Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.5 Texcoco Jun./Ago. 2017
https://doi.org/10.29312/remexca.v8i5.107
Articles
Join process of splice graft in tomato(Solanum lycopersicum L.)
1Departamento de Fitotecnia-Universidad Autónoma Chapingo. Carretera México-Texcoco km 38.5. Chapingo, Estado de México. CP. 56230.
2Recursos Genéticos y Productividad-Genética-Colegio de Postgraduados, Campus Montecillo. Carretera México-Texcoco km 36.5. Montecillo, Texcoco, Estado de México. CP. 56230.
The tomato grafting technique represents a sustainable alternative for disease control, increasing plant vigor, improving fruit quality and yield. The first important factors in the grafting process are the anatomical compatibility and the time of establishment of vascular continuity between the two plant tissues, the latter determining the moment of transplantation. The objective of this paper was to evaluate the process of graft joining with the splicing method in tomato, by means of anatomical cuts in the attachment point; as well as to determine the time of planting time between the ‘Multifort’ and ‘El Cid’ genotypes (rootstock and graft, respectively) to synchronize the stem diameter. 100 seeds of both genotypes were sown, from these seedlings the stem diameters were measured daily. Tissue samples were taken from the graft attachment site at 5, 10 and 15 days after grafting (DDI), anatomical sections were 10 μm thick obtained with a rotary microtome. The results indicated that it is necessary to plant the graft or spike two days before the rootstock to in order to homogenize the stem diameter to 2 mm (ideal for grafting). In the images of the anatomical sections it was found that at 5 DDI the demarcation line began to be reabsorbed and at the same time the formation of new conduction tissue (xylem and phloem) began, the complete vascular connection was carried out at the 10 DDI.
Keywords: Solanum lycopersicum L.; rootstock/graft; vascular continuity
La técnica de injerto en jitomate representa una alternativa sustentable para el control de enfermedades, para incrementar el vigor de planta, mejorar la calidad de fruto y rendimiento. Los primeros factores importantes en el proceso de injerto son la compatibilidad anatómica y el tiempo de establecimiento de la continuidad vascular entre los dos tejidos vegetales, este último determina el momento del trasplante. El objetivo del presente estudio fue evaluar el proceso de unión del injerto con el método de empalme en jitomate, mediante cortes anatómicos del punto de unión; así como, determinar el tiempo de desfasamiento en la siembra entre los genotipos ‘Multifort’ y ‘El Cid’ (portainjerto e injerto, respectivamente) para sincronizar el diámetro de tallo. Se sembraron 100 semillas de ambos genotipos, de esas plántulas se midieron los diámetros de tallo diariamente. Se tomaron muestras de tejido del punto de unión del injerto a los 5, 10 y 15 días después de la injertación (DDI), los cortes anatómicos fueron de 10 µm de espesor obtenidos con un microtomo rotatorio. Los resultados indicaron, que se requiere sembrar el injerto o púa, dos días antes que el portainjerto para homogenizar el diámetro de tallo a 2 mm (ideal para injertar). En las imágenes de los cortes anatómicos se encontró que a los 5 DDI la línea de demarcación comenzó a reabsorberse y al mismo tiempo dio inicio la formación de nuevo tejido de conducción (xilema y floema), la completa conexión vascular se llevó a cabo a los 10 DDI.
Palabras clave: Solanum lycopersicum L.; continuidad vascular; portainjerto/injerto
Introduction
The use of grafted seedlings in the production of vegetables is a new technique, compared to its use in fruit species. In recent years it has become important for the control of stressful factors in the production of tomato (S. lycopersicum L.), pepper (Capsicum annuum L.), watermelon (Citrullus lanatus Thunb) and melon (Cucumis melo L.) (Fernández et al., 2004; Goto et al., 2013). To promote growth, improve agronomic traits and increase tolerance and resistance to biotic and abiotic factors (Colla et al., 2006; Colla et al., 2010; Vitale et al., 2014. Edelstein and Ben-Hur, 2015; Velasco et al., 2016). However, in order to maximize the benefits of using grafted seedlings, it is necessary to study the rootstock/graft interaction; such as anatomical compatibility to determine the time and vascular establishment level, as well as to know the effect on plant vigor that is expressed through variations in growth, yield and fruit quality (Teruo and Hiromichi, 1994).
The evaluation of the grafting fixation in vegetables allows to know the time necessary for vascular reestablishment and transplantation, ideally, this event should be rapid, since once the vascular continuity is stablished, the conduction of water, nutrients and organic substances begins between materials, thus the new plant can resume its growth and development (Turquois and Malone, 1996). This process is complex and involves first the formation of callus tissue followed by the restoration of a functional vascular system (Fernández et al., 2004). The percentage of stuckage of plantlets grafted on vegetables has been improved, by knowing the ideal environmental conditions in the post-graft phase such as temperature, relative humidity and luminosity; factors that favor cell division and the rapid formation of callus. Also the use of special clips for the attachment of the join point improves the stuckage success and facilitates the work (Lee and Oda, 2003).
Commercial tomato rootstocks, in most cases, have a faster seedling growth than those of the graft or spike; product of heterotic effect of the cross between a wild relative of tomato with a cultivated line; so it is necessary to homogenize the stem diameter, by the early sowing of the genotype that functions as a graft; since when having stems with similar diameters it is easier to match the cut parts, so that a considerable portion of the cambial regions are in contact and cohesion between rootstock/graft is given (Andrews and Márquez, 1993; Hartmann et al., 2002), and consequently a more successful engraftment. Getting a similar diameter stem when grafting is necessary if the grafting process is performed with the use mechanized robot (Kubota et al., 2008). In order to investigate and generate information on the new production techniques of tomato, such as grafting, this paper had as objective to determine the gap time of sowing between the rootstock and graft and to describe the join process with the splicing method in tomato, by means of anatomical cuts, to determine the time of the vascular restoration, which indicates the ideal moment for the transplant.
Materials and methods
The research was carried out in a greenhouse with polyethylene roof (600 gauge, 80% light transmission), tunnel type with overhead ventilation located at Chapingo Autonomous University (19° 29’ north latitude, 98° 53’ west longitude at 2 240 masl), in the spring-summer cycle of 2013. A polyethylene fishing chamber (600 gauge, 4 m long x 3 m wide x 2 m high) was built inside, eight microaspersores were placed inside (using 0.00833 L s-1). Having the following conditions during the day: average temperature of 25 °C, HR of 80 to 100% and photon flux density of 111 µmol m-2 s-1. The spray frequency was gradually decreased, the first three days after grafting (DDI) the micro sprinklers were operated 1 s/min; the next 3 days, 1 s/3 min and finally 1 s/8 min.
The rootstock was the interspecific hybrid ‘Multifort’ (Solanum lycopersicum x S. habrochaites) recommended for soilless cultivation and short cycle, which has high yield and balance between vegetative and reproductive growth (De Ruiter SeedTM), which is also one of the rootstocks most currently used in the production of grafted tomatoes As graft the commercial hybrid ‘El Cid’ was used, with saladette type fruits and indeterminate growth habit (Harris MoranTM), a popular material in the production of tomato in greenhouse.
For the synchronization of stem diameter in seedlings of rootstock and graft, 100 seeds of each genotype were seeded the same day in polystyrene trays with 200 cavities with peat moss (®Kekkila, Finland) as substrate. Once the seedlings emerged, the stem diameter was measured daily for 23 days with a digital vernier (Mitutoyo®, In/mm professional) also the minimum and maximum temperatures were recorded, giving a total of 427 units heat. For fertilization the Steiner universal nutrient solution was used at 20% (Steiner, 1984), the application started 8 days after the emergency. With the data, a simple linear regression analysis was performed aiming of making inferences about the behavior of the stem diameter data, with this analysis the mathematical model for the dataset of each genotype was obtained, the independent variable was number of days and the dependent was stem diameter (mm).The terms of the models were, where, Yi = β1Xi + ei where Yi is the i-th value of the dependent variable, Xi the i-th value of the independent variable, β1 the slope of the line and ei the i-th random value.
Once the stem diameter was synchronized, 80 seeds of each genotype (‘Multifort’/‘El Cid’) were planted, the rootstock was planted two days after the grafting to obtain equal diameters in the genotypes. The grafts were made at 26 days after sowing with the splicing method (Lee, 1994), when the seedlings reached 9 cm in height and 2 mm in stem diameter, for the attachment of the join point, special 2 mm wide silicone clips were used.
The post-grafting phase lasted 15 days, during which samples were performed at 5, 10 and 15 DDI. Each one of them consisted of sampling the join point of 10 grafted seedlings, cutting 2 mm above and below the join point. These were fixed in FAA (50% of 96% ethanol + 5% glacial acetic acid + 10% formaldehyde + 35% distilled water). Dehydration was carried out with ethanol (50, 70, 96 and 100%) and transparency with 100% xylene using an automatic processor (Histokinette, 2000). The inclusion was made in paraffin and the tissue was cut on a rotary microtome Leica® brand (Jung Histocut 820), making 10 µm thick cuts.The staining was performed with safranin and fast green. Photographs were taken in a Carl Zeiss®optical microscope with Canon® digital camera adapted to the microscope.
Results and discussion
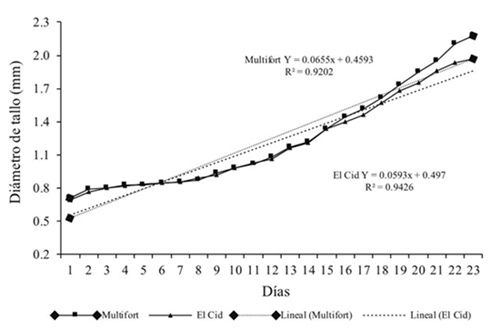
According to linear regression models (Figure 1), the ‘Multifort’ rootstock stem diameter grew 0.0655 mm-1 day and ‘El Cid’ engraftment or spike grew 0.0593 mm-1 day. The stem diameter ideal for the grafting process in tomato is 2 mm, usually obtained at 30 days after sowing (Lee et al., 2010). Considering this recommendation and replacing the value of 2 mm in the models, it stays as follows:
Portainjerto ‘Multifort’
Injerto ‘El Cid’
Therefore, the difference of days in the sowing is of 25.3-23.5= 1.8 days. That is, in order to obtain 2 mm diameter in both components for grafting, it is required to plant ‘El Cid’ graft genotype about two days before the ‘Multifort’ rootstock. This allowed greater contact surface between rootstock/graft, greater overlap of cambial parts, rapid cohesion between tissue and callus tissue forming, these are the initial consideration in the grafting process (Hartmann et al., 2002; Pina and Errea, 2005).
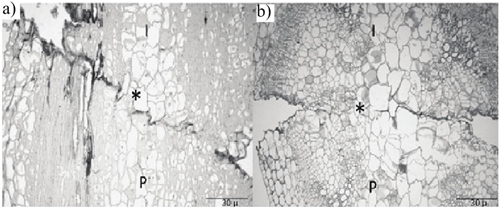
In tomato, the cohesion between the rootstock and grafting, as the first event of the join is related to the secretion of pectic substances, that provide initial mechanical support at the join point (Jeffree and Yoeman, 1983; Parkinson et al., 1987). Figure 2a shows the accumulation of these substances and the remains of dead cells caused by the cut, forming a demarcation line in the junction area, Fast Green staining shows a more intense region.
As the callus develops, the necrotic layer is gradually reabsorbed. In the obtained sections the reabsorption of the demarcation line is observed in the inter phase at 5 DDI, even in the longitudinal section (Figure 2b), it is observed that the demarcation line has been reabsorbed in 50%, it is seen at the same time the entanglement of rootstock/graft cells, due to the increase in size of the new callus cells, this confers greater mechanical strength at the join point. In grafts of fruit species, the reabsorption demarcation line begins two to three weeks after grafting (Parkinson et al., 1987). Oda (2002) reported in pea (Pisum sativum) grafts, the reabsorption of the demarcation line at four DDI.
In this paper, it was observed that at 5 DDI the join point is not yet fully filled, starting from the center towards the peripheries (Figure 2a and 2b). However, the initiation of the formation of new vascular tissue was observed. Which coincides with that reported by Lee et al. (2016), who found a 50% functionality of vascular tissue at 5 DDI. Meanwhile, Hartmann et al., 2002, indicated that the formation of callus tissue occurs in both materials (rootstock/graft), filling the spaceof the inter phase and from these parenchymal cells it begins the formation of new vascular tissue.
At 10 DDI, even when the binding line was present, vascular redifferentiation was notorious, establishing the continuity of xylem elements between rootstock/graft (Figure 3a and 3b). Turquois and Malone (1996) found an increase in the hydraulic connection at 5 DDI, in other herbaceous grafts, such as that reported by Oda (2002) in peas, the xylem connection occurred at 7 DDI and phloem occurred at 8 DDI.

Figure 3 a) 30 μ cross section (10 DDI); b) 15 μ cross-section (10 DDI); P= rootstock; I= graft; --- vascular redifferentiation zone.
Figure 3a shows the vascular continuity, noting that in the rootstock/graft interphase phase the xylem elements were oriented obliquely. From this point the graft was completely healed and therefore, the plant was functional.Which differs from that reported by Fernández García et al.(2004), who reported that a grafted tomato seedling is fully functional at 15 DDI. In watermelon (Citrullus lanatus) grafts, Yang et al. (2016) reported the complete connection at 11 DDI.
At 10 DDI, a more advanced connection of vascular tissue was observed, with seedlings able to survive without the conditions of the stall chamber; at that point the clamping clip was also withdrawn. Figure 3b shows the vascular continuity in the join region, on the sides the remains of the demarcation line can be observed. Lindsay et al. (1974) divided the development of vascular continuity in tomato grafts into two phases: first (4 DDI), intense cellular activity and increased number of tracheids in xylem and second (7 DDI), differentiation of tracheids formed in the first phase. In other solanaceous grafts such as peppers (Capsicum annuum L.), Chang et al. (2012) reported the vascular establishment at 14 DDI, time required to perform the transplant. In this paper at 15 DDI the vascular connection was observed fully developed (Figure 4). This means that the transplant can be performed from the 10 or 15 DDI, without dehydration or tissue detachment risk.The process of vascular restoration is influenced largely by the brightness in the post-grafting period (Lee et al., 2016) and the night temperature in the same stage (Yang et al., 2016).
Conclusions
For the Multifort/El Cid combination it is necessary to plant the grafting two days before, to homogenize the stem diameter, it is necessary to perform the synchronization of the stem thickness between rootstocks/grafts for each combination in order to improve the success of the attachment.
After 5 days of grafting, the entanglement of the new cells of the callus was observed between the rootstock/graft, at the same time it was observed the beginning of the reabsorption of the demarcation line at the attachment point, along with the identification of the beginning of vascular continuity, even with limited functionality. The complete connection of the vascular tissue was obtained at 10 DDI, which was also when the plants were abe to survive wihout the conditions of the camera, indicating the momento when the transpant can be performed. The results of this paper provide information on the grafting technique in tomato, as well as basic instructions for the success in obtaining grafted tomato seedlings.
Literatura citada
Andrews, K. P. and Marquez, C. S. 1993. Graft incompatibility. Horticultural reviews. John Wiley and Sons Inc. 15:467. [ Links ]
Chang, Y. C.; Chen, S.; Chiu, Y. C.; Lin, L. H. and Chang, Y. S. 2012. Growth and union acclimation process of sweet pepper grafted by a tubing-grafting robotic system. Hort. Environ. Biotechnol. 53(2):93-101. [ Links ]
Colla, G.; Rouphael, Y. and Cardarelli, M. 2006. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. Hortsci. 41:622-627. [ Links ]
Colla, G.; Rouphael, Y.; Leonardi, C. and Bie, Z. 2010. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 127:147-155. [ Links ]
Edelstein, M. and Ben, H. M. 2015. Grafting: a useful tool to increase tolerance to toxic elements in vegetables under arid and semiarid conditions. Acta Hortic. 1086:133-140. [ Links ]
Fernández, G. N.; Carvajal, M. and Olmos, E. 2004. Graft union formation in tomato plants: peroxidase and catalse involvement. Ann. Bot. 93:50-60. [ Links ]
Goto, R.; de Miguel, A.; Marsal, J. I.; Gorbe, E. and Calatayud, A. 2013. Effect of different rootstocks on growth, chlorophyll a fluorescence and mineral composition of two grafted scions of tomato. J. Plant Nutr. 36:825-835. [ Links ]
Hartmann, H. T.; Kester, D. E.; Davies, F. T. and Geneve, R. L. 2002. Plant propagation. Principles and practices, seventh ed. Prentice Hall, Upper Saddle River, NJ. 849 p. [ Links ]
Jeffree, C. E. and Yoeman, M. M. 1983. Development of intercellular connections between apposing cells in a graft union. New Phytol. 93:491-509. [ Links ]
Kubota, C. M.; McClure, N.; Kokalis, B. M. G. and Rosskopf, E. N. 2008. Vegetable grafting: history, use, and current technology status in North America. HortScience. 43(6):1664-1669. [ Links ]
Lee, J. 1994. Cultivation of grafted vegetables I. Current status, grafting methods, and benefits. HortScience. 29(4):235-239. [ Links ]
Lee, J. M. and Oda, M. 2003. Grafting of herbaceous vegetable and ornamental crops. In: horticultural reviews. John Wiley & Sons. USA, New York. 28:478 p. [ Links ]
Lee, K. M.; Lim, C. S.; Muneer, S. and Jeong, B. R. 2016. Functional vascular connections and light quality effects on tomato grafted unions. Scientia Hortic. 201:306-317. [ Links ]
Lindsay, D. W.; Yeoman, M. M. and Brown, R. 1974. An analysis of the development of the graft union in Lycopersicon esculentum. Ann. Bot. 38:639-646. [ Links ]
Oda, M. 2002. Grafting of vegetable crops. Sci. Rep. Agr. Biol. Sci. Osaka Pref. Univ. 54:49-72. [ Links ]
Parkinson, M.; Jeffree, C. E. and Yoeman, M. M. 1987. Incompatibility in cultured explant-grafts between members of the solanaceae. New Phytol. 107:489-498. [ Links ]
Pina, A. and Errea, P. 2005. A review of new advances in mechanism of graft compatibility-incompatibility. Scientia Hortic. 106:1-11. [ Links ]
Steiner, A. A. 1984. The universal nutrient solution. In: Proc. Sixth International Congress on Soilles Culture. International Soc. Soilless Culture . The Netherlands. 633-647 pp. [ Links ]
Teruo, M. and Hiromichi, H. 1994. Mineral contents in melon plants (Cucumis melo L. cv. ‘Prince’) and fruit quality influenced by grafting on squash root stocks and calcium applications in soil. Environ. Contr. Biol. 32:119-123. [ Links ]
Turquois, N. and Malone, M. 1996. Non-destructive assessment of developing hydraulic connections in the graft union of tomato. J. Exper. Bot. 47:701-707. [ Links ]
Velasco, A. M. J.; Castro, B. R.; Castillo, G. A. M.; Avitia, G. E.; Sahagún, C. J. y Lobato, O. R. 2016. Composición mineral, biomasa y rendimiento en tomate injertado. Interciencia. 41:703-708. [ Links ]
Yang, X.; Hu, X.; Zhang, M.; Jinhu, X.; Ren, R.; Liu, G.; Yao, X. and Chen, X. 2016. Effect of low night temperature on graft union formation in watermelon grafted onto bottle gourd rootstock. Scientia Hortic. 212:29-34. [ Links ]
Vitale, A.; Rocco, M.; Arena, S.; Giuffrida, F.; Cassaniti, C.; Scaloni A.; Lomaglio, T.; Guarnaccia, V.; Polizzi, G.; Marra M. and Leonardi, C. 2014. Tomato susceptibility to Fusarium crown and root rot: effect of grafting combination and proteomic analysis of tolerance expression in the rootstock. Plant Physiol. Biochem. 83:207-216. [ Links ]
Received: February 2017; Accepted: May 2017











 texto em
texto em