Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.4 Texcoco jun./jul. 2017
https://doi.org/10.29312/remexca.v8i4.14
Articles
Determination of lysine, tryptophan and protein in germinated of creole and QPM maize
1Instituto Tecnológico de Roque. Carretera Celaya-Juventino Rosas, km 8. Celaya, Guanajuato, México. CP. 38110. (garcia-2956@hotmail.com; frcervantes@itroque.edu.mx; ceaguirre@itroque.edu.mx; drjgrp2004@yahoo.com.mx; grodriguez263@hotmail.com; francisca790201@hotmail.com.
The objective of this research was to determine the lysine, tryptophan, protein and yield of green fodder in maize QPM and creole. The research was carried out at the CINVESTAV and Technological Institute of Roque, Celaya, México, in 2014. Two maize genotypes, one with high protein content (QPM) and a normal one, were evaluated. The production of green fodder was carried out according to the FAO method (2001). Samples were taken at 9, 11, 13, 15, and 17 days after sowing and seedling height, fresh weight, dry matter, lysine, tryptophan, protein and forage yield were taken as variables. The yield of green fodder reached its highest values at 17 days, creole 25.4 kg m-2 and QPM 27.14 kg m-2. For the lysine and tryptophan content the highest value was determined at 13 days in both genotypes, 0.55 and 1.17% and 1.18 and 0.56%, respectively. It was also concluded that the QPM maize protein was higher at 17 days with a value of 19.86% and for creole maize at 15 days with 14.45%.
Keywords: fodder; genotype; high protein content maize; yield
El objetivo de la presente investigación fue determinar la lisina, triptófano, proteína y rendimiento de forraje verde en maíz QPM y criollo. El trabajo se realizó en el CINVESTAV e Instituto Tecnológico de Roque, Celaya, México, en el año 2014. Se evaluaron dos genotipos de maíz, uno de alto contenido de proteína (QPM) y otro normal. La producción de forraje verde se realizó de acuerdo al método de la FAO (2001). Se realizaron muestreos a los 9, 11, 13, 15, y 17 días después de la siembra y se tomaron como variables la altura de plántula, peso fresco, materia seca, lisina, triptófano, proteína y rendimiento de forraje. El rendimiento de forraje verde alcanzó sus valores más altos a los 17 días, el criollo 25.4 kg m-2 y QPM 27.14 kg m-2. Para el contenido de lisina y triptófano el valor más alto se determinó a los 13 días en ambos genotipos, 0.55 y 1.17% y 1.18 y 0.56%, respectivamente. Asimismo, se concluye que la proteína del maíz QPM fue más alta a los 17 días con un valor de 19.86% y para el maíz criollo a los 15 días con 14.45%.
Palabras claves: forraje; genotipo; maíz de alto contenido de proteína; rendimiento
Introduction
At global and national levels, maize cultivation occupies the first place in planted area (FAO, 2015). The US Department of Agriculture (USDA) in June 2015 estimated global production of 989.3 million tonnes. México has the seventh place with a production of 2.3% (USDA, 2015). Guanajuato state has a large area for maize grain production; however, the state is considered as a semi-arid region with severe water shortage problems; it is worth mentioning that of the 370 742 ha planted previously, 68.23% is rainfed and 31.77% is irrigated (SIAP, 2015).
The importance of maize is that per capita consumption in the country is 330 g day-1, with a contribution to nutrition of 32 to 55% protein. Corn in the rural sector contributes 65% of calories to nutrition, 53% of proteins, 69% of calcium, 51% of iron, 15% of vitamin A, 62% of thiamine, 36% of riboflavin and 54% of niacin (SAGARPA, 2014). The maize protein, and in general that of cereals, is of low nutritional value compared to animal origin protein. This deficiency is the result of an imbalance of amino acids and a low protein content (Azevedo et al., 2006). In maize the highest amount of protein (75-85%) is found in the endosperm and has deficient two essential amino acids lysine and tryptophan (Huang et al., 2004). High-quality protein maize (QPM) are cultivars carrying the opaque-2 gene, which promotes a reduction of zein content and increase of glutelin. They duplicate the amounts of lysine and tryptophan, making it a maize with higher nutritional value than conventional maize (Ortega et al., 1986).
In this sense, maize germination is considered as a simple and low cost method to improve the quality of protein matter and nutritional grain value, since in the early stages of maize germination the concentration of lysine and tryptophan increases considerably. In germinated maize, there is a 19% increase in lysine and tryptophan concentration, and an overall modification of their chemical composition during the grain germination process (Arámbula, 2015).
In México, forages account for approximately 50% of the total feed ration of cattle. In this area there are better possibilities of reducing production costs, through the use of more productive and higher quality forages. A good alternative for México is the cultivation of hydroponic green forages of maize. In terms of fodder, it is necessary that it has a good quality for silage, where the neutral detergent fiber (NDF) is involved, for this, it is necessary to know the ideal stage for its cutting (Herrera and Saldaña, 1999).
Therefore, the identification of the nutritional quality of the grain and forage in breeding programs requires the integral analysis of fast and reliable methods that allow to compare adequate parameters in the largest possible number of samples. Among the parameters that can measure the nutritional value of maize, the quality of the protein (Poey, 1978) stands out, which is why the bromatological analysis is so important. This information would allow the maximum information, in order to establish possible selection criteria. Regarding to the forage is necessary to make a proximal analysis, which would help in the nutritional content.
On the other hand, Llanos (1984) indicates that almost all forage plants are cultivated exclusively to take advantage of their stems and leaves, whereas their seeds usually lack of nutritive value that justifies its use. Forage maize is an exception, especially the cultivars that reach the maximum carbohydrates yield after they bloom. In addition, when the grain is milky, the leaves and stems are still green and the whole plant then has a high nutritional value for livestock.
In response to the high costs of animal feeding, used for the production of meat and milk, new feeding techniques have been evaluated at low cost and with high nutritional value such as the use of hydroponics. Which is a production technology of vegetal biomass obtained from the initial plants growth by the germination from viable seeds and by its time no longer than 16 days. In addition, the use of hydroponics or the production of hydroponic green forage (FHV) has the advantage of being obtainable at any time of the year and in any geographic location, the FVH could solve the problem of the high feeding cost, which represents the 70% of the costs in animal production (Less, 1983).
Maize has the highest conversion rate to meat, milk and eggs, due to its high content of starch and low fiber concentration, it is an excellent source of energy. In tropical countries, on average, the same percentage (44%) is used for fodder than for human consumption. Generally, the whole or milled grain of yellow maize is preferred for cattle feeding (Paliwal, 2001).
Hydroponic green forage is a production technology of vegetal biomass obtained from the initial plants growth in the states of germination and early growth of seedlings from viable seeds. The forage obtained has high digestibility, nutritional quality and is very suitable for animal feed (Palomino, 2008).
Hydroponic green forage is the result of the germination process of cereals grains or legumes (barley, maize, sorghum, soybeans, etc) which takes place in a period of 9 to 15 days, capturing energy from the sun and assimilating minerals dissolved in a nutrient solution. Hydroponic forage is included in a new production concept, since it does not require large land extensions, long production periods or conservation and storage forms. This fodder is destined for the feeding of dairy cows, passage and race horses, whose, sheep, etc (Tarrillo, 2007).
An alternative is the production of FVH to prevent productive losses (abortions, weight loss, low milk volume, fertility problems) especially at the small producers level (FAO, 2001). It has been shown that it is possible to partially substitute the dry matter provided by the forage obtained by conventional methods, as well as that from dry grains or concentrated feed, by its equivalent in FVH. Milk stables that supply about 18 to 20 kilos of FHV per head have been reported to have increased their milk production from 10 to 12.5%, fertility, reduced incidence of mastitis and reduced abortion rate, also the cost of feeding per head has decreased (Rodríguez, 2003).
In the FHV production system, water losses due to evapotranspiration, surface runoff and infiltration are minimal compared to conventional production conditions in forage species, whose efficiencies range from 270 to 635 liters of water per kg of dry matter. For the production of 1 kilo of FVH, 2 to 3 liters of wáter are required with a percentage of dry matter that varies, depending on the forage species and the variety, between 12 and 18% (Lomeli, 2000). This translates into a total consumption of 15 to 20 liters of water per kilogram of dry matter obtained in 14 days.
In this sense, Mendoza et al. (2007) mention that in México there is a great genetic diversity of maize, and the various national and international breeding programs constantly release materials. However, the added value of protein has not been considered until today as important to nutrition. Therefore, studies on fodder quality are required because lysine and tryptophan are the limiting amino acids in protein quality. In addition, high-cost soy protein is considered in the diets of cattle and other animals, with QPM maize part of the protein and economic situation is solved, since the cost of maize with high protein content is the same than common grain.
Therefore, the objective of this research was to determine the significant changes in lysine, tryptophan, protein and yield in normal maize and QPM maize through germinate-seedling sampling.
Materials and methods
This research was carried out at the Centro de Investigación de Estudios Avanzados (CINVESTAV), Querétaro Unit, located in Juriquilla, Querétaro and in the biotechnology laboratory of the Technological Institute of Roque, located in Celaya, Guanajuato. Two maize genotypes were used: as standard, a) western creole (red grain), plant height 3.6 m, physiological maturity 132 days, with 12 rows per cob of the Instituto Tecnológico de Roque (ITR) and b) QPM hybrid (of high protein, white grain) of the maize program of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) Bajío Experimental Field, located in Celaya, Guanajuato.
Green fodder production was done according to the method reported by the Food and Agriculture Organization of the United Nations (FAO) (2001), with some modifications (seedlings were watered 4 to 6 times per day depending on the climate, first irrigation was at 8:00 am and the last irrigation at 4:00 pm. If the weather was very hot, up to 6 irrigations were applied).
Previously a germination test was performed obtaining for the genotype of Creole maize 96%, while for the QPM maize genotype was of 94%.
A density of 300 g of seed/tray (0.53 m*0.27 m) was distributed in plastic trays for both evaluated maize genotypes (QPM and normal or creole). Trays were placed on shelves and continuous irrigation with bottle water was applied during the first nine days of growth at the rate of 4.2 L m-2. In order to measure the studied variables, different samples were taken, the first one was at 9, then at 11, 13, 15 and 17 days after sowing in both genotypes.
Some components of fresh weight were evaluated, such as, seedling height, that was evaluated measuring from the seedling base until the vegetative shoot apex. In each treatment six plants were identified, its height was expressed in mm, the reading was performed every other day until day 17 of growth. The stem diameter was measured randomly with a vernier diameters of 6 plants in mm. The total number of leaves was counted from the beginning of the main stem to the fifth sample (9, 11, 13, 15, and 17 days). The total fresh weight of the seedlings in grams was also recorded, from day nine until day 17.
To determine the percentage of dry matter (DM), portions of 250-350 g of green forage were taken per tray and placed in a pre-weighed paper bag. Samples were then placed in a forced air oven at 60 °C for 72 hours. After three days, the samples were weighed and the weight of the bag was subtracted to obtain the net dry weight. Finally, the variable was calculated using the following formula: (%) MS= ((net dry weight)/(fresh weight))*100. The biomass yield was the ratio of kg of produced forage/kg of used seed, obtained by dividing the net weight of FV per tray between the amount of seed used per tray (0.3 kg), both data expressed in kg.
The formula used was kg FV/kg seed= kg fresh FV per tray /0.3 kg. The yield of fresh biomass (kg fresh weight/m2) corresponded to the production (kg) of fresh FV, obtained in 1 m2. It was calculated by dividing the net weight of material per tray between the container area (0.53 m*0.27 m= 0.1431 m2); that is; fresh biomass= kg FV per tray/0.1431 m2 and dry biomass (kg dry weight m-2), referred to dry weight (kg) of FV produced in 1 m2. It was obtained by multiplying the fresh biomass by MS %. That is: dry biomass= fresh biomass (kg fresh weight/m2)*(%) (MS/100).
Proximal chemical analyzes were performed on finished products using the following techniques: for protein it was used 46-16 (AACC, 1995), fat, 30-20 (AACC, 1995), moisture, 40-16 (AACC, 1995), ashes, 08-01 (AACC, 1995).
The tryptophan determination was by the Opienska-Blauth method modified by Hernández and Bates (1969); Hartcamp et al. (2000). The sample of defatted and pulverized 80 mesh was mixed with a papain solution, incubated for 16 h at 63 ±2 °C, cooled and centrifuged at 2500 rpm for 5 m, 1 mL of hydrolyzate was transferred into a solution of ferric chloride and sulfuric acid, then incubated at the above temperature, allowed to cool and the reading was taken at 560 nm to calculate on a standard curve the amount of tryptophan, relative to the protein.
Lysine was analyzed by the method of Tsai et al. (1975), and modified by Villegas (1984). The procedure consisted in preparing a hydrolysed sample of maize with papain at 65 ±2 oC for 16 h, once the sample was cold, 1 mL was placed into a solution of carbonate and copper phosphate, shaken for 5 min and centrifuged at 2 000 rpm for 5 min, 1 mL of the supernatant was taken to mix it with 0.1 mL of 2-chloro-3,5-dinitropyridine solution. It was then stirred vigorously, and allowed to stand for 2 h, shaken every 30 min, hydrochloric acid was added and the mixture was stirred again until homogenized. Subsequently ethyl acetate extracting solution was added, inversion mixed for 10 times and the upper phase was extracted, repeating three times, reading at 390 nm against a blank, then the lysine content was calculated with base on a standard curve to report it according to the estimated protein.
Statistical analysis of the results was performed using the statistical package SAS version 9.0, where data were subjected to a variance analysis with a completely random statistical design with factorial arrangement, being factor A maize and factor B, the samplings of 9, 11, 13, 15, and 17 after sowing. The comparison of means was performed according to the DMS test (p≤ 0.05).
Results and discussion
The analysis of variance showed significant differences at 1 and 5% probability in the source of variation for seedling height, stem diameter, fresh seedling weight, dry matter, and biomass yield, except leaf number. For the samples source differences for all evaluated variables were found; however, for the interaction there was significance in the leaf width variable, the rest were not significant. These differences found in the different variables are mainly attributed to the genetic constitution or diversity that exists between QPM maize and creole maize, in addition of the influence on samples of the phenological crop stage.
The behavior of the two evaluated genotypes is shown in Table 1, where QPM maize was higher in all variables, except for seedling height. This response is due to the fact that the creole cultivar is more precocious, that is to say its growth and development speed is much faster, in order to an early development of its phenological stages.
Table 1 Means comparison of green forage yields and its components under greenhouse conditions in QPM and Creole maize in Celaya, Guanajuato, México, 2014.
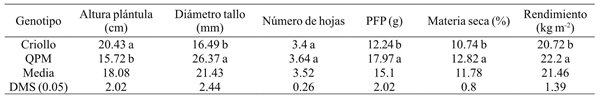
DMS= diferencia mínima significativa. Valores que comparten la misma letra en la misma columna son estadísticamente iguales. PFP= peso fresco de plántula.
Among the most important forage yield components are dry matter and biomass yield, where the QPM genotype was superior for both traits with values of 12.82% and 22.2 kg m-2 with respect to the creole cultivar that showed yields of 10.74% and 20.72 kg m-2 and 10.65%, respectively. These results are superior to those reported by Vargas (2008) who report a production of 17.2 kg with 4 kg of seed in 720 cm2 trays. Also, Tarrillo (2007) mentions that 1 kg of seed can produce a forage mass of 6 to 8 kg and Elizondo (2005) reports that from 1 kg of seed, up to 9 kg of biomass can be harvested.
Table 2 shows the average values of seedlings height at harvesting from 9 to 17 days of age. According to the DMS test, the T17 treatment reached the highest value with 21.76 cm and 27.96 cm in QPM and creole genotypes. For stem diameter are shown values of 24.73 mm for creole maize in the last sampling, while QPM genotype shows an average of 31.85 mm. For leaves number an average of 4.8 was recorded in the creole genotype, while for the QPM genotype its average was 5. According to Sheehy and Cooper (1973) the number of leaves and foliar architecture vary among plants, within the same species, as well as between varieties.
Table 2 Average yield of green forage and its components in two maize types under greenhouse conditions in Celaya, Guanajuato, México in 2014.
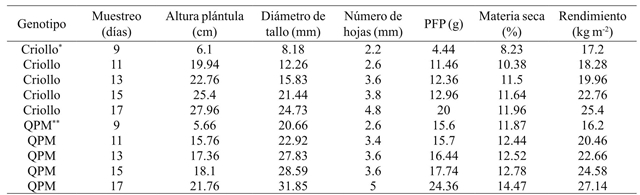
*Criollo= maíz normal; **QPM= maíz de alto contenido de proteína; PFP= peso fresco de plántula.
The number of leaves is directly related to the efficiency of interception and absorption of photosynthetically active radiation, as well as to the spatial organization of leaves and angle of leaf insertion (Moreira et al., 2005), making it an importan morphological variable, as it is frequently used for topological arrangements in crops, to determine the ideal population density, to intercept the maximum solar radiation, and to reduce competition for light (Oliveira et al., 2010). Regarding to fresh seedling weight, the QPM genotype reached an average of 24.36 g and the creole cultivar of 20 g.
In dry matter, the creole genotype showed an average of 10.74% with respect to the QPM genotype that showed 12.82%. Dry weight increased as seedling development progressed, obtaining the highest values at 17 days. Results that coincide with Teixeira et al. (2009) who report that with the plant maturity, the dry weight increased. A significant increase in dry weight was also found by increasing the harvest days in both genotypes.
In green forage yield, the creole genotype reported an average of 20.72 kg m-2 while for QPM maize an average of 22.2 kg m-2. These results were similar to those from Bayardo (2006), who points out that the yield of fresh forage depended to a greater extent on the harvest day and the genotype fertilization. Based on the normal development of any plant, as age increases, growth increases until reaching an asymptotic value. Therefore, if the seed quantity is uniform, this variable will be higher as plant gets older. The values of these results are superior to those reported by Rodríguez (2003); Castro (2006); Izquierdo (2003), who established relationships of 10, 6, and 9 kg of green forage/kg of seed, respectively, to the 14 and 15 days, although the last author reported it at 9 days.
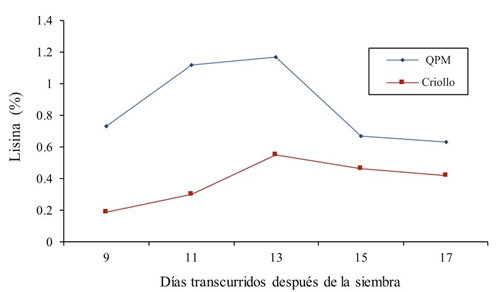
Figure 1 shows the values of the genetically contrasting genotypes. For the lysine content in green forage, in the first treatments it was shown that in both cultivars of QPM and creole maize there is an increase in the lysine percentage, that is to say, at 9, 11 and 13 days after sowing, values of 0.73, 1.12 and 1.17% for QPM maize were found and for the creole genotype the data are 0.19, 0.3 and 0.55% of lysine, later in the samples of 15 and 17 days a decrease in the two maize types was observed. In this regard, Dale (1997); Arano (1998) report values of 0.29% of lysine in grain.
The mean through sampling was 0.864 and 0.384 for QPM and creole, respectively; which means a 43.43% superiority of the improved maize. In this regard, Arámbula (2015) reports a 16% increase in lysine in pregerminated maize. In this sense, Mendoza et al. (2006) in a lysine and tryptophan bioavailability study in high protein quality and normal maize report that lysine in QPM grain was superior from 45 to 66% with respect to the normal variety. They also concluded that for the genotype Opaco-2 58.74% of lysine was found, characteristic strongly influenced by genotype, population density and nitrogen fertilization. In this sense, Poehlman and Allen (2003) report that the main advantage of QPM maize over Creole maize is that it contains approximately twice as much lysine and tryptophan, essential amino acids for human and animal nutrition. On the other hand, Fufa et al. (2003) report that QPM maize contains between 30 and 82% more lysine than normal maize. Likewise, higher values of arginine, tryptophan, histidine, threonine, cysteine and valine are also reported by Dale (1997) with 0.29% of lysine in grain. All these results are inferior to those found in this research.
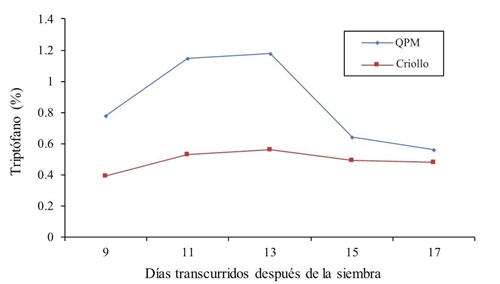
The results of tryptophan percentage through the five vegetative stages are shown in Figure 2. Such as in lysine, the tryptophan percentage is higher in all samples for QPM maize versus creole maize. The same behavior pattern was observed at 9, 11 and 13 days after sowing in both genotypes. There is an increase in this aminoacid content the growth and development vegetative days pass, however, at 15 and 17 days of sampling there is a decrease in the cultivars tryptophan content.
The average of the five samples shows a mean of almost doublé of this amino acid in QPM (0.862) compared to normal maize (0.49), which represents a 56.84% superiority. These results agree with Ortega et al. (2001) who mention that the expression of the Opaque-2 gene doubles the amounts of lysine and tryptophan, making it a maize with higher nutritive value than normal maize. On the other hand, Mendoza et al. (2006) showed results of 0.41 and 0.44 g for grain and endosperm in normal maize and also add that the QPM maize for this characteristic surpasses in 46 and 73%, respectively. Poey (1978) also found 0.85 g of tryptophan in 100 g of protein in QPM maize and 0.45 in the creole control. Bantte and Prasanna (2004) report that QPM genotypes showed higher protein content and that there was high significance for lysine and tryptophan between both maize types. It is also mentioned that in germinated maize there is a 19% increase in the concentration of lysine and tryptophan, and an overall modification of its chemical composition during the grain germination process (Arámbula, 2015).
Regarding the total protein in all treatments, a superiority of QPM maize versus common corn was observed (Figure 3). Values for the high protein genotype ranged from 7.49% in the first sampling at 9 days to 19.86% protein at 17 days after sowing, ie there is a linear response. In normal maize (creole) results elevated constantly 15 days, after which there was a reduction of 3.24% with respect to the previous treatment. An average protein is also shown through sampling for QPM maize of 12.56% and for normal maize of 8.172%, this indicates an increase of 34.94% for the QPM genotype. Similar results are reported by Cuevas-Rodríguez et al. (2004) who describe in their paper protein values ranging from 9.1 to 9.13%. In another similar study, Fufa et al. (2003) found that the protein oscillates from 7 to 11.8% among normal hybrids and QPMs, respectively. Mendoza et al. (2006) report from a tryptophan study a 11% of protein in QPM maize, this advantage in the percentage of QPM protein is attributed to the gene Opaque-2 (Ortega et al., 2001).
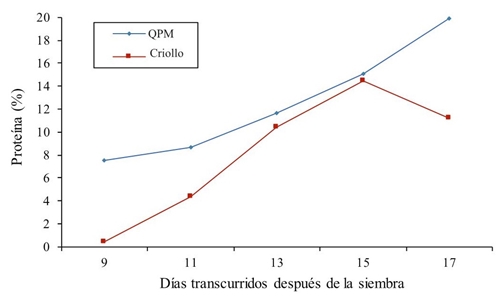
Figure 3 Protein percentage in creole and high protein (QPM) maize forage in different samples in 2014.
Figure 4 shows the forage yield, it is shown that in the sampling at 9 days after sowing the normal genotype of western maize was slightly higher than the QPM maize surpassing by 1 kg m-2, which is attributed to the precocity and emergence speed, later on samples of 11, 13, 15, and 17 days, cultivar QPM was superior, reaching yields from 20.46 kg m-2 to 27.14 kg m-2. Both genotypes peak at 17 days after planting; however, the recommendation for superiority in this research is to exploit the genetically improved material (QPM), which also showed a higher content of lysine, tryptophan and protein. In this regard, Castro (2006) and Rodríguez (2003) obtained up to 10 kg of green fodder per kilogram of seed at 14 and 15 days after sowing. Although the forage yield was higher at 17 days after sowing, the quality of lysine and tryptophan reached their maximum at 13 days in both maize types.
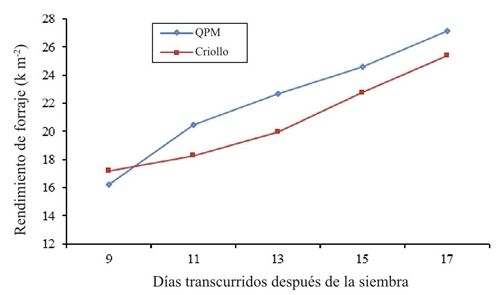
Figure 4 Average yield of forage in Creole and high protein (QPM) maize in different samples in 2014.
In relation to the protein QPM maize shows a linear response, however, normal maize presents its highest value at 15 days, in this sense Mendoza et al. (2006); Vivek et al. (2008) in a related research mention that the protein content shows a moderately strong, positive and highly significant correlation with lysine and tryptophan content, so an increase in protein would also imply an increase in lysine and tryptophan. The crude protein in common maize barely reached 10% (Paliwal et al., 2001).
In relation to the crude protein Taiz and Zeiger (2003) and Muller et al. (2006) describe that the quality decrease of crude protein in the hydroponic green forage production is due to the plant maturation, because during the development of structural organs such as stems and petioles the nitrogen moves towards the youngest parts, decreasing the active biomass fraction and promotes a nitrogen dilution, but is particular to each species and is an element of high mobility in the plant and in the early stages of development is concentrated in the growth parts, it is a function of cultivation and depends on the physiological development state of the plant. Carbadillo (2005) reported 19.41% of crude protein in hydroponic green forage, a very similar value to QPM maize at 17 days after sowing.
Conclusions
The yield of green forage reached its highest values at 17 days after sowing, however, the highest lysine and tryptophan content was determined at 13 days in both genotypes. It was also concluded that the QPM maize protein was higher at 17 days with a value of 19.86% and for creole maize at 15 days with 14.45%. It should be noted that with the pregerminates or hydroforrage technique in both genotypes the amount of lysine, tryptophan and protein was improved.
Literatura citada
AACC A (pproved Methods of the Association of Cereal Chemists). 1995. 9th (Ed.). The Association, St. Paul, MN. [ Links ]
Arámbula, V. G. 2015. Elaboración de tortillas de maíz de mejor calidad nutricional mediante el uso de grano germinado utilizando el método tradicional de nixtamalización. http://www.siac.org.mx. 5 p. [ Links ]
Arano, C. 1998. Forraje verde hidropónico y otras técnicas de cultivo sin tierra. Buenos Aires. 52 p. [ Links ]
Azevedo, R. A.; Lancien M. and Lea P. J. 2006. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 30: 143-162. [ Links ]
Bantte, K. and Prasanna, B. M. 2004. Endosperm protein quality and kernel modification in the quality protein maize inbred lines. J. Plant Biochem. Biotechnol. 13(1)57-60. [ Links ]
Bayardo, P. R.; Cigales, R, M. R.; Lorenzana, S. J. G. y Urquiaga, S. 2006. Caracterización de variedades de maíz mediante 15N como marcador en tres etapas fenológicas. Rev. Fitotec. Mex. 29:13-17. [ Links ]
Carballido, C. 2005. Forraje verde hidropónico. Artículos silvoagropecuarios: forraje verde hidropónico (en línea). Chile. http://www.ofertasagricolas.cl/articulos/88. [ Links ]
Castro, A. 2006. Forraje hidropónico para alimentar cabras. MAG. Costa Rica. http://www.mag.go.cr/bibliotecavirtuaanimal/cabra.hidro.html. [ Links ]
Cuevas, R. E. O.; Milan, C. J.; Mora, E. R.; Cardenas, V. O. G. and Reyes, M. C. 2004. Quality protein maize (Zea mays L.) tempeh flour through solid state fermetation process. Lebensmittel-Wissenschaftund-Technologie. 37(1)59-67. [ Links ]
Dale, N. 1997. Ingredient analysis table: 1997 edition. Feedstuffs Reference Issue. 69(30):24-31. [ Links ]
Elizondo, J. 2005. Forraje verde hidropónico. Una alternativa para la alimentación animal. Revista ECAG Informa. (32):36-39. [ Links ]
FAO. 2001. Manual técnico forraje verde hidropónico. Oficina Regional de la FAO para América Latina y el Caribe. 70 p. http://www. fao.org./a-ah472s.pdf. [ Links ]
FAO. 2016. http://www.fao.org/worldfoodsituation/csdb/es/. [ Links ]
Fufa, H.; Akalu, G.; Wondimu, A.; Taffesse, S.; Gebre, T.; Schlosser, K.; Noetzold, H. and Henle, T. 2003. Assessment of protein nutritional quality and effects of traditional processes: a comparison between Ethiopian quality protein maize and five Ethiopian adapted normal maize cultivars. Ethiopian Health and Nutrition Research Institute. 47(4):269-273. [ Links ]
Hartcamp, A. D.; White, J. W.; Rodríguez, A. A.; Bänzinger, M.; Hernández, G. and Bates, H. 2000. Modified method for rapid tryptophan analysis in maize. CIMMYT. Research Bulletin. 13:3-6. [ Links ]
Hernández, H. H. and Bates, L. S. 1969. A modified method for rapid tryptophan analysis of maize CIMMYT. Research Bulletin. 13:3-7. [ Links ]
Herrera, Y. y Saldaña, R. 1999. La importancia de los maíces y sorgos mejorados para producción de ensilaje. In: Memorias de 2do taller de especialidades de maíz. UAAAN. Saltillo, Coahuila, México. 135 pp. [ Links ]
Huang, S.; Whitney, R. A.; Zhou, Q.; Kathleen, P. M.; Dale, A. V.; Jan, A.; Alan L. K. and Luethy M. H. 2004. Improving nutritional quality of maize proteins by expressing sense and antisense zein genes. J. Agric. Food Chem. 52(7):1958-1964. [ Links ]
Izquierdo, J. 2003. El forraje verde hidropónico (FVH) como tecnología apta para pequeños productores agropecuarios. http://www.fao.org/3/a-ah472s.pdf. [ Links ]
Less, P. 1983. Ganadería hidropónica. Agricultura de las Américas. 32(10):16-41. [ Links ]
Llanos, C. M. 1984. El maíz: su cultivo y aprovechamiento. Ed. Mundi-prensa. Madrid, España. 43-50 pp. [ Links ]
Lomelí, Z. H. M. 2000. Forraje verde hidropónico. El forraje del futuro… Hoy. Agricultura. 63:15-18. [ Links ]
Mendoza, E. M.; Andrio, E.; Juárez, E.; Mosqueda, C.; Latournerie, L.; Castañón, G.; López, A. y Martínez E. 2006. Contenido de lisina y triptófano en genotipos de maíz de alta calidad proteica y normal. Universidad y Ciencia. 22(2):153-161. [ Links ]
Mendoza, E. M.; Morán, N.; Andrio, E.; López, A.; Rodríguez, S. A. y Castañón, G. 2007. Efecto de nitrógeno y la densidad de población en el contenido de lisina en la semilla de maíz en México. Agron. Mesoam. 18(2):177-183. [ Links ]
Moreira P, A.; Marchetti M, E.; Vieira M, C.; Novelino J, O.; Gonçalves M, C. e Robaina, A. 2005. Desenvolvimento vegetativo e teor foliar de macronutrientes da calêndula (Caléndula officinalis L.) adubada com nitrogênio e fósforo Rev. Bras. Pl. Med., Botucatu. 8(1):18-23. [ Links ]
Muller, L. S.; Osmar S. M. and Augusto, P. 2006. Hydroponic millet forage: production and nourishing quality in different sowing densities and harvest ages. Cienc. Rural. 36:1094-1099. [ Links ]
Oliveira, A. P.; Oliveira, F. J. V.; Silva, J. A.; Oliveira A. N. P.; Santos R. R. e Silva, D. F. 2010. Parcelamento e fontes de nitrogênio para produção de maxixe. Hortic. Bras. 28:218-221. [ Links ]
Ortega, C. A.; Cota, A. O.; Vasal, S. K.; Villegas, M. E.; Córdoba, O. H.; Barreras, S. M. A.; Reyes, M. C. A.; Preciado, O. E. R.; Terrón, I. A. y Espinoza, C. A. 2001. H-441C, H-442C y H-469C, híbridos de maíz de calidad proteínica mejorada para el noroeste y subtrópico de México. Ed. INIFAP. Folleto Técnico Núm. 41:4-15. [ Links ]
Ortega, E.; Villega, E. and Vasal, S. K. 1986. A comparative study of protein changes in normal and quality protein maize during tortilla making. Cereal Chem. 63:446-451. [ Links ]
Paliwal, R. L. 2001. Maíz en los trópicos. Mejoramiento y producción. FAO. Producción y protección vegetal. 56-78 pp. [ Links ]
Palomino, K. 2008. Producción de forraje hidropónico. Primera edición. Empresa, editora Macro EIRL. Miraflores Perú. 5-59 pp. [ Links ]
Poehlman, J. M. y Allen, S. D. 2003. Mejoramiento genético de las cosechas. Trad. Guzmán, O. M. 2da edición. Ed. LIMUSA. México, D. F. 509 p. [ Links ]
Poey, D. F. R. 1978. Mejoramiento integral del maíz: rendimiento y valor nutritivo, hipótesis y métodos. Tesis Doctoral, Colegio de Posgraduados, Chapingo, México. 206 p. [ Links ]
Rodríguez, S. A. C. 2003. Como producir con facilidad, rapidez y óptimos resultados forraje verde hidropónico. Editorial Diana. México, D. F. 113 p. [ Links ]
SAGARPA (Servicio de Información Agroalimentaria y Pesquera de la Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). 2014. Sistema producto, maíz, producción, costos. http://www.campomexicano.gob.mx ;http://www.sagarpa.gob.mx/agronegocios/documents/ebespañol300909.pdf. [ Links ]
Sheehy, J. E. and Cooper, J. P. 1973. Light interception, photosynthetic activity, and crop growth rate in canopies of six temperate forage grasses. J. Appl. Ecol. Oxford. 10(1):239-250. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2014. http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/. [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2015. http://infosiap.siap.gob.mx/aagricola-siap-gb/icultivo/index.jsp. [ Links ]
Taiz, L. y Zerge, E. 2003. Fisiología vegetal. 3ra (Ed.). Editorial Artemed, Porto Alegre, Brasil. 720 p. [ Links ]
Tarrillo, H. 2007. Producción de forraje verde hidropónico. Arequipa, Perú. http://www.forrajehidroponico.com/art001.htm. [ Links ]
Teixeira, V. C.; Miranda, D; Coser, C.; Martins, E.; Nascimento, D. D. e Ribeiro, E. J. 2009. Producao do materia seca e valor nutritivo de pastagem de capim-elefante sob irrigacao e edubacao nitrogenada. Rev. Bras. Zoot. 38:435-442. [ Links ]
Tsai, C. Y.; Dalby A. and Jones, R. A. 1975. Lysine and tryptophan increases during germination of maize seed. Cereal Chem. 52:356-360. [ Links ]
USDA (United States Department of Agriculture-Foreign Agricultural Service). 2015. Producción mundial de granos y cereales. http://www.usda.gov/wps/portal/usda/usdahome. [ Links ]
Vargas-Rodríguez, C. F. 2008. Comparación productiva de forraje verde hidropónico de maíz, arroz y sorgo negro forrajero. Agron. Mesoam. 19(2):233-240. [ Links ]
Vivek, B. S.; Krivanek, A. F.; Palacios, R. N. y Twumasi, A. S. 2008. Breeding quality protein maize (QPM): protocols for developing QPM cultivars. CIMMYT, Mexico, D. F., Mexico. 50 p. [ Links ]
Villegas, E.; Ortega, E. and Bauer, R. 1984. Chemical methods used at CIMMYT for determining protein quality in cereal grains. Folleto Técnico. CIMMYT. México 35 p. [ Links ]
Received: January 2017; Accepted: March 2017











 texto en
texto en