Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.4 Texcoco Jun./Jul. 2017
Investigation notes
Penetration pathways for a foliar fertilizer in Agave tequilana Weber var. Azul
1Centro Universitario de Ciencias Biológicas y Agropecuarias-Universidad de Guadalajara. Carretera a Nogales, km 15.5. Predio Las Agujas, Zapopan, Jalisco, México. CP. 45110. Tel. 01(33)37771150, ext. 33040. (galdinobejines@herradura.com.mx; diegonz@cucba.udg.mx; ramonrod@cucba.udg.mx).
2Posgrado en Edafología-Colegio de Postgraduados. Carretera México-Texcoco, km 36.5. Montecillo, Texcoco, Estado de México. CP. 56230. Tel. 01(595) 9520200, ext. 1262. (marinie@colpos.mx).
This research aimed to contribute to the knowledge of the penetration pathway of the foliar fertilizer 4-17-17 (NPK) in blue agave. In 1.5-year-old blue agave plants, histological sections were made to identify the main morphological characteristics of the leaf. Leaves were sprayed with the fertilizer-dye fast green solution plus naphthol blue black (F-VR-ANN) and separately with the fertilizer-dye calcofluor solution (FC). Histological sections were made at 30, 60 and 120 min after application. The activities were conducted at the Laboratory of Plant Histopathology of the Postgraduate College in Agricultural Sciences, Campus Montecillo, during the months of August and September 2013. Small vascular bundles scattered in the palisade parenchyma and spongy parenchyma were observed. The thickness of the cuticle varied according to the sampling site within the leaf, on average, 17.1 μm in the apical part and 19.1 μm in the middle part of the leaf. In the palisaded parenchyma crystals were observed, probably of calcium oxalate in the form of prisms. Evidence on the accumulation of the F-VR-ANN solution suggests that the penetration route was stomatal and cuticular, since accumulation was observed after 30 min after application to the cuticle and 120 min after in the parenchyma in palisade. Also, the application of the F-C solution suggests a cuticular and stomatal penetration, as there was fluorescence within the epidermal cells 30 min after the application and 120 min after it was observed between and within the palisade parenchyma cells. In this way, the results show that foliar fertilization may be a viable option to supplement soil fertilization.
Keywords: Agave tequilana Weber var.Azul; cuticle; fluorescence
La presente investigación tuvo como objetivo contribuir al conocimiento de la ruta de penetración de un fertilizante foliar 4-17-17 (NPK) en agave azul. En plantas de agave azul de 1.5 años de edad se hicieron cortes histológicos para identificar las principales características morfológicas de la hoja. Se asperjaron hojas con la solución fertilizante-colorante verde rápido más azul negro de naftol (F-VR-ANN) y por separado con la solución fertilizante-colorante calcofluor (F-C). Se hicieron cortes histológicos a los 30, 60 y 120 min después de la aplicación. Las actividades se realizaron en el laboratorio de Histopatología Vegetal del Colegio de Postgraduados en Ciencias Agrícolas, Campus Montecillo, durante los meses de agosto y septiembre de 2013. Se observaron haces vasculares pequeños dispersos en el parénquima en empalizada y en el parénquima esponjoso. El grosor de la cutícula varió de acuerdo al sitio de muestreo dentro de la hoja, en promedio, de 17.1 μm en la parte apical y 19.1 μm en la parte media de la hoja. En el parénquima en empalizada se observaron cristales, probablemente de oxalato de calcio en forma de prismas. Las evidencias en la acumulación de la solución F-VR-ANN sugieren que la ruta de penetración fue vía estomática y cuticular, ya que se observó acumulación a partir de los 30 min después de la aplicación en la cutícula y 120 min después en el parénquima en empalizada. Asimismo, la aplicación de la solución F-C sugiere también una penetración vía cuticular y estomática, ya que hubo fluorescencia dentro de las células epidérmicas 30 min después de haber realizado la aplicación y 120 min después se observó entre y dentro de las células del parénquima en empalizada. De esta manera, los resultados evidencian que la fertilización foliar puede ser una opción viable para complementar la fertilización al suelo.
Palabras clave: Agave tequilana Weber var. Azul; cutícula; fluorescencia
The objective of this paper was to perform a foliar characterization of the anatomy of the agave leaf in a general way and to identify the penetration pathways of a foliar fertilizer. Based on methodologies used for these purposes (Nava et al., 2003; García et al., 2013), two plants of Agave tequilana of 1.5 years old, from the company Casa Herradura, in Amatitán Jalisco, Mexico were used. Taking into account the parts of the leaf that mostly capture the applied solutions, samples were obtained from the middle and apex of fully expanded leaves, located in both the central and radial parts of the bud. The research was developed in the Laboratory of Plant Histopathology at the College of Postgraduates Campus Montecillo, Mexico, in August and September of 2013. In the first stage, the leaf structure was characterized and in the second stage the penetration pathway of the fertilizer was determined.
Leaf fragments from the apical and middle part of about 0.5 mm2 were dissected, which were cut on a freezing microtome (American Optical, model 880), to a thickness of 25 μm (Sandoval, 2005). To measure the cuticle thickness, the obtained sections were stained with Sudan IV (C24H20N4O) for 25 min and were mounted in coverslips and slides using water. Sudan IV is characterized by coloring lipids when it is solubilized (Martínez and Gragera, 2008). Also, other sections were stained with Toluidine Blue (C15H16N3S+) for 20 min to differentiate vascular bundles (D’Ambrogio, 1986). Photographs were taken with a digital camera integrated into a light microscope (Velab brand model Ve-B6); cuticular measurement was made with the Motic Images Plus 2 M1 software, considering three visual fields per section and cutting (Guerrero et al., 2008; Alejo-Plata et al., 2011).
The foliage fertilizer used was 4-17-17, based on urea, ammonium phosphate and potassium phosphate 1%, supplemented with DAP-PLUS® as surfactant. The Fast green dye (C37H34N2O10S3Na2) + naphthol blue black (C22H14N6Na2OS2) were used at a concentration of 0.05% as non fluorescent tracers for location of the fertilizer solution and calcofluor (Fluorescent Brightener 28, C40N42O10S2Na2) at 0.01% as a fluorescent tracer, these dyes were prepared appart from the fertilizer (Sandoval, 2005). The dyes were individually sprinkled with the fertilizer and the control plant was sprayed only with water.
The leaves were then sampled at 30, 60, 90 and 120 min after the leaf fertilizer was applied and 0.6*0.4 cm (Sandoval, 2005) fragments were dissected and transversely cut with a freezing microtome (American Optical, Model 880) at 25 μm thickness. The cuts were mounted on slides and coverslips. The samples were treated with fertilizer plus rapid green + black blue naftol (F-VR+ANN) dyes and were observed in a light microscope of Velab brand VE-B model and those sprayed with fertilizer plus calcofluor (FC) were observed in a Carl Zeiss fluorescence microscope to visualize penetration through fluorescence of the leaf’s anatomical structures (Nava et al., 2003; Sandoval, 2005; García et al., 2013); it should be noted that calcofluor is a non-specific fluorescent dye which binds to the cellulose and chitin of the cell walls, so that in case it penetrates into the leaf, there will be a blue fluorescence in its anatomical structures.
The results showed that the agave leaf is iso-lateral, since on the two surfaces of the lamina there were pluriestratified strata of palisade parenchyma, while in the central portion of the mesophyll parenchymal cells of isodiametric appearance were observed, among which the vascular bundles were distributed. In the photomicrograph A (Figure 1) (10 X), the arrows show the position of the vascular bundles in the central portion of the mesophyll (PCM) and the fibers surrounding the vascular bundles, it can also be observed that the leaf is amphistomatic. Photomicrograph B (Figure 1), shows the smaller diameter vascular bundles at the inner border of the palisade parenchyma (PE) and at the central part of the mesophyll (PCM) (40 X).
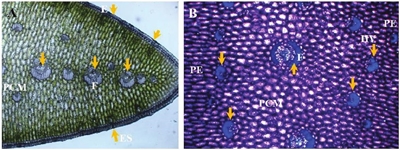
Figure 1 Photomicrographs of cross section of agave leaf. A= vascular bundles in the central portion of the mesophyll (PCM), fibers (F), epidermis (E) and stomas (ES). B=vascular bundles (HV) at the mesophile internal border (arrows), fibers (F), central portion of the mesophile (PCM), palisade parenchyma (PE).
On the other hand, both photomicrographs show that the epidermal tissue was formed by a layer of slightly elongated cells in a perpendicular direction to the leaf surface; no plastids were observed, which are cell organelles characteristic of plants in which lipids, proteins, pigments, etc, depending on the type (chloroplasts, amyloplasts and proplasts) accumulate, and which is a typical characteristic of plants adapted to aridity conditions (Nobel, 1988). Photomicrograph A of Figure 2 shows that the outer tangential wall had a thick cuticle (C) projected towards the periclinal walls and on a layer of epidermal cells (EC).
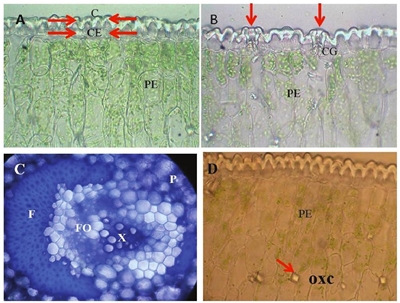
Figure 2 Photomicrographs of cross section of agave leaf A= unstratified epidermis with ordinary cells without plastids; cuticle (C), epidermal cell (CE), palisade parenchyma (PE). B= stomatal apparatus; guard cell (CG), palisade parenchyma (PE). C= vascular tissue; fibers (F), parenchyma (P), xylem (X), phloem (FO). D= palisade parenchyma (PE) where prismatic structures are indicated, which may be crystals whose probable chemical composition is calcium (OXC).
The guard cells (CG) were distributed slightly lower than the rest of the ordinary epidermal cells, as shown in photomicrograph B (Figure 2). Apparently, the cells of the palisade parenchyma (PE) had more chloroplasts than the parenchymal cells of the central region of the lamina (photomicrographs A and B, Figure 2). Each vascular bundle showed a large number of fibers (F) and was surrounded by thin-walled parenchymal cells (P), as well as xylem (X) and phloem (FO) (photomicrography C, Figure 2); there are also abundant prismatic structures that probably correspond to calcium oxalate crystals (photomicrograph D, Figure 2).
The images above show how agave leaf has a thick cuticle, with waxy coating and an accumulation probably of calcium oxalate shaped bodies, allowing to isolate the leaf surface and impenetrable to gases such as CO2 and water vapor, as well as protection against adverse environmental agents. It can also be noted that the cells contain large vacuoles for the accumulation of organic acids and water.
In the mesophyll, in palisade cells, what were probably prismatic crystals of calcium oxalate (Figure 2, photomicrograph D) were observed. In plants of 1.5 years old, the average thickness of the adaxial cuticle varied according to the region where the sample was taken: at the apex it was 17.1μm, while in the middle part it was 19.1μm.
Regarding the penetration routes, 30 minutes after the application of foliar fertilizer with rapid green and naphthol black blue (F-VR-ANN), the F-VR-ANN accumulated in the cuticle, which is a layer found above the epidermis (E) (photomicrograph A, Figure 3), at 60 minutes in the ostioles of the stomata (ES) (photomicrograph B, Figure 3), and at 90 and 120 min in the stomatal chamber and parenchymal cells in palisade (PE), respectively (photomicrographs C and D, Figure 3). The use of rapid green and black blue Naftol dyes as tracers allowed to observe its accumulation in the stomata and in the cuticle, to later find them in the parenchyma cells, with these evidences it can be pointed out that the solutes penetration is via stoma and cuticular; however, it is necessary to perform more tests for more consistent information about these penetration pathways in Agave tequilana.
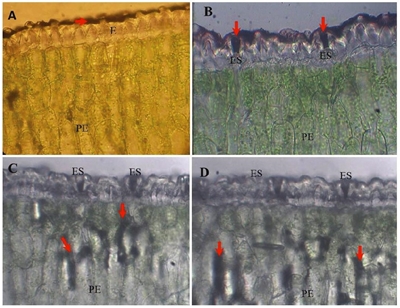
Figure 3 Photomicrographs of cross-sections in agave leaves sprinkled with 4-17-17 fertilizer plus rast green and naphthol lue black. A= the arrow indicates the presence of fertilizer at 30 minutes after application to the leaf surface; epidermis (E), palisade parenchyma (PE). B= at 60 min the fertilizer was placed in the stomates (ES), palisade parenchyma (PE). C= at 90 min the fertilizer was located in the palisade parenchyma (PE), stomata (ES). D= at 120 min the fertilizer remained at the same place; stomata (ES), palisade parenchyma (PE).
In the application of the foliar fertilizer with calcofluor (FC), in the photomicrographs A and B of Figure 4, cross sections of leaf are shown, seen under the light and fluorescence microscope respectively, the cuticle(C) and the palisade parenchyma (PE) are shown. At 30 minutes after sprinkling, the F-C accumulated in the cuticle after the application and there was little fluorescence in the mesophile cells (photomicrography C, Figure 4). At 60 min, fluorescence was observed in the epidermal cells (photomicrograph D, Figure 4). At 90 min after application, a slight increase of fluorescence was observed in some mesophile cells (photomicrograph E, Figure 4); and at 2 h, in all mesophyll tissues, including the sub-static chambers (photomicrograph F, Figure 4).
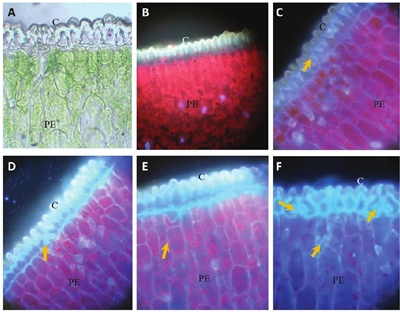
Figure 4 Photomicrographs of cross sections in agave leaves sprayed with 4-17-17 fertilizer with calcofluor. A= leaf section without fertilizer application observed with light microscope; cuticle (C), palisade parenchyma (PE). B= leaf section without fertilizer application observed with fluorescence microscope; cuticle (C), palisade parenchyma (PE). C= Arrows point fluorescence where fertilizer plus calcofluor is found at 30 min, cuticle (C), palisade parenchyma (PE). D= 60 min; cuticle (C), palisade parenchyma (PE). E= at 90 minutes; cuticle (C), palisade parenchyma (PE). F= at 120 min after application; cuticle (C), palisade parenchyma (PE).
Stomas are found in both the bundle and the underside of the leaf and the larger vascular bundles are found in the central portion of the mesophyll, while the smaller bundles in the inner border of the palisade parenchyma. In the palisade parenchyma, the probable presence of crystals, possibly of calcium oxalate, was observed in the form of prisms.
There are differences in the cuticle thickness, which varies depending on the region of the leaf; in the apical region with an average value of 17.1 μm, while in the middle part it was 19.1 μm.
Conclusions
Penetration of the fast-green fertilizer solution + naphthol black blue through the stomata was observed and subsequently visualized in the parenchyma. With the fertilizer-calcofluor penetration of the solution was observed, as it practically stained the cuticle, the stomata, the epidermis, the palisade cells and the cells of the spongy parenchyma.
Literatura citada
Alejo, P. C.; Gómez, J. L. y Salgado, U. I. H. 2011. Edad y crecimiento del dorado Coryphanea hippurus, en el Golfo de Tehuantepec, México. Revista de Biología Marina y Oceanografía. 2(46):125-134. [ Links ]
D´Ambrogio, A. A. 1986. Manual de técnicas de histología vegetal. Ed. Hemisferio Sur, Argentina. 83 p. [ Links ]
García, G. V.; Rodríguez M. M. N.; Valdovinos P. G.; Pedraza S. M. E.; Trejo T. L. I. y Soto H. M.; 2013. Rutas de la penetración foliar en la fertilización de la orquídea Cymbidium sp. (Orchidaceae). Rev. Mex. Cienc. Agríc. Pub. Esp. Núm. 5. [ Links ]
Guerrero, C. B.; Ramírez, S. H. U.; Varela, O. R.; Mondragón, E. J. D.; Meléndez, R. J. L.; León, C. J. M. y López, A. M. 2008. Evaluación del sellado apical de sistemas resinosos en la obturación de conductos radiculares: “estudio in vitro”. Acta Odontológica. Venez. 1(48):1-11. [ Links ]
Martínez, R. R. y Gragera, R. R. M. 2008. Fundamentos teóricos y prácticos de la Histoquímica. Vol.40 de Textos universitarios; Ed. CSIC-CSIC Press; España. p. 425-426. [ Links ]
Nava, S. R.; Almaguer, V. G.; Pérez, G. M.; Maldonado, T. R. y Cárdenas, S. E. 2003. Fertilización foliar en cebolla. Revista Chapingo. Serie Horticultura. 10(2):159-163. [ Links ]
Nobel, 1988. Environmental biology of agaves and cacti. Cambridge University Press. p. 40-41. [ Links ]
Sandoval, Z. E. 2005. Técnicas aplicadas al estudio de la anatomía vegetal. Instituto de Biología. Colección: Cuadernos del Instituto de Biología. Universidad Nacional Autónoma de México. p. 64-66. [ Links ]
Received: April 2017; Accepted: May 2017











 texto em
texto em 


