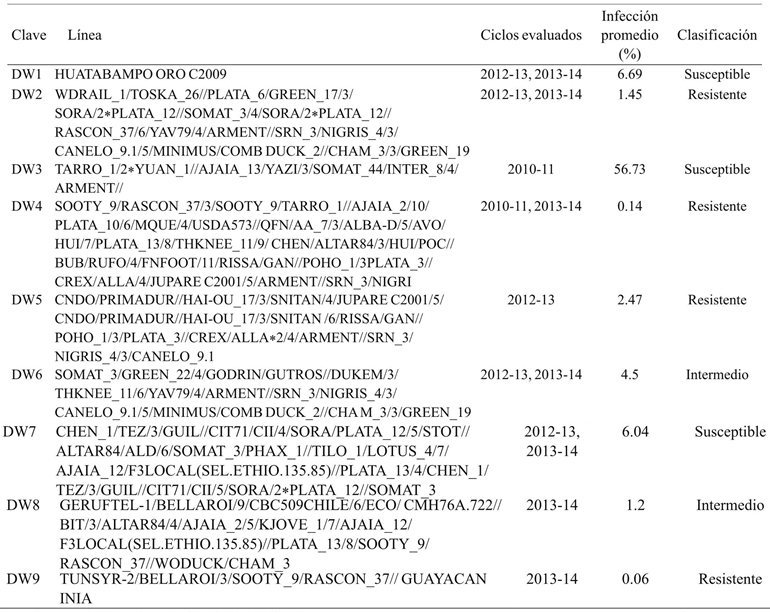
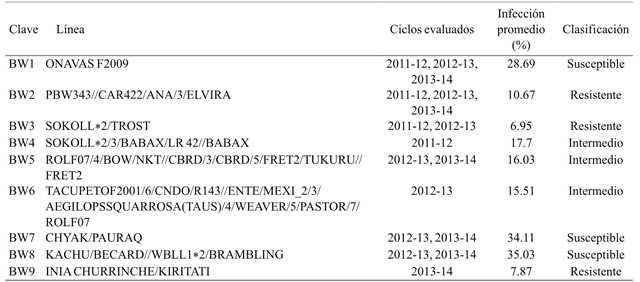
The experiment was carried out at the Campo Experimental Norman E. Borlaug (CENEB) located in the Yaqui Valley, Sonora (27° 22’ 10.2” N, 109° 55’ 51.5” W) during the autumn-winter cycle 2014- 2015. The trial consisted of nine lines of wheat flour (Table 1) and nine lines of durum wheat (Table 2) selected according to the susceptibility degree to karnal blunt manifested in three previous cycles. These were inoculated with T. indica sporidia during step boot stage or GS41-45 of Zadoks et al. (1974) scale. The inoculum production (isolation, propagation and sporidial concentration) was carried out using the methodology of Fuentes-Dávila and Figueroa-López (2009).
Fuente: Programa de Mejoramiento Genético de Trigo (CENEB).
Table 1 Selected lines of bread wheat and its historical phenotypic response to T. indica
Evaluation of phenotypic response
To the crop, 30 spikes inoculated by variety were manually harvested and the percentage of infection was determined by the count of infected and healthy grains per spike per experimental unit and per line; the average percentage corresponds to the phenotypic response of each variety.
Oligonucleotides design
The sequences of the used oligonucleotides are listed in Table 3. Various programs were used for both editing and sequence alignment such as CLC Sequence Viewer and the NCBI Primer Designing Tool, adjusting the amplicon size to 200 bp. Part of the non-coding 3’ chain regions were included to ensure specificity between cystatine genes. Amplification of the oligonucleotides (sense and antisense) was tested by end-point RT-PCR and 2% agarose gel electrophoresis.
Sampling
Spikes and foliar tissue were collected in duplicate for each line during three stages of phenological development, vegetative stage (SV), post inoculation (S1Pi), and post- anthesis (S2), corresponding to GS16-19, GS41-45 and GS69 respectively. These samples were immediately frozen with liquid nitrogen and stored at -80 °C until processing.
RNA extraction
Extraction of total RNA from 100-200 mg of frozen plant tissue was performed with Trizol® (Invitrogen) reagent, following the manufacturer’s instructions with modifications. These consisted of the addition of a saline solution (Sodium Citrate 0.8 M + 1.2 M NaCl) during the precipitation phase in a 1:1 v/v ratio with isopropanol and during the purification phase with LiCl (8 M) per hour at 4 °C. The RNA was analyzed by electrophoresis to verify its integrity and its concentration and purity were determined by spectrophotometry in the NanoDrop 1000 (NanoDrop Products). Subsequently, the RNA was retrotranscribed to generate complementary DNA (cDNA) using the enzyme SuperScript (Invitrogen) according to the manufacturer’s instructions.
Real-time polymerase chain reaction (PCR)
Amplifications were performed using chemical detection with SYBR Green in reactions of 20 μl in 96-well plates in a C1000 thermocycler with CFX96 (Bio-Rad) real-time system.
Analysis of gene expression and correlation
The normalized expression of each gene was calculated using the comparative method of Ct (Livak and Schmittgen, 2001). The abundance data of the transcripts were normalized with the average abundance of endogenous reference gene transcripts to obtain the normalized expression with the formula 2^-∆Ct, where: ΔCt= (Ct of the white gene - average Ct of the reference genes).
Pearson correlation analyzes were performed, considering percentage infection data against normalized expression values (2^-ΔCt) individually in leaf tissue and spikes. The Pearson correlation coefficient (R), the coefficient of determination (R2) and the level of significance at 95% confidence were obtained using the software package STATGRAPHIC Centurion XVI.
Results and discussion
Percentage of infection
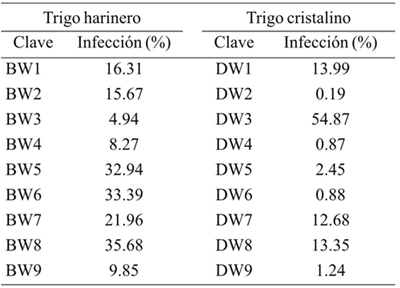
30 spikes per line were analyzed, classifying grains as “infected” and “healthy” according to the presence of typical damage by karnal bunt, then the percentage of spike infection and the average percentage per line were calculated. Table 4 shows that the wheat flour line BW3 showed the lowest percentage of infection (4.94%), while the line with the highest susceptibility was BW8 with 35.68%. In addition, the lines classified as intermediate response BW4, BW5 and BW6 were resistant (8.27%), susceptible (33.39%) and susceptible (32.94%) respectively.
Durum wheat has historically shown lower levels of infection compared to flour wheat. The percentages of karnal bunt infection in durum wheat are shown in Table 4, where the DW2 line shows a 0.19% infection. In contrast, the DW3 line was the most susceptible (60.33%). It is notorious the grouping of the varieties in 3 levels of resistance to T. indica, resistant (DW2, DW4, DW5 and DW9), susceptible (DW1, DW3 and DW7) and intermediate, which tend to be classified as resistant (DW6) and susceptible (DW8). It is possible to attribute phenotypic variations to genetic interactions in wheat lines, these variations may suggest the partial dominance of the partial carbon resistance attribute.
Standard expression (ΔCt)
For the calculation of gene expression it is required to normalize using expression data from one or more endogenous genes. These were selected from the literature based on homogeneity at different stages of development and tissues; EF1A standing out in various tissues under biotic stress conditions and TaWIN1 by its stability in flag leaves until the formation of milky grain (Tenea et al., 2011).
The WC1, WC3 and WC5 transcripts were differentially expressed in resistant and susceptible genotypes at all development stages, and the normalized expression patterns were different in leaf tissue and spikes. This was determined using 95% Tukey multiple rank tests.
In leaf tissue, cystatin was differentially expressed during plant development (Figure 1). Expression levels of WC1 were significantly (p< 0.05) higher in the resistant lines BW3 in SV (1.015) and BW2 in S1 (1.61) and S2 (1.132). This pattern is repeated in the WC3 expression, with BW3 in SV (1.182) and BW2 in S1 (2.88) and S2 (2.186). On the other hand, the behavior of the WC5 levels was mixed, since it is observed that the greater expression in SV corresponds to the lines BW1 (0.003), BW3 (0.0026) and BW2 (0.002), the first one having a susceptible response value (16.31%). On the other hand, in the S1 the BW8 line was the one that most expressed WC5 (0.007), being this one the one that showed greater susceptibility (35.68%), whereas in S2 the lines BW1 and BW2 showed the highest values, 0.0043 and 0.0036 respectively.
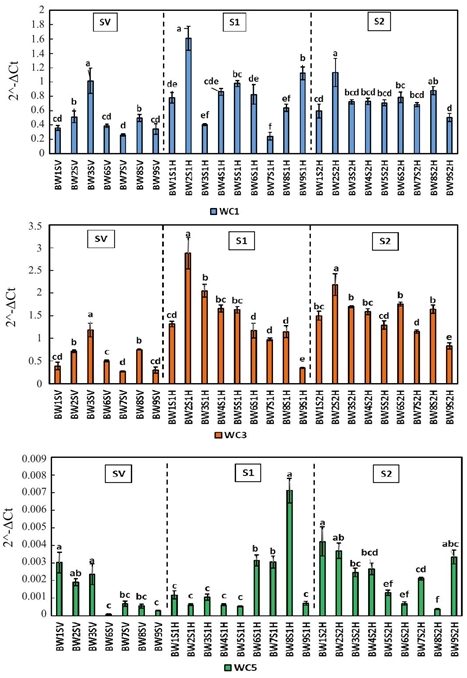
Figure 1 Relative expression of cystatins WC1, WC3 and WC5 in wheat flour leaves during stages SV, S1 and S2. Literals indicate significant difference (p< 0.05).
The spike constitutes a focal point of activity in wheat, the nutrients directed to the formation of the grain are mobilized and regulated towards here, is also where T. indica finds appropriate conditions to proliferate or reproducing; so that the level of expression of the cystatin can be affected by both the phenological stage and the presence of the pathogen. As shown in Figure 2, the normalized expression of cystatin was comparatively lower than that shown in leaf tissue (Figure 1) for WC1 and WC3, while WC5 was higher.
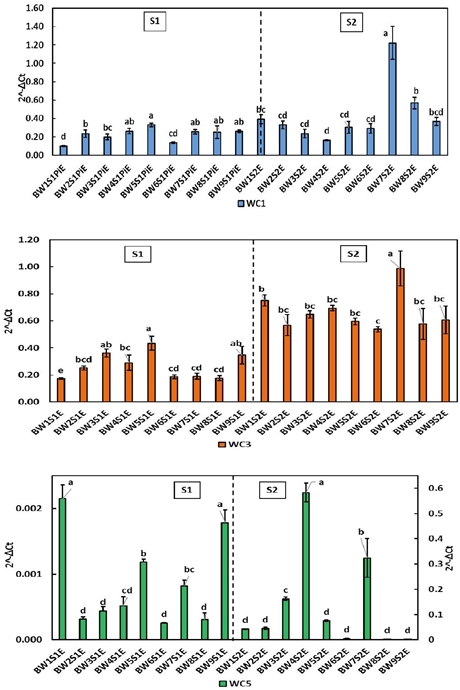
Figure 2 Relative expression of cystatins WC1, WC3 and WC5 in wheat flour leaves during S1 and S2 stages. Literals indicate significant difference (p< 0.05).
The WC1 gene was more expressed in the BW5 susceptible line with 0.33 during S1, also during S2 the highest values were in the susceptible BW7 (1.222) and BW8 (0.568). In the expression of WC3 it is observed that during S1 the susceptible line BW5 followed by the resistant BW3 and BW9 showed the highest values (0.434, 0.362 and 0.346 respectively). The significant increase in the normalized expression values in S2 (post-anthesis stage) implies an inherent gene activity to the plant development in all evaluated varieties. However, since the normalized expression values of WC3 are greater than those observed for WC1 and WC5, it could suggest that the presence of the pathogen is affecting the expression of WC3, probably as part of a defensive system in the plant. The expression of WC5 decreases from S1 to S2 in most of the lines except for the resistant BW4 (0.583), the susceptible BW7 (0.324) and the resistant BW3 (0.163). When comparing these values with the controls, it was observed that in the controls the normalized expression of WC5 is higher, so that WC5 levels decrease in the presence of the pathogen.
In durum wheat (Figure 3), the resistant lines DW2, DW4, DW6 and DW9 showed significantly higher expression levels in SV and S1 for WC1, WC3 and WC5. During S2 stage the expression levels of WC1 and WC3 (including controls) decreased, so that in durum wheat the levels of WC1 and WC3 appear to be more affected by phenological development than by the interaction with the fungus. The expression patterns of WC5 varied little from S1 to S2; standing out the DW4 and DW6 resistant lines in S1, as well as DW8 and DW5 in S2. The above could indicate a background transcript of WC5 lower or similar to that of the endogenous genes.
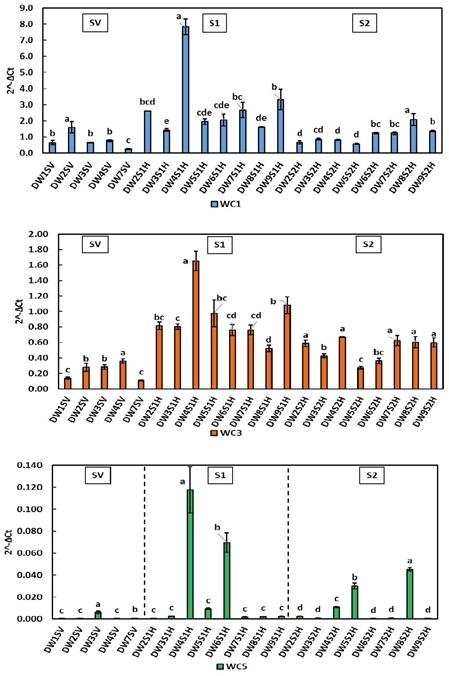
Figure 3 Relative expression of cystatins WC1, WC3 and WC5 in durum wheat leaves during stages SV, S1 and S2.Literals indicate significant difference (p< 0.05).
The expression patterns of cystatin in spikes of durum wheat (Figure 4) differ markedly from that shown in foliar tissue; WC1 as WC3 reached significantly lower levels. WC5 expression in spikes exceeded the levels reached in leaf tissue during S2, this behavior was also observed in flour wheats. The DW5 and DW9 lines excelled in the two growth stages in all three genes. The latter are resistant (2.45% and 1.24%).
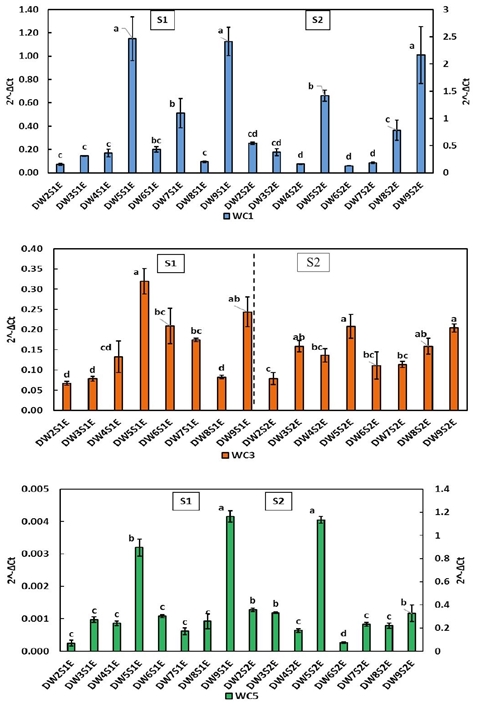
Figure 4 Relative expression of cystatins WC1, WC3 and WC5 in durum wheat spikes during stages S1 and S2. Literals indicate significant difference (p< 0.05).
In both, bread wheat and durum wheat the intense reaction produced during S2 was remarkable, this is in agreement with numerous investigations that have reported the occurrence of expression peaks of cystatin during the post-fertilization stage; also this expression is significantly stronger in resistant genotypes than in the susceptible ones (Gupta et al., 2010; Purwar et al., 2010). These results indicate that increased expression of WC1, WC3 (in spikes) and WC5 (leaves) could be related to resistance due to the increase in S2, in the stage of greatest vulnerability to infection of T. indica, since the grain where the pathogen will colonize is formed.
Correlation analysis
The post-inoculation embedding step (S1) was considered focal point of cystatin gene activity; this behavior was correlated with the resistance level to karnar bunt in bread wheat spikes with the WC3 gene. This correlation follows the linear model with a coefficient of -0.709, a coefficient of determination of 50.30% and an equation of the line of WC3= 0.357966 - 0.0056264*(%) infection at 95% significance. The above allows to attribute 50.30% of the variation of infection percentage to the gene expression; that is, the increase in the relative expression of WC3 causes a decrease in the percentage of infection.
In the post anthesis stage, the behavior of the relative expression of WC5 in bread wheat leaves showed significantly correlation (p< 0.05) with the infection percentage, it was adjusted to the linear model with a line equation as follows: % Infection= 35.9286 - 4937.86*WC5. The correlation coefficient indicates that there is a moderately strong inverse relationship (-0.789) and a significant coefficient of determination (62.34%).
These results evidenced a significant relationship between the expression of cystatin and the karnal bunt resistance attribute; it also suggests some synergy between WC1, WC3 and WC5 by regulating the activity of cysteine proteases during seed maturation (Purwar et al., 2012), when proteins must be accumulated for storage in response to the infection of T. indicates.
Conclusions
The cystatin genes are differentially expressed as a function of the phenological stage and the tissue, reaching a sustained intensity in the post-fertilization period. The presence of it T. indica induces a response in the expression of cystatin from 12 h after inoculation (S1), suggesting that cystatins may form part of the defense mechanism of the plant.
Cystatins can be considered as good candidates for markers of the defense mechanism of plants, not only against T. indica but also to other pathogens and even aphids. Therefore, it is feasible that the expression patterns of cystatin elucidate the resistance mechanism to karnal bunt and an efficient tool for the genetic improvement of wheat. However, it is necessary to determine which cystatin acts only on pathogens and which have endogenous activity, with the purpose of not eroding the inherent genetic machinery of the plant.
REFERENCES
Azzouz, H.; Campan, E.; Cherqui, A.; Saguez, J.; Couty, A.; Jouanin,L.; Kaiser, L. and Giordanengo, P. 2005. Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae). J. Insect. Physiol.51(8):941-951. [ Links ]
Bhat, R.; Deosthale, Y. G.; Roy, D. N.; Vijayaraghavan, M. and Tulpule,P. G. 1980. Nutritional and toxicological evaluation of Karnal bunt affected wheat. Ind. J. Exp. Biol. 18:1333-1335. [ Links ]
Camacho, C. M. A.; Chávez, V. G.; Figueroa, L. P.; Fuentes, D. G.;Peña, B. R. J.; Valenzuela, H. V.; Félix, F. J. L. y Mendoza, J.A. 2010. Samayoa c2004, nueva variedad de trigo cristalino para el sur de Sonora, México. Rev. Mex. Cienc. Agríc.1(5):657-661. [ Links ]
Christova, P. K.; Christov, N. K. and Imai, R. 2006. A cold inducible cystatin from winter wheat inhibits growth of the snow mold fungus Microdochium nivale. Planta. 223:1207-1218. [ Links ]
Figueroa, L. P.; Fuentes, D. G.; Valenzuela, H. V.; Chávez, V. G.; Félix,F. J. L. y Mendoza, L. J. A. 2012. ‘Sáwali Oro C2008’: nueva variedad de trigo cristalino para el noroeste de México. Rev.Mex. Fitopatol. 30(1):91-94. [ Links ]
Fuentes, D. G. y Figueroa, L. P. 2009. Técnicas de laboratorio para la producción de inóculo de Tilletia indica. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP).Cd. Obregón, Sonora. 32 p. [ Links ]
Fuentes, D. G. and Singh, R. P. 2007. Evaluation of advanced bread wheat (Triticum aestivum) lines and synthetic hexaploid wheat derivatives (T. turgidum/Aegilops squarrosa/T. aestivum) for resistance to karnal bunt (Tilletia indica). Rev. Mex. Micol. (24):56-58. [ Links ]
Gottwald, S.; Samans, B.; Lück, S. and Friedt, W. 2012. Jasmonate and ethylene dependent defence gene expression and suppression of fungal virulence factors: two essential mechanisms of Fusarium head blight resistance in wheat?. BMC Genomics. 13:369. [ Links ]
Gupta, V.; Jaiswal, J.; Sharmac, I. and Kumar, A. 2010. Investigating the role of cystatin in conferring stage dependent resistance against Karnal bunt of wheat. Food Agric. 21(1):65-79. [ Links ]
Livak, K. and Schmittgen, T. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the DDCT Method. Methods. 25:402-408. [ Links ]
Margis, R.; Reis, E. M. and Villeret, V. 1998. Structural and phylogenetic relationships among plant and animal cystatins. Arch. Biochem.Biophys. (359):24-30. [ Links ]
Munger, A.; Coenen, K.; Cantin, L; Goulet, C.; Vaillancourt, L. P.;Goulet, M. C.; Tweddell, R.; Sainsbury, F. and Michaud, D.2012. Beneficial ‘unintended effects’ of a cereal cystatin in transgenic lines of potato. Solanum tuberosum. BMC Plant Biol. 12:198. [ Links ]
Peña, R. J.; Amaya, A. y Del Toro, E. 1992. Efecto del almacenamiento y del lavado de grano en las características de calidad de muestras de trigo (variedad Seri 1982) con diferentes niveles de Carbón Parcial (Tilletia indica). In: estado actual de la investigación sobre el carbón parcial en México. Fuentes-Dávila, G. y Hettel, G. P. (Eds.). Reporte Especial de trigo Núm. 7. Centro Internacional de Mejoramiento de Maíz y Trigo (CIMMYT).El Batán, Estado de México. 24-32 pp. [ Links ]
Purwar, S.; Marla, S.; Singh, U. and Kumar, A. 2010. Basal expression studies of cystatins during specific growth stages of wheat spikes for defining their possible role in differential and stage dependent immunity against Karnal bunt (Tilletia indica). Mol Biol Rep. 37:1377-1389. [ Links ]
Purwar, S.; Sundaram, S.; Verma, P.; Srivastava, S. and Kumar, A. 2012.A physiologically regulated multidomain cystatin of wheat shows stage-dependent immunity against karnal bunt (Tilletia indica). Appl. Biochem. Biotechnol. 168:2344-2357. [ Links ]
Santamaria, M. E.; Hernández, C. P.; Ortego, F.; Grbic, V.; Grbic, M.;Diaz, I. and Martinez, M. 2012. Cysteine peptidases and their inhibitors in Tetranychus urticae: a comparative genomic approach. BMC Genomics.13:307. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2016. Atlas agroalimentario 2016. Secretaría de Agricultura Ganadería Pesca y Alimentación (SAGARPA). Primera edición. México,D. F. 160-162 pp. [ Links ]
Tenea, G.; Peres, B. A.; Cordeiro, R. F. and Maquet, A. 2011. Reference genes for gene expression studies in wheat flag leaves grown under different farming. BMC Research Notes. 4:373. [ Links ]
Valdés, R. S.; Cedro, T. A.; Aguilar, H. V.; Cortes, O. E.; Blanco, L. A. and Guerrero, R. A. 2010. Recombinant amaranth cystatin (AhCPI)inhibits the growth of phytopathogenic fungi. Plant Physiol.Biochem. 48:469-475. [ Links ]
Zadoks, J.; Chang, T. and Konzak, C. 1974. A decimal code for the growth stages of cereals. Weed Res. 14:415-421 [ Links ]
Received: March 2017; Accepted: June 2017











 texto en
texto en