Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.3 Texcoco abr./may. 2017
https://doi.org/10.29312/remexca.v8i3.40
Essays
Application of jasmonic acid as an inducer of plant resistance to pathogens
1Departamento de Parasitología Agrícola-Universidad Autónoma Agraria Antonio Narro. Calzada Antonio Narro núm. 1923. Buena Vista, Coahuila México. (elan-laredo@hotmail.com.).
2Facultad de Ciencias Químicas-Universidad Autónoma de Coahuila. Saltillo, Coahuila, México. (jose-martinez@uadec.edu.mx; anna-ilina@hotmail.com).
3Centro de Investigación en Química Aplicada. Blvd. Enrique Reyna Hermosillo, núm.140. Saltillo, Coahuila México. CP. 25294. (lourdes.guillen@ciqa.edu.mx).
Jasmonic acid (JA) is an endogenous phytohormone that regulates plants growth. This is found in many plant species and is involved in various functions including resistance and senescence, is produced by the plant after damage caused by a pathogen, which can be a microorganism or an insect, resulting in an increase in the production of resistance compounds. The natural resistance of plants to pathogens is based on the combined effects of preformed barriers and inducible mechanisms. A complex network of hormone signals has been shown to control the plant’s response to pathogen attack. Depending on the type of inducing agent, there are two types of resistance induction: biotic and abiotic, each activated depending on the damage type.
Keywords: phytohormone; jasmonates; plant growth regulator
El ácido jasmónico (AJ) es una fitohormona endógena reguladora del crecimiento de plantas. Este se encuentra en muchas especies vegetales y está involucrado en diversas funciones de resistencia y senescencia, es producido por la planta después del daño ocasionado por un patógeno, el cual puede ser microorganismo o insecto, dando como resultado un incremento de la producción de compuestos de resistencia. La resistencia natural de las plantas a patógenos se basa en efectos combinados de barreras preformadas y mecanismos inducibles. Se ha comprobado que una compleja red de señales hormonales controla la respuesta de la planta frente al ataque de patógenos. Dependiendo del tipo de agente inductor, existen dos tipos de inducción de resistencia: biótica y abiótica y se activan dependiendo del tipo de daño generado.
Palabras clave: fitohormona; jasmonatos; regulador de crecimiento vegetal
In their natural environment the attack of a pathogen on plants is of minor importance; however, agriculture has generated a culture of monoculture and implementation of various agronomic techniques which has led to an ecosystem imbalance and pests proliferation (Cruz et al., 2006).
A wide variety of plant species are affected by different pathogens, among which are: fungi, bacteria, nematodes and viruses. A single plant can be attacked by hundreds of thousands of individuals from the same pathogen class. However, although plants may suffer considerable or minor damage, many of them survive and continue their normal development and good yields (Agrios, 2002).
Natural resistance of plants to pathogens is based on the combined effects of preformed barriers and inducible mechanisms. Plants use physical and biochemical defenses against invaders (Rangel et al., 2010). Theoretically, plants possess the necessary genes to respond to the pathogen’s aggression; This response can be in a constitutive form, being present in a permanent way in the plant or not constitutive and induced, when the attack of the pest or the interaction between these is enough to trigger toxic thresholds of substances that block the installation of the pest (Riveros, 2001). A complex network of hormone signals has been shown to control the plant's response to pathogen attack.
Plant hormones are a group of small molecules of diverse chemical nature that control processes ranging from the growth and plant’s development, to its response to biotic and abiotic stress. Ethylene, jasmonic acid (JA) and salicylic acid (SA) are plant growth regulators with a well-documented role in plant response to biotic stress (Lumba and Culter, 2010). The purpose of this paper is to review the role of JA during the pathogen defense responses as well as to present the characteristics and qualities of jasmonic acid as resistance inducing hormone against the attack of pathogen in various types of plant tissue.
Main mechanisms of plant defense against pathogens
Plants are continually in contact with other organisms, in natural conditions they interact with a wide range of potentially pathogenic microorganisms. However, plants remain healthy normally due to the manifestation of various defense mechanisms (Kenneth, 2002). Vegetables have developed physical and chemical mechanisms that reduce the possibility of infection or prevent the access of pathogens to the host. There are several mechanisms involved in plants’ defense against pathogens, there are those that may present a passive or preformed (pre-existing) defense, if it is determined by already existing properties before the pathogen’s infection attempt; also called constitutive factors, or an active or induced, dynamic defense if it results from structures or substances produced in response to the penetration of the pathogen (Cruz et al., 2006).
Passive defense
Within this type are: the structural or physical barriers of plants, formed by all physical systems that are innate to the plant, without any stimulus to produce them, among which are waxes, cuticle, cell walls, size, shape and location of natural openings among others (Agrios, 2005). Preformed biochemical barriers, this mechanism includes the inhibitors that are released in order to remove or interrupt the reproduction of pathogens, just as the preexistence factors may be due to lack of biochemical substances or nutrients that allow the recognition of the plant with the pathogen, which makes it not susceptible to its infection, also substances that are capable to degrade the cell wall of the pathogens are produced in the cell, like glucanase and chitinasa, the concentrations of these substances may vary depending on the age of the plant tissue (Lundstedt and Valdés, 2013).
Active or induced defense
Active defense mechanisms, also referred to as induced resistance, are only activated in response to a pathogen attack (Collinge et al., 2001). This type of resistance is an active defense mechanism that involves changes in metabolism caused by differential gene expression. The induction of biochemical defenses in plants, caused by a pathogen attack, is activated by several mechanisms that depends on several factors, among which: plant susceptibility, type of pathogen, environmental conditions, etc, are of greater importance.
Within the response mechanisms are hypersensitivity reactions, synthesis of PR proteins (pathogenicity related), production of phytoalexins, a phenolic compounds increase, conversion of glycosides to toxic phenols to the pathogen, production of oxidizing phenols enzymes (polyphenoloxidase, peroxidase, phenylalanine ammonia-lyase) which make some phenols even more toxic and accelerate the synthesis of compounds such as lignin that strengthen the cell walls, it is also possible to activate the synthesis of other defensive system complex substances such as pectins, proteins, cyanogenic products (cyanides), enzymes inhibiting substances and toxins produced by the pathogen among others (Agrios, 2005; Robledo et al., 2012).
Resistance inductors
The induction of resistance against a pathogen is mainly based on transforming a compatible interaction into an incompatible one; that is, the susceptible to disease plant is resistant (Riveris, 2010). Inducers act on the plant by preventing or delaying the entry of the pathogen, and consequently limiting its activity in the infected tissue or organ. They have no direct effect or specific activity on phytopathogens (Gómez and Reis, 2011), several mechanisms of induction exist so far among the most outstanding are: acquired systemic resistance (ASR) and induced systemic resistance (ISR) (Camarena and Torre, 2007).
ASR is activated locally and systemically following plant infection by necrotical pathogens (virus, bacterial or fungal). ASR is characterized as a broad-spectrum resistance, ie, it confers resistance not only to the pathogen that has activated it, but also to other pathogens (Ryals et al., 1996). It has been proven that ASR is a durable resistance (active for days or weeks) in both natural and laboratory conditions, which makes it very attractive from an agronomic point of view (Molina and Rodríguez, 2008). While ISR is activated by soil pathogens that are able to colonize the plants roots.
Like ASR, ISR is a systemic, broad-spectrum resistance (conferring protection against bacteria, fungi, and some viruses), and is durable under laboratory and field conditions (Pieterse and Loon, 2004). Their similarity is based on the fact that plants, after being exposed to an inducing agent, activate their defense mechanisms both at the site of infection and in distant areas (systemic responses) in a more or less generalized way (Cavalcanti et al., 2005).
Depending on the type of inductor, there are two types of resistance induction. One where the resistance can be activated by the presence, on the plant tissue, of organisms such as fungi, viruses, bacteria, nematodes and even herbivorous insects, known as biotic induction. While the other type of induction imitates the presence of a pathogen in order to generate resistance by the presence of synthetic molecules deposited on the plant organs, called abiotic induction (Kuc, 2001). The signaling pathways of responses caused by a biotic agent may depend on both salicylic acid, in association with the accumulation of proteins related to pathogenesis (PRP), as well as jasmonic acid and ethylene, not being associated, in this case, with PRPs accumulation, known as acquired systemic resistance (ASR). In contrast, the signal cascade generated by an abiotic inductor only follows the pathway of jasmonic acid and ethylene, called induced systemic resistance (ISR) (Vallad and Goodman, 2004).
Jasminonic acid
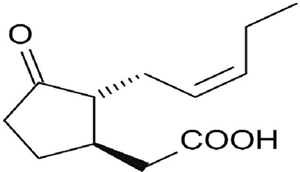
Jasmonic acid (JA), related molecules and their derivatives, all called jasmonates (JAs), are phytohormones of lipid origin with molecular structure similar to prostaglandins in animals (Farmer, 2003; Howe, 2004). They act as signal molecules of plant responses to various stress situations and participate in various growth and development processes (Avanci et al., 2010; Ting, 2014). JA is a cyclopentenone that has a pentenyl chain and a carboxylic chain (Figure 1). The chemical name and the empirical formula of jasmonic acid are cis-2-pent-2’-enyl 3-oxo-cyclopentenylacetic acid and C12H18O3, respectively (Abdala, 2006). JA is a viscous yellow oil, soluble in chloroform, ethyl acetate, acetone and ether; sparingly soluble in water, its boiling point is 125 °C/0.001 mm Hg (López, 1985).
The biosynthesis pathway, also called the octadecanoid pathway, has been extensively studied, and several enzymes involved in various steps are now known (Berger, 2002; Agrawal, 2004). The jasmonates are formed from the linoleic and linolenic unsaturated fatty acids that are released from the phospholipids of the cell membranes by the lipases action, a mechanism that occurs mainly in the leaves (Jordán and Casaretto, 2006) (Figure 2).
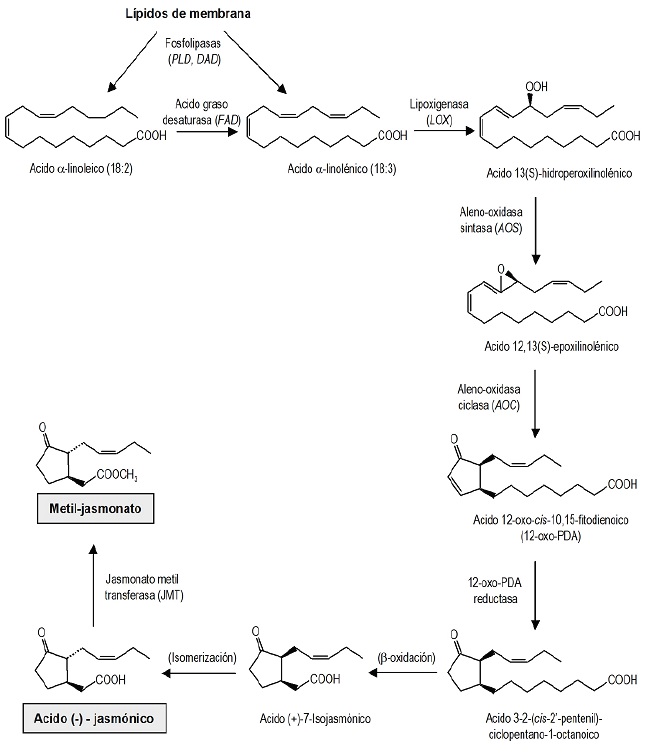
Figure 2.Biosynthesis of jasmonate. Route of octadienoic acids (18:3) which describes the formation of jasmonic acid and methyl jasmonate from membrane phospholipids (Jordán and Casaretto, 2006).
Jasmine acid is produced by the plant after damage caused by a pathogen which can be a microorganism or insects and results in increased production of resistance compounds such as salicylic acid and ethylene, among others (Chavez et al., 2012). Among stress situations that regulate it are wounds (mechanical or biotic), exposure to ozone, drought and attack by pathogens and pests. Among the developmental processes in which jasmonates participate are root growth, tuberization, fruit maturation, senescence and pollen development, bud and flower formation, among other effects (Rojo et al., 2002; Red et al., 2003; Wasternack 2007; Chunmei, 2013).
Salisbury and Ross (1992) reported that this type of compound has been found in 150 families and 206 plant species, including fungi, mosses and ferns. It has also been shown that JA causes physiological effects on plants similar to those of abscisic acid (ABA) (Schilmiller and Howe, 2005).
This organic acid is generally in the picomolar range per gram of fresh weight in leaf tissue and can rapidly increase under external stimuli. Some organs and tissues have above 10 times what is found in leaves, suggesting that these elevated levels indicate different functions in the regulation of certain developmental processes (Wasternack and Hause, 2002).
Jasmonic acid as resistance inducer against pathogens
Jasminonic acid and its derivatives are considered components of the signal transduction pathway in the defense mechanisms of plants and increases in their endogenous levels have been shown in plants subjected to stress (Fonseca et al., 2009; Yhan et al., 2013). These compounds induce the expression of genes encoding specific proteins, among which we can mention: protease inhibitors, enzymes involved in the biosynthesis of flavonoids, osmotins and lipoxygenases, and different proteins related to pathogenesis (Andrade et al., 2005). Several papers have shown that JA and JAs induce the biosynthesis of several enzymes involved in resistance reactions in plants. Among the most important are the key enzymes to produce phyloxins such as polyphenol oxidase, chalcone synthase, phenylalanine ammonium lyase (PAL) and HMG-Co-reductase (HMGR) (Jankiewicz, 2003; Leszek, 2003).
Jasmonic acid, like any other hormone, does not participate alone in the activation of the processes it regulates, but interacts with other signaling molecules (Lorenzo and Solano, 2005). A large number of interactions between JA and other hormone signaling pathways such as ethylene, AS, auxins or ABA have been described (Turner et al., 2002; Rojo et al., 2003). When the plant is injured by some pathogen, the increase in the production of ethylene is induced. This results in the formation of elicitors (consisting mainly of the elements of destroyed cell walls) (Anderson et al., 1989). These cause several reactions: that can go from hypersensitivity reactions connected with the oxidative explosion and the apoptosis of the cells around the infected place.
When the hypersensitivity reaction and the oxidative explosion are shown, the oxygen formation that can help the neighboring cells alive for the oxidation of linolenic acid, the latter being a precursor of the jasmonic acid by the octadecanoids route (Leszek, 2003). It has been observed that although JA and ethylene cooperate in the activation of defense gene expression against pathogens or ozone exposure, both hormonal signals may be antagonistic in response to phytophagous caused injuries or mechanical damage (Rojo et al., 2003).
Studies on tomato plants showed that not only the plant that produces them responds to stress, but also in neighboring plants, due to the presence of volatile methyl-jasmonate ester (Me-JA) (Ryan, 2000). It has been observed that healthy plants exposed to methyl jasmonate are able to accumulate proteinase inhibitors similarly to insect-damaged plants, suggesting that volatile methyl jasmonate is transmitted through the air. Later studies deciphered that the attack of foliar organs by herbivores caused an 18 amino acid polypeptide, the systemin, that is released and transported by the phloem to other leaves where a membrane lipase would induce an increase of the JA fatty acid precursor and this would induce the expression of genes encoding defense proteins such as the proteinase inhibitors already mentioned (Wasternack et al., 2006). The production of systemin once the damage has occurred would be induced by electric currents (Peña, 2000). Systemin has not been identified in most families, so it is thought that in these the induction of JA would occur in a more directly way.
The accumulation of the protease inhibitor by the application of JA occurs in several plants, an example of which is alfalfa and tobacco, these respond positively to the exposure of Me-JA to accumulate their respective trypsin inhibitors in the Leaves (Wasternack, 2006). On the other hand, it has been demonstrated that JA biosynthetic precursors, such as linolenic acid, also induce the protease inhibitor in leaves of various plant tissues similar to JA and Me-JA (Abdala, 2006). Schweizer (1993), observed that JA efficiently protects oats against infection by Erysiphe graminis f. sp. hordei, and similarly protects the tomato and potato plants against Phytophthora infestan. Jaiti (2009), demonstrated that JA has the capacity to induce the production of polyphenoloxidase and peroxidase in date palm seedlings, these enzymes are able to generate resistance against Fusarium oxysporum f. sp. albedinis.
The exogenous application of JA and Me-JA in a wide variety of plants such as lettuce, cotton (Omer et al., 2001), potato (Halim et al., 2006), tobacco and tomato (Thaler et al., 2001; Rohwer and Erwin, 2010) stimulated direct resistance against various kinds of insects. This direct resistance was caused by reduced fecundity, growth and survival of these insects (Stout et al., 2002). It has also been shown that the exogenous addition of these phytohormones induces the production of floral nectars with insecticidal properties, for example in cotton and Macaranga tanarius (Rodríguez-Saona et al., 2001; Heil et al., 2001; Liechti and Farmer, 2002). Soybean cultures treated with JA achieved the activation of enzymes encoding genes such as phenylalanine ammonium lyase and chalcone synthase. These proteins are involved in plant defense against pathogens, herbivores and physical and chemical stress (Rohwer and Erwin, 2008).
A growing trend is the application of JA by extracts in aqueous media on seeds, in order to generate protection of various types of crops at least ten weeks after germination (Wasternack and Hause, 2002). Similarly the treated seeds can be stored and seeded at a later stage. It was hypothesized that extracts of JA act as immunizers, thus generating protection for plants for an extended period after treatment (Wasternack, 2007).
Conclusions
Jasmonic acid is a widely studied hormone due to its participation in different physiological effects in plants, such as the induction of local and systemic resistance to disease. The JA seems to use different mechanisms to develop the induction of these responses, in addition it interacts in different ways with other hormonal signaling pathways to generate the induction of defense responses. During the last decade, it has been sought to accelerate the response of the plant to a pathogen attack and one of the alternatives for this problem could be the application of systemic resistance markers, which at the same time would generate an alternative of biological and environmental protection, as well as being compatible with current methods of pathogen control. The use of jasmonic acid is expected to allow access to a powerful new pest control agent to help plants to defend itself against its enemies. However, a series of laboratory and field investigations are still required to understand the biological behavior of this acid.
Literatura citada
Abdala, G. y Cenzano, A. 2006. Biosíntesis de jasmonatos y participación en procesos del desarrollo vegetal. Temas de fisiología vegetal. 56-87 pp. [ Links ]
Agrawal, G.; Tamogami, S.; Han, O.; Iwahashi, H. and Rakwal, R. 2004. Rice octadecanoid pathway. Biochem. Biophy. Res. Comm. 317:1-15. [ Links ]
Agrios, G. 2002. Fitopatología. Editorial Limusa, S. A. de C. V. Grupo Noriega Editores. 98-109 pp. [ Links ]
Anderson, J.; Spilatro, S.; Klauser, S. and Franceschi, V.1989. Jasmonic acid-dependent increase in the level of vegetative storage proteins in soybean. Plant Sci. 62:45-52. [ Links ]
Andrade, A.; Vigliocco, A.; Alemano, S.; Miersch, O.; Botella, M. and Abdala, G. 2005. Endogenous jasmonates and octadecanoids in hypersensitive tomato mutants during germination and seedling development in response to abiotic stress. Seed Sci. Res. 15:309-318. [ Links ]
Avanci, N.; Luche, D.; Goldman, G. and Goldman, M. 2010. Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Gen. Mol. Res. 9:484-505. [ Links ]
Berger, S. 2002. Jasmonate-related mutants of Arabidopsis as tools for studying stress signaling. Planta. 214:497-504. [ Links ]
Camarena, G. y Torre, R. 2007. Resistencia sistémica adquirida en plantas: estado actual. Rev. Chapingo Ser. Cienc. Forest. Amb. 13(2):157-162. [ Links ]
Cavalcanti, L.; Piero, R.; Cia, P.; Pascholati, S.; DeResende, M. y Romeiro, R. 2005. La inducción de resistencia de las plantas a los patógenos e insectos. FEALQ. Piracicaba. 11-153 pp. [ Links ]
Chávez, L.; Álvarez, A. y Ramírez, R. 2012. Apuntes sobre algunos reguladores del crecimiento vegetal que participan en la respuesta de las plantas frente al estrés abiótico. Cultivos Tropicales. 33:47-56. [ Links ]
Chunmei, Z. and Zhi, H. 2013. Effects of endogenous abscisic acid, jasmonic acid, polyamines, and polyamine oxidase activity in tomato seedlings under drought stress. Sci. Hortic. 159:172-177. [ Links ]
Collinge, D.; Borch, J.; Madriz, A. and Newman, K. 2001. The responses of plants to pathogens. In: Hawkesford, M. J. Buchner, p. molecular analysis of plant adaptation to the environment. Dordrecht, Holanda, Kluwer Academic Publishers. 390 p. [ Links ]
Cruz, M.; Hernández, Y. y Rivas, E. 2006. Temas de ciencia y tecnología. 29(10):45-54. [ Links ]
Farmer, E.; Almeras, E. and Krishnamurthy, V. 2003. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Current Opinion in Plant Biology. 6:372-378. [ Links ]
Fonseca, F.; Chico, J. and Solano, R. 2009. The jasmonate pathway: the ligand, the receptor and the core signalling module. Current Opinion in Plant Biology. 12:539-547. [ Links ]
Glazebrook, J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann. Rev. Phytopatol. 43:205-227. [ Links ]
Gómez, D. y Reis, E. 2011. Inductores abióticos de resistencia contra fitopatógenos. Rev. QuímicaViva. 1:1. [ Links ]
Halim, V.; Vess, A.; Scheel, D. and Rosahl, S. 2006. The role of salicylic acid and jasmonic acid in pathogen defense. Plant Biol. 19:203-208. [ Links ]
Heil, M.; Koch, T.; Hilpert, A.; Fiala, B.; Boland, W. and Linsenmair, K. 2001. Extrafloral nectarie production of the ant-associated plant, Maracanga tanarius, is an induced, indirect, defense elicited by jasmonic acid. Proc. Natl. Acad. Sci. 98:1083-1088. [ Links ]
Howe, G. 2004. The roles of hormones in defense against insects and disease. In: Davies, P. J. (Ed.). Plant hormones: biosynthesis, signal transduction, action. Cornell University, NY, USA. 610-634. [ Links ]
Jaiti, F.; Verdeil, J. and Hadrami, I. 2009. Effect of jasmonic acid on the induction of polyphenoloxidase and peroxidase activities in relation to date palm resistance against Fusarium oxysporum f. sp. albedinis. Physiol. Mol. Plant Pathol. 74:84-90. [ Links ]
Jankiewicz, L. 2003. Reguladores del crecimiento, desarrollo y resitencia en plantas. Editorial Mundi-Prensa. México. Madrid. 1:163-1190. [ Links ]
Jordan, M. y Casaretto, J. 2006. Hormonas y reguladores del crecimiento: etileno, ácido abscísico, brasinoesteroides, poliaminas, ácido salicílico y ácido jasmónico. Fisiología Vegetal. Ediciones Universidad de la Serena, La Serena. 16 p. [ Links ]
Kuc, J. 2001. Concepts and direction of induced systemic resistance in plants its application. Eur. J. Plant Pathol. 107:7-12. [ Links ]
Liechti, R. and Farmer, E. 2002. The jasmonate pathway. Science. 296:1649-1650. [ Links ]
López, R.; Dathe, W.; Miersch, O. y Sembdner, G. 1985. Determinación del ácido jasmónico en semillas inmaduras de soya glycinemax. Ciencias de la Agricultura. 23:123-124. [ Links ]
Lorenzo, O. y Solano, R. 2005. Señalización de ácido jasmónico e interacciones con otras hormonas. Biojournal.net.1. 59 p. [ Links ]
Lumba, S. and Cutler, S. 2010. Plant nuclear hormone receptors: a role for small molecules in protein-protein interactions. Annu. Rev. Cell. Dev. Biol. 26:445-469. [ Links ]
Lundstedt, J. y Valdés, R. 2013 Mecanismos de defensa de las plantas e inducción de resistencia. Boletín Técnico. Núm. 11. Departamento Técnico ASP Chile S. A. 86 p. [ Links ]
Madriz, K. 2002. Mecanismos de defensa en las interacciones planta-patógeno. Manejo Integrado de Plagas. 63:22-32. [ Links ]
Cruz, M.; Hernández, Y. y Rivas, E. 2006. Temas de ciencia y tecnología. 10:45-54. [ Links ]
Molina, A. y Rodríguez, P. 2008. Resistencia sistémica inducida: ¿una herramienta bio-ecológica. II Conferencia internacional sobre eco-biología del suelo y el compost. 43 pp. [ Links ]
Mysore, K. and Ryu, C. 2004. Nonhost resistance: how much do we know? Trends Pl. Sci. 9(2):97-104. [ Links ]
Omer, A.; Granett, J.; Karban, R. and Villa, E. 2001. Chemically-induced resistance against multiple pests in cotton. Int. J. Pest. Mgmt. 47:49-54. [ Links ]
Peña, H. 2000. Ácido jasmónico. In: Barrueto-Cid, L. P. (Eds.). Introduçao aos hormônios vegetais: 131-157. [ Links ]
Pieterse, M. and Loon, L. 2004. Curr. Opinion Plant Biol. 7:456-464. [ Links ]
Rangel, G.; Castro, E.; Beltran, E.; Reyes H y García E. 2010. El ácido salicílico y su participación en la resistencia a patógenos en plantas. Biológicas. 12(2):90- 95. [ Links ]
Rao, M.; Lee, H.; Creelman, R.; Mullet, J. and Davis, K. 2000. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. The Plant Cell. 12:1633-1646. [ Links ]
Riveris, A. 2010. Inducción de resistencia en plantas. Interacción planta-patógeno. Universidad de Tolima. 261. [ Links ]
Riveros, A. 2001. Moléculas activadoras de la inducción de resistencia, incorporadas en programas de agricultura sostenible. Manejo Integrado de Plagas. 61:4-11. [ Links ]
Robledo, M.; Lozoya, H. y Colinas, M. 2012. Inducción de defensa en papa (Solanum tuberosum L.) contra Phytophthora infestans Mont. de bar y por fungicidas. Interciencia. 37:688-695. [ Links ]
Rodríguez, C.; Crafts, S.; Pare, P. and Henneberry, T. 2001. Exogenous methyl jasmonate induces volatile emission in cotton plants. J. Chem Ecol. 27:679-695. [ Links ]
Rohwer, C. and Erwin, J. 2008. Horticultural applications of jasmonates: a review. J Hortic. Sci. Biotechnol. 83(3):283-304. [ Links ]
Rohwer, C. and Erwin, J. 2010. Spider mites (Tetranychus urticae) perform poorly on and diperse from plants exposed to methyl jasmonate. Entomol. Exp. Appl. 1:1-10. [ Links ]
Rojo, E.; Solano, R. and Sanchez, J. 2003. Interactions between signaling compounds in volved in plant defense. J. Plant Growth Reg. 22:82-98. [ Links ]
Ryals, J.; Neuenschwander, A.; Willits, H.; Molina, G.; Steiner, A. and Hunt, M. 1996. Plant Cell 8:1809-1819. [ Links ]
Ryan, C. 2000. The systemin signaling pathway: differential activation of plant defensive genes. Biochimica et Biophysica Acta 1477. 112-121. [ Links ]
Salisbury, F. and Ross, C. 1992. Plant physiology. Wasdworth Publishing Company. Fourth Edition, California, USA. 321 pp. [ Links ]
Schaller, F.; Schaller, A. and Stintzi, A. 2005. Biosynthesis and metabolism of jasmonates. J. Plant Growth Regul. 23:179-199. [ Links ]
Schilmiller, A. and Howe, G. 2005. Systemic signaling in the wound response. Current Opinion in Plant Biol. 8:369-377. [ Links ]
Schweizer, P.; Gees, R. and Mosinger, E. 1993. Effect of jasmónico acid on the interaction of barley (Hirdeum vulgare L.) with the powdery mildew Erysiphe graminis f. sp. hordei. Plant physiol. 102:503-511. [ Links ]
Stout, M.; Zehnder, G. and Baur, M. 2002. Potential for the use of elicitors of plant resistance in arthropod management programs. Archi. Insect Biochem. Physiol. 51:222-235. [ Links ]
Thaler, J.; Stout, M.; Karban, R. and Duffey, S. 2001. Jasmonate-mediated induced plants resistance affects a community of herbivores. Ecol. Entomol. 26:312-324. [ Links ]
Ting, L.; Kun, M.; Hong-Li, M.; Yang, L.; Ling, L. and Yang, H, 2014. Jasmonic acid enhancement of anthocyanin accumulation is dependent on phytochrome A signaling pathway under far-red light in Arabidopsis. Bioch. Biophy. Res. Comm. 1:78-83. [ Links ]
Turner, J.; Ellis, C. and Devoto, A. 2002. The jasmonate signal pathway. Plant Cell. 14:153-164. [ Links ]
Vallad, G. and Goodman, R. 2004. Systemic Acquired resistance and induced systemic resistance in conventional agriculture. Crops Sci. 44:1920-1934. [ Links ]
Wasternack, C. 2006. Oxylipins: biosynthesis, signal traduction and action. In: plant hormone signaling. Annual Plant Reviews. 1:185-222. [ Links ]
Wasternack, C. 2007. Jasmonates: anupdate on biosynthesis, signal transduction and action in plant stress reponse. Growth and development. Ann. Bot. 100:681-697. [ Links ]
Wasternack, C. y Hause B , 2002. Jasmonates and octadecanoids: signals in plant stress responses and development. Progress in nucleics acid. Res. Mol. Biol. 72:165-221. [ Links ]
Wasternack, C.; Stenzel, I.; Hause, B.; Hause, G.; Kutter, C.; Maucher, H.; Neumerkel, J.; Feussner, I. and Miersch, O. 2006. The wound response in tomato-role of jasmonic acid. J. Plant Physiol. 163:297-306. [ Links ]
Yhan, G.; Ying, Y.; Xiao, H.; Yu, C. and Jian, W. 2013. Imaging of jasmonic acid binding sites in tissue. Analy. Biochem. 440(2013):205-211. [ Links ]
Received: February 2017; Accepted: April 2017











 texto en
texto en