Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.5 Texcoco jun./ago. 2017
https://doi.org/10.29312/remexca.v8i5.112
Articles
Characterization of oil and meal obtained from wild grape seeds (Vitis tiliifolia)
1Instituto de Ciencias Básicas-Universidad Veracruzana. Av. Dr. Luis Castelazo Ayala s/n. Col. Industrial-Animas, Xalapa, Veracruz. México. CP. 91000. Tel. 01 (228)8418900, Fax: 01 (228) 8418932.
2Facultad de Instrumentación Electrónica-Universidad Veracruzana. Xalapa, Veracruz. México.
3Red de estudios Moleculares Avanzados, Clúster Biomimic®, Instituto de Ecología, A. C. Xalapa, Veracruz, México.
Vitis tiliifolia is a wild grape that is usually consumed as wine, but the seeds are discarded, so in this paper the physicochemical properties of flour and oil of grapes seeds Vitis tiliifolia were evaluated, the phenolic compounds were identified and quantified by UPLC-ESI-MS. Seed meal had 75% moisture, 0.23% protein, 1.43% ash, 3.15% total dietary fiber and 17.5% oil.The characterization of polyphenols in flour revealed the presence of (-)-epicatechin (1318.66 ug g-1), (+)-catechin (703.12 µg g-1) and trans-resveratrol (32.88 µg g-1) as the major polyphenols. The oil featured bioactive compounds such as carotenoids and polyphenols. The fatty acids that predominated in the oil were linoleic (Ω-6, 84.73%), oleic (Ω-9, 5.83%) and stearic acid (2.21%). In addition the extracts, ethanol: water (3:1; v: v) and methanol: HCl (0.01%) of the oil, resulted in a high concentration of polyphenols (125 mg GAE g-1) and a high antioxidant activity. The iodine value was 68.56 g I2/100 g oil and the peroxide was 20.0 meq O2 kg-1 oil. The seeds had a considerable amount of polyphenols and linoleic acid that confers a high antioxidant activity, so it could be suitable for use in food and industrial applications and encourage the production and consumption of this grape as a source of nutrients and phenolic compounds suitable for incorporation into functional foods.
Keywords: fatty acids; polyphenols and antioxidant activity; wild grape
Vitis tiliifolia es una uva silvestre que se consume generalmente en forma de vino, pero las semillas son desechadas, por lo que en este trabajo las propiedades fisicoquímicas de la harina y el aceite de las semillas de uvas de Vitis tiliifolia fueron evaluadas, se identificaron y cuantificaron los compuestos fenólicos mediante UPLC-ESI-MS. La harina de la semilla tuvo 75% de humedad, 0.23% de proteínas, 1.43% de cenizas, 3.15% de fibra total dietaria y 17.5% de aceite. La caracterización de polifenoles en la harina reveló la presencia de (-)-epicatequina (1318.66 µg g-1), (+)-catequina (703.12 µg g-1) y trans-resveratrol (32.88 µg g-1) como los polifenoles mayoritarios. El aceite presentó compuestos bioactivos como carotenoides y polifenoles. Los ácidos grasos que predominaron en el aceite fueron el ácido linoleico (Ω-6, 84.73%), oleico (Ω-9, 5.83%) y esteárico (2.21%) Adicionalmente los extractos, etanol: agua (3:1; v: v) y metanol: HCl (0.01%) del aceite, resultaron tener una alta concentración de polifenoles (125 mg GAE g-1) y una alta actividad antioxidante. El índice de yodo fue de 68.56 g I2/100 g aceite y el índice de peróxidos fue de 20 meqO2 kg-1 aceite. Las semillas tuvieron una cantidad considerable de polifenoles y ácido linoleico que le confieren una alta actividad antioxidante, por lo cual podría ser adecuada para su uso en aplicaciones alimentarias e industriales y fomentar la producción y consumo de esta uva como fuente de nutrientes y compuestos fenólicos adecuados para su incorporación en alimentos funcionales.
Palabras claves: ácidos grasos; polifenoles y actividad antioxidante; uva silvestre
Introduction
Vitis tiliifolia is a wild grape that has not yet been adequately recognized by researchers and oenologists. It is a shrub that can be small to very large, with woody stems that can measure between 10 and 35 m long and up to 20 cm in diameter. It grows commonly in humid, dry forests and thickets, it is often found in pine and oak forests, although it is more abundant at low elevations. Its presence extends to 1 700 msnm. This grape is native to the southern states of México and grows in a range from the Antilles to Colombia (Fernández, 2009). In México, it grows in the states of Chiapas, Colima, Guerrero, Hidalgo, Nuevo León, Oaxaca, Querétaro, San Luis Potosí, Tabasco y Veracruz where it is known by different names according to where it grows, such as mountain grape, water hunter liana, broncadora, conduj, Gunhi, loobabi-chuli, sanalo todo, tecamate, xocomecatl, and others (Arellano et al., 2003). Vitis tiliifolia flowers from May to June and bears fruit from August to November (Ibarra-Manriquez and Sinaca, 1996). Its fruits have been used as raw materials to produce juices and handcrafted wines (Lascurain et al., 2010). Fresh grapes are used to make vinegar and non-alcoholic beverages (Fernández, 2009), while the roots and leaves are used empirically against hemorrhoids. The grape contains a single seed that can be considered as an unconventional source of lipids; but so far this seed has not been exploited.
Grape seeds are usually discarded as part of the winemaking process; however, extracting and selling grape seed oil and grape seed extract can be a profitable business as well as an efficient use of byproducts. Grapeseed oil of other varieties is known for the benefits of its nutritional value due to its high linoleic acid content (about 72-76%), this is commonly used for frying foods (due to its high smoke point) or is included in dressings and sauces. It is also used in cosmetics because of its moisturizing skin characteristics (Da Porto et al., 2013). Grape seeds are a rich source of monomeric phenolic compounds such as (+)-catechin, (-)-epicatechin, (-)-epicatechin-3-O-gallate and dimers, trimers and tetramers of procyanidins (Saito et al., 1998). Also, grape oil and meal contain polyphenols and other bioactive compounds which are attractive to the industry due to its antioxidant properties (Crews et al., 2006). These compounds are of great interest to the pharmaceutical and food industries as it possess anti-aging, anti-inflammatory, anti-carcinogenic, anti-mutagenic, anti-ulcer and anti-viral effects, besides being associated with a lower risk cardiovascular disease (Da Porto et al., 2013).
However, to our knowledge there is no information on the physicochemical properties and composition of seed oil from Vitis tiliifolia. Therefore, the objective of this study was to evaluate the composition and physicochemical properties antioxidants oil and flour obtained from the seed of Vitis tiliifolia. This information is essential for a comprehensive assessment of the use of oil in possible industrial applications.
Materials and methods
Reagents
2,2’-diphenyl-1-picrylhydrazyl (DPPH), Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid), Folin-Ciocalteu reagent, 2,4,6- 2 - pyridyl) -1, 3,5-triazine (TPTZ), gallic acid, 4-hydroxybenzoic acid, (+)-catechin, vanillic acid, chlorogenic acid, caffeic acid, (-)-epicatechin, vanillin, acid-4- coumaric, quercetin-3-glucoside, quercetin, rutin, ferulic acid, trans-cinnamic acid, mangiferin, umbelliferone, quercetin 3,4-di-O-glucoside, scopoletin, ferulic acid, quercetin-3-D-glucoside, luteolin 7-O-glucoside, kaemferol 3-O-glucoside, 2,4-dimethoxy-6-methylbenzoic acid, cirsimarine, luteolin, angelicin, apigenin, kaempferide, piperine and dehydroabietic acid were purchased from Extrasynthese (Lyon, France) and Sigma- Aldrich (USA). The solvents used for extraction were analytical grade and those used for Ultra High Resolution Liquid Chromatography (UPLC) processes were purchased from Sigma-Aldrich (USA). Reference solutions, samples, solvents and reagents were filtered through a PTFE 0.20 μm membrane filter (Phenomenex, USA) prior to separation or injection into the equipment.
Collection of samples
Vitis tiliifolia samples were collected from the “Cafetal” ranch, located in the state of Veracruz, México. Located at coordinates 19° 37’ 0.4” north latitude and 96° 50’ 2.7” west longitude, at an elevation of 734 masl. The grape varieties were harvested at optimum ripening stage, with soluble solids ranging between 12-14 brix. The seeds were obtained by manual separation from 5 kg of sample, washed several times with water, dried in a vacuum oven (Shel Lab, USA) at 50 °C, then passed through a mill (Glen Mills, USA) several times until obtaining a smaller size of 0.5 mm. The sample was divided into 100 g portions, placed in vacuum bags, frozen at -40 °C and protected from light for further analysis.
Determination of physicochemical properties of flour
The total nitrogen content was determined by the micro-Kjendahl method and the protein was calculated using the factor of 6.25, the ash content was determined by weighing the product incinerated in a muffle at 550 °C. Titratable total acidity, reducing sugars and total dietary fiber concentration were determined according to the methodology described in AOAC (2000).The oil was extracted from the dry flour using Soxhlet and hexane (60-80 °C) for 6 h. The extracts were filtered and concentrated in vacuo to obtain the crude extracts which were stored in a desiccator until they were used.
Determination of physicochemical parameters of oil
The peroxide index, acidity percentage, iodine number, saponifiable matter and unsaponifiable matter were evaluated according to the Official and Recommended Methods of AOCS (1998). The refractive index was determined with an Abbe refractometer at 40 °C and the viscosity was measured with an Anton Paar viscometer at 25 °C. The titratable acidity was determined by titration of an oil solution dissolved in an ethanol: ether mixture (1:1, v: v) with a solution of 0.1M potassium hydroxide in ethanol. Spectroscopic indices, K232, K268, in the UV region were determined with a UV/Vis spectrophotometer (JENWAY, Model 6305, Japan), in the oil diluted with isooctane [10]. Determining the fatty acid profile of oil of Vitis tiliifolia was performed according to the method described by Lopez et al. (2001).
Analysis of antioxidant activity
The antioxidant activity was analyzed in the extracts methanol: water (3:1; v: v) and methanol: 0.1% HCl (v: v) obtained from the flour. 5 g aliquots of the pulverized samples (flour) were weighed in Erlenmeyer flasks and extracted with 100 mL of the aforementioned solvents for 24 h with shaking. Subsequently they were washed with nitrogen in order to avoid oxidation. The extracts were centrifuged (10 min, 5 366 g) and the solids were re-extracted with 100 mL of respective solvents for 60 min. Finally, the extracts were combined and vacuum filtered through Whatman num. 1 filters, and then concentrated in a rotavaporator at 35 °C (R-210; Büchi, Flawil, Switzerland).
Total phenol content
The total content of total phenols was determined using the Folin-Ciocalteu method (Singleton and Rossi, 1965). The grape extracts were mixed with Folin-Ciocalteu reagent and subsequently a sodium carbonate solution (10%) was added. The mixture was reacted at room temperature in the dark for 120 minutes, then the absorbance at 765 nm was measured. The result was compared with a calibration curve (R2= 0.89) obtained with a similar range of concentrations of gallic acid, and expressed as mg gallic acid equivalents (EAG) per g of extract.
DPPH free radical capture capability
The ability of DPPH free radical capture was estimated by the method of Brand-Williams et al. (1995). The extracts previously obtained from the seeds were mixed with DPPH methanolic solution and kept in the dark for 30 min. The absorbance of the reaction mixture was measured at 517 nm on a UV/VIS spectrophotometer (Jenway, model 6305, Japan).
Antioxidant activity (%) expressed in oxidation percentage of β-carotene/linoleic
The antioxidant activity was evaluated through of β-carotene bleaching according to reports from Oboh et al. (2009). The absorbance of the samples and control were measured at 470 nm using a UV/Vis spectrophotometer (JENWAY, Model 6305, Japan) against a blank consisting of β-free carotene emulsion.
Reducing power
Reducing power was determined according to the method of Yen and Duh (1993). A 0.125 mL aliquot of the sample at a concentration of 1 mg mL-1 was placed in a test tube and 1.25 mL of phosphate buffer (200 mM, pH 6.6) and 1.25 mL of potassium ferrocyanide (1%) were added. The mixture was incubated at 50 °C for 20 min. Then, 1.25 mL of 10% trichloroacetic acid were added, and then centrifuged at 650xg for 10 minutes. A 2.5 mL aliquot of the supernatant was transferred to a new tube and 2.5 mL of distilled water and 0.5 mL of ferric chloride were added. The optical density was measured at 700 nm on a UV/VIS spectrophotometer (Jenway, model 6305, Japan).
Identification and content of polyphenols in grape seed meal
For phytochemical analysis, the extracts were prepared using a solvent-accelerated extraction system (ASE 350, Thermo Scientific, USA), 3.0 g of dry material were dispersed in 1 g of diatomaceous soil and placed in a 34 mL cell. The cell was filled with methanol to a pressure of 1500 psi and heated at 60 °C for 5 min then the cells were washed with 30% of that volume. The extract was concentrated with a rotary evaporator (Büchi RII, Switzerland). 10 mg of crude extract were redissolved in 1 mL MeOH with 0.1% formic acid (MS grade, Sigma-Aldrich), filtered and placed in a 1.5 mL vial. The sample was analyzed in triplicate. The identification and quantification of individual phenolic compounds was established by ultra high resolution chromatography (UPLC) (Agilent series 1290) and multiple dynamic reaction monitoring (dMRM) following the conditions of protocol-Hullack Durand et al. (2015).
Results and discussion
Characterization of meal obtained from seeds of Vitis tiliifolia
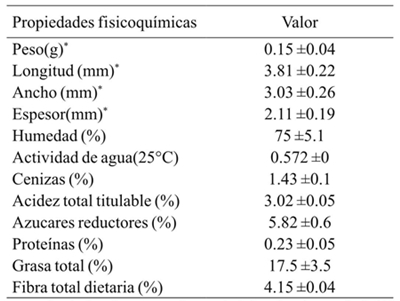
Some physicochemical parameters of the seed of Vitis tiliifolia grape are described in Table 1. Seeds of Vitis tiliifolia are round, have an average length of 3.81 mm and a width of 3.03 mm. Its average weight is 0.15 g, with a moisture content of 75%. The percent of total titratable acidity (3.02%) is less than that reported for other varieties of grapes (Jiang-Fei et al., 2012). This seed has a smaller amount of total dietary fiber (4.15%) than that reported for other grape seed varieties (Bravo and Saura-Calixto, 1998) and the oil content of the grape seeds was 17.5%, which is in the range of 9.9 to 20% reported for other grape varieties (Bravo and Saura-Calixto, 1998; Ohnishi et al., 1990).
Analysis of chemical compounds present in the seed meal
In order to obtain compounds that contribute to the high antioxidant activity, the polyphenols present in the seed meal were identified and quantified (Table 2). Seven compounds were isolated, and their chemical structures were identified with the basis of the spectral data of ultra high resolution chromatography-mass spectrometry (UHPLC-MS). A mass spectrometry protocol was used in multiple reaction monitoring mode of operation (MRM, MS), in a retention time range of 1.5 to 46 min, collision energy between 10 and 30, with values of R2> 0.971 linearity in the concentration range of 0.1 to 14 mM. From the methanolic extract, the two major compounds were: (-)-epicatechin (1 318.66 µg g-1) and (+)-catechin (703.12 µg g-1), followed by trans-resveratrol (32.88 µg g-1), quercetin-3-glucoside (14.65 µg g-1), gallic acid (3.32 ± 0.49 µg g-1), quercetin-3-D-galactoside (1.38 µg g-1) and lastly vanillin (0.83 µg g-1), all contribute to antioxidant activity in other types of grapes (Maier et al., 2009).
Table 2 Polyphenolic compounds (μg kg-1) identified in Vitis tiliifolia flour using ultra high resolution chromatography-mass spectrometry.
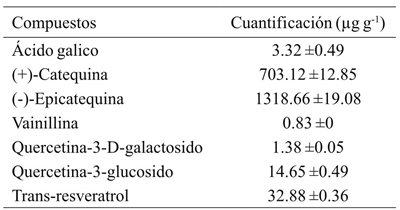
Los datos son expresados en promedio ± desviación estándar (n=3).
It has been reported that phenolic groups present in natural products are responsible for electron delocalization to which the free radical harvesting capacity is attributed (Rice-Evans et al., 1996). Likewise, the antioxidant activity of polyphenols is mainly attributed to their potent activity as a metal chelator and antiradical activity. It has often been reported that these antioxidant compounds are present in grapes and provide antimutagenic, anti-inflammatory and antiproliferative activity. Similarly, it has been shown that resveratrol is a cyclooxygenase and hydroperoxides inhibitor in several experimental systems (Aziz et al., 2003).
Physicochemical properties of oil
Table 3 shows the physicochemical and quality properties of the oil extract that were prepared using the Soxhlet extractor. The color parameters of L*, a* and b* were 1.64, 1.06 and 1.72, respectively. There are no color standards for Vitis seed oil and these values could be used for quality assessment. The parameter a* is within the same range as that reported for chia seed oil (Ixtana et al., 2011). The samples showed positive values for parameter b*, characteristic of light yellow colors. This color is associated with the total content of pigments, such as carotenoids. Density of Vitis tiliifolia seed oil was 0.909 g mL-1. This value is similar to that reported for soybean oil, but is slightly lower tan the density range (0.92-0.926) for oil from the Vitis vinifera L. seeds (FAO/WHO, 1999). This difference in density may be due to oil extraction method or the difference in the origin and type of seed (Tsaknis et al., 1999). Water activity (0.65) and specific weight (0.926 g mL-1) were higher than those reported for other oils such as canola and linseed oil (Yin and Sathivel, 2010).
Table 3 Physicochemical parameters of oil extracted by Soxhlet from Vitis tiliifolia.
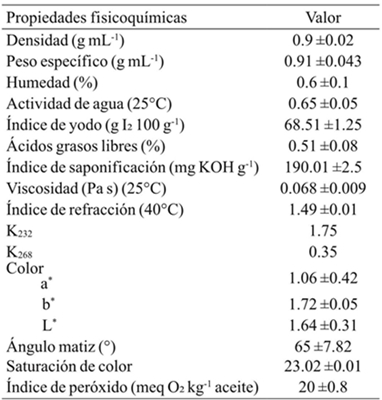
Los datos son expresados en promedio ± desviación estándar (n= 3).
The moisture content (0.6%) of the oil was slightly higher than that reported (0.4 to 0.58%) for edible oils, indicating greater susceptibility to degradation such as hydrolysis of lipids that may cause undesirable oxidation reactions (Bhattacharya et al., 2008; Adedokun and Onuegbu, 2011). The refractive index of Vitis tiliifolia seed oil was similar to Chia oil (1.49) and jatropha oil (1.47), indicating that it contains similar amount of free fatty acids (Eromosele and Pascal, 2003; Iztaina et al., 2011). Furthermore, the saponification indexof Vitis tiliifolia oil was 190.01 mg KOH g-1, this value is similar to the saponification value of canola oil (Eskin et al., 1996). The viscosity at 25 °C found for this oil (0.068 Pa s) was found within the range reported for some edible oils (Hernández et al., 2013) range. The oil had an acid value (1.02%) slightly higher than that reported for other oils such as Dacryodes edulis edible oil (Adedokun and Onuegbu, 2011), but lower than the refined oils and cold pressed oils (FAO-OMS, 1999).
Champe and Harvey (1994) reported that the acidity measurement is relative to the amount of free fatty acids and suggests a good post-harvest handling and a low moisture content in the oil. The iodine index found (68.75 g I2/100 g) is lower than the reference for grapeseed oils (FAO-OMS, 1999), but is higher than what has been reported for other types of edible oils (Adedokun and Onuegbu, 2011), and for Vitis spp. wild grape oils (57.9 g I2/100 g) (Franco-Mora et al., 2015), which could mean that this behavior is characteristic of this grape. In turn, the peroxide value measures hydroperoxides produced by oils oxidation (Abbas et al., 2008).
The values obtained for the peroxide index were higher than the minimum established as the reference standard (up to 10 meq O2 kg-1 oil) and than the values set for the cold pressed and virgin oils (up to 15 meq O2 kg-1 of oil) (FAO-OMS, 1999). According to this, some authors report that it is important to refine the oils in order to obtain products with desirable and acceptable properties that would extend its useful life (Yin and Sathivel, 2010). Furthermore, specific extinction coefficients had values of 1.72 and 0.35 for K232 and K268, respectively, revealing the oxidative deterioration of the oil (Yoon et al., 1985). The specific extinction coefficient at 232 nm (K232) is related to the grade of oil oxidation. This ratio is also an indicator of the polyunsaturated fatty acid conjugation, while K268 is related to secondary oxidation products responsible for off-flavors.
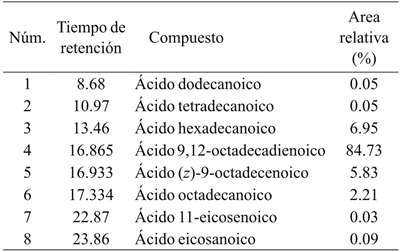
Oil fatty acid profile
Table 4 shows the fatty acid composition of Vitis tiliifolia seed oil. The content of total saturated fatty acids was approximately (9.35%). However, a high level of unsaturated fatty acids (90.59%) was found in the oil. The concentration of saturated fatty acids was of palmitic acid (6.95%), stearic acid (2.21%), arachidonic acid (0.09%), lauric acid (0.05%) and myristic acid (0.05%). Among the polyunsaturated fatty acids, linoleic acid (Ω-6, 84.73%), followed by oleic acid (Ω-9, 5.83%) and gondoic acid (Ω-11, 0.03%) were found in the highest proportion. The linoleic acid content was close to that reported for seed oil Oecopetalum mexicanum (Hernández et al., 2013), however, for its use as an additive or dietary supplement it is necessary to consider that there must be a certain balance between fatty acids (Ω-6/(Ω-3) (Pisani et al., 2015).
Compounds and antioxidant activity of oil
Table 5 shows the chemical components and antioxidant properties of extracts made with ethanol: water [3:1; v:v] and methanol: HCl (0.01%). The antioxidant activity in vitro of Vitis tiliifolia seed oil was evaluated through the capability of capturing DPPH radical; antioxidant activity is expressed as the percentage of β-carotene/linoleic oxidation and through the reducing power.
Table 5 Chemical composition and antioxidant activity in Vitis tiliifolia seed of extracts obtained using different solvent mixtures.
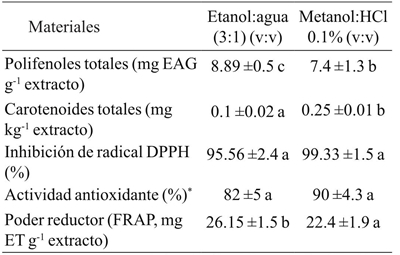
* = expresada como porcentaje de oxidación of β-caroteno/linoleico. EAG= equivalentes de ácido gálico; ET= mg equivalentes de Trolox. Valores con letras diferentes en la misma fila indican diferencias significativas (p< 0.05).
Concentration of polyphenols
Total phenolic compounds are an important group of secondary metabolites present in seeds. They are usually abundant in grapes and play an important role in the quality and nutritional value of grape seeds. The range of total phenolic compounds in the extract of ethanol: water (8.89 mg EAG g-1 extract) was higher than in methanol: HCl extract (7.4 mg EAG g-1 extract). These values were lower than what has been reported for oil extracts from Vitis vinifera and oil of red grape variety (Apostolou et al., 2013). These differences in the concentration of phenolic compounds in the liquid extracts could depend on the affinity of the polyphenolic compounds to a particular solvent, which in turn depends on the grape variety. Moreover, this concentration may be influenced by climatic and geographical factors, cultural practices and the maturation status (Burin et al., 2014), and by the type and proportion of solvents used in extraction, the type of extraction and treatment used.
Carotenes concentration
Another important class of compounds derived from grape seed oil are carotenoids. These compounds have antioxidant activity and are present in amounts ranging from 0.25 mg kg-1 to 0.1 mg kg-1 for the ethanol:water (3:1; v:v) extract and methanol:HCl (0.01%) respectively. These values are similar to those reported for soybean (0.3 mg kg-1), sunflower (0.1 mg kg-1) and flaxseed (0.7 mg kg-1), but less than rapeseed (1.9 mg kg-1) (Tuberoso et al., 2007).
Inhibition of DPPH radical of the oil
The DPPH radical capture activity was 95.56% and 99.33% for the extract of ethanol:water and methanol:HCl, respectively. Both extracts showed no significant differences in DPPH radical capture, which can be expected from the concentrations of bioactive compounds, such as total polyphenols and carotenoids. Therefore, the data obtained reveal that these compounds act as inhibitors of free radicals and confer antioxidant activity on this grape seed oil.
Antioxidant activity of the oil (oxidation of β-carotene/linoleic acid)
Antioxidant activity was determined based on the inhibition of the oxidation of β-carotene and linoleic acid. This activity varied from 82% to 90% in the ethanol: water and methanol: HCl extracts respectively, indicating in both samples that the presence of antioxidant compounds may hinder the degree of β-carotene discoloration by neutralizing the free radical of linoleate and other free radicals formed in the system (Jayaprakasha et al., 2001).
Reducing power (FRAP) in the oil
The FRAP test showed high values for both seed oil extracts. FRAP values ranged from 22.4 to 26.15 mg ET g-1 ethanol: water and methanol:HCl extract, respectively. The results indicate that the antioxidant activity of the extracts of Vitis tiliifolia seems to be due to the presence of polyphenols. These polyphenols can act in a similar way to reductones, by electron donation and react with free radicals to become more stable products and thereby terminating the chain reaction of free radicals (Jayaprakasha et al., 2001). Similarly, the reducing power was greater in the ethanol:water extract, indicating the presence of compounds which act as reducing agents, as evidenced by the reduction of ferric ion (Fe3+) to ferrous ion (Fe2+). A high correlation between polyphenol content with the ability to capture the DPPH radical (R2= 0.86) and with antioxidant activity estimated by β-carotene-linoleic acid oxidation (R2= 0.89) was found. Both extracts showed antioxidant activity statistically equal (p>0.05), indicating that the compounds present in both extracts have the ability to trap the DPPH radical and inhibit lipid peroxidation. The data obtained reveal that the compounds present in the extracts are inhibitors of free radicals and primary antioxidants that react with free radicals.
Conclusions
Oil and flour from the Vitis tiliifolia seeds have compounds with functional properties that propose it for possible commercial and industrial uses. The oil is rich in linoleic acid and other compounds that give it antioxidant activity. The physicochemical properties are suitable for use in the food industry and other related areas. Seed flour is rich in polyphenols, such as catechin, epicatechin and trans-resveratrol that were the most abundant. These antioxidants present in the seed can be extracted using either a mixture of ethanol:water (3:1; v:v) or methanol:HCl (0.01%).
Literatura citada
Abbas, M.; Abu, M.; Kumar, R.; Yeasmin, S. and Mohal, A. 2008. Comparative study on characteristics of seed oils and nutritional composition of seed from different varieties of Tobacco (Nicotiana tabacum L.) cultivated in Bangladesh. Asian J. Biochem. 3(4):203-212. [ Links ]
Adedokun, I. I. and Onuegbu, N. C. 2011. The physical properties of pulp and chemical characteristics of edible oil extracted from the pulp of African pear (Dacryodes edulis). Pakistan J. Nutr. 10(6):558-560. [ Links ]
Association of Official Analytical Chemists (AOAC). 2000. Official methods of analysis. Maryland, USA. [ Links ]
AOCS (American Oil Chemists Society). 1998. Official methods and recommended practices of the AOCS. 5th Ed.. Champaign, IL. [ Links ]
Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, K.; Trizoglou, I.; Hayes, A. W.; Tsatsakis, A. M. and Kouretas, D. 2013. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chemical Toxicol. 61:60-68. [ Links ]
Arellano, J. A.; Flores, J. S.; Tun, J. and Cruz, M. M. 2003. Nomenclatura, forma de vida, uso, manejo y distribución de las especies vegetales de la Península de Yucatán. Etnoflora Yucatanense Fasículo 20. Yucatán: UADY. Mérida, México. 1-815 pp. [ Links ]
Ayoola, P. B. and Adeyeye, A. 2010. Effect of heating on the chemical composition and physico-chemical properties of Arachis hypogeal (groundnut) seed flour and oil. Pak. J. Nutr. 9(8):751-754. [ Links ]
Aziz, M. H.; Kumar, R. and Ahmad, N. 2003. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (Review). Inter. J. Oncology. 23:17-28. [ Links ]
Bhattacharya, A. B.; Sajilata, M. G. and Sibghal, R. S. 2008. Lipid profile of foods fried in thermally polymerized palm oil. Food Chemistry. 109:808-812. [ Links ]
Brand, W. W.; Cuvelier, M. E. and Berset, C. 1995. Use of a free-radical method to evaluate antioxidant activity. LWT-Food Science and Technology. 28:25-30. [ Links ]
Bravo, L., and Saura, C. F. 1998. Characterization of dietary fiber and the in vitro indigestible fraction of grape pomace. Am. J. Enol. Viticulture. 49:135-141. [ Links ]
Burin, V. M.; Ferreira, L. N. E.; Panceri, C. P. and Bordignon, L. M. T. 2014. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: evaluation of different extraction methods. Microchemical J. 114:155-163. [ Links ]
Champe, P. C. and Harvey, R. A. 1994. Lippincott’s illustrated reviews: biochemistry. 2nd (Ed.). Lippincott Raven Publishers. New Jersey, USA. 303-340 pp. [ Links ]
Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S. and Winkelmann, W. 2006. Quantitation of the main constituents of some authentic grape seed oils of different origin. J. Agric. Food Chem. 54:6261-6265. [ Links ]
Da Porto, C.; Porretto, E. and Decorti, D. 2013. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrasonics Sonochemistry. 20:1076-1080. [ Links ]
Eromosele, C. O. and Pascal, N. H. 2003. Characterization and viscosity parameters of seed oils from plants. J. Bio. Technol. 86:203-205. [ Links ]
Eskin, N. A. M.; McDonald, B. E.; Przybylski, R.; Malcolmson, L. J.; Scarth, R.; Mag, T.; Ward, K. and Aldoph, D. 1996. Canola Oil. In: Bailey’s industrial oil & fat products. Y. H. Hui, (Eds.). Edible oil and fat products: oils and oil seeds. 5th edition. John Wiley and Sons. Inc. New York, USA. (2):1-96. [ Links ]
FAO/OMS (Food and Agriculture Organization /Organización Mundial de la Salud). 1999. CODEX Stan 210. Norma del codex para grasas y aceites comestibles no regulados por normas individuales, codex alimentarius official standards, Canadá. 1-14 pp. [ Links ]
Fernández, C. C. 2009. Plantas comestibles de Centroamérica. Instituto Nacional de Biodiversidad. Santo Domingo de Heredia. Costa Rica. http://www.inbio.ac.cr/webca/biodiversidad/regional/plantascomestibles ca-ve.pdf. [ Links ]
Franco, M. O.; Salomón, C. J.; Morales, A. A.; Castañeda, V. A. and Rubí, A. M. 2015. Fatty acids and parameters of quality in the oil of wild grapes (Vitis spp.). Sci. Agrop. 6(4):271-278. [ Links ]
Göktürk, N. and Akkurt, M. 2001. Oil content and oil quality properties of some grape seeds. Tur. J. Agric. Fores. 25:163-168. [ Links ]
Hernandez, B.; Luna, G.; García, O.; Mendoza, M. R.; Azuara, E.; Beristain, C. I. and Jimenez, M. 2013. Extraction and characterization of Oecopetalum mexicanum seed oil. Industrial Crops and Products. 43:355-359. [ Links ]
Ibarra, M. G. y Sinaca, C. S. 1996. Lista comentada de plantas de la estación de biología tropical. Los Tuxtlas, Veracruz, México: (Violaceaea-Zingiberaceae). Rev. Biol. Trop. 427-447. [ Links ]
Ixtaina, V. Y.; Martínez, M. L.; Spotorno, V.; Mateo, C. M.; Maestri, D. M.; Diehl, B. W. K.; Nolasco, S. M. and Tomás, M. C. 2011. Characterization of chia seed oil obtained by pressing and solvent extraction. J. Food Comp. Anal. 24:166-174. [ Links ]
Jayaprakasha, G. K.; Singh, R. P. and Sakariah, K. K. 2001. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry. 73:285-290. [ Links ]
Jiang, F. M.; Yu, L. F.; Min, Y. Q.; Xi, F. Z. and Zhen, W. Z. 2012. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County. Food Chemistry. 134:2049-2056. [ Links ]
Lascurain, M.; Avendaño, S.; Del Amo, S. y Niembro, A. 2010. Guía de frutos silvestres comestibles en Veracruz.Fondo Sectorial para la Investigación, el Desarrollo y la Innovación Tecnológica Forestal, Conafor- Conacyt, México. 119p. [ Links ]
López, A.; Castellote, A. I. and López, M. C. 2001. Comparison of two direct methods for the determination of fatty acids in human milk. Chromatographia. 54(11-12):743-747. [ Links ]
Maier, T.; Schieber, A.; Kammerer, D. R. and Carle, R. 2009. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chemistry. 112:551-559. [ Links ]
Oboh, G.; Raddatz, H. and Henle, T. 2009. Characterization of the antioxidant properties of hydrophilic and lipophilic extracts of jute (Corchorus olitorius) leaf. Inter. J. Food Sci. Nutr. 60:124-134. [ Links ]
Ohnishi, M.; Hirose, S.; Kawaguchi, M.; Ito, S. and Fujino, Y. 1990. Chemical composition of lipids. Especially triacylglycerol in grape seeds. Agric. Biol. Chem. 54(4):1032-1042. [ Links ]
Oluba, O. M.; Ogunlowo, Y. R.; Ojieh, G. C.; Adebisi, K. E.; Eidangbe, G. O. and Isiosio, I. O. 2008. Physicochemical properties and fatty acid composition of Citrullus lanatus (Egusi Melon) seed oil. J. Biol. Sci. 8(4):814-817. [ Links ]
Pardo, J. E.; Fernández, E.; Rubio, M. and Alvarruiz, A. 2009. Characterization of grape seed oil from different grape varieties (Vitis vinifera). Eur. J. Lipid Sci. Technol. 111:188-193. [ Links ]
Pisani, D. F.; Amri, E. Z. and Ailhaud, G. 2015. Disequilibrium of polyunsaturated fatty acids status and its dual effect in modulating adipose tissue development and functions. Oilseeds and Fat Crops and Lipids. 22(4):D405. [ Links ]
Rice, E. C.; Miller, N. J. and Paganga, G. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 20:933-956. [ Links ]
Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S. and Yamaji, N. 1998. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 46(4):1460-1464. [ Links ]
Singleton, V. L. and Rossi, J. A. 1965. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144-158. [ Links ]
Tsaknis, J.; Lalas, S.; Gergis, V.; Dourtoglou, V; Spilitois V. 1999. Characterization of Moringa oleifera variety Mbololo seed oil of Kenya. J. Agric. Food Chem. 47:4495-4499. [ Links ]
Tuberoso, C.; Kowalczyk, A.; Sarritzu, E. and Cabras, P. 2007. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chemistry, 103:1494-1501. [ Links ]
Yen, G. C. and Duh, P. D. 1993. Antioxidative properties of methanolic extract from Peanut Hull. J. Am. Oil Chem. Soc. 70:383-386. [ Links ]
Yin, H.; and Sathivel S. 2010. Physical properties and oxidation rates of unrefined menhaden oil (Brevoortia patronus). Food Engineering and Physical Properties. 75(3):163-168. [ Links ]
Yoon, S. H.; Kim, S. K.; Shin, M. G. and Kim, K. H. 1985. Comparative study of physical methods for lipid oxidation measurement. J. Am. Oil Chem. Soc. 68:1487-1489. [ Links ]
Received: January 2017; Accepted: April 2017











 texto en
texto en