Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.3 Texcoco Abr./Mai. 2017
https://doi.org/10.29312/remexca.v8i3.30
Articles
Physical and microbiological factors in the deterioration of huitlacoche in post-harvest
1Ingeniería Agroindustrial, Parasitología Agrícola. Universidad Autónoma Chapingo. Carretera México-Texcoco, km 38.5. Texcoco, Estado de México, México. CP. 56230. (lopez.geliza@gmail.com; aperezl.dia@gmail.com; acostam14@gmail.com; mary-0613@hotmail.com; t.espinosa.s@taurus.chapingo.mx).
2Fitopatología, Colegio de Postgraduados. Carretera México-Texcoco, km. 36.5. Montecillo, Texcoco, Estado de México, México. CP. 56230. ( rojas@colpos.mx).
The huitlacoche (Ustilago maydis (DC) Corda) is a good source of nutrients in the diet. During post-harvest handling, the product shows a rapid senescence, attributed to the high rates of dehydration and respiration of the product, in addition to its composition. In order to prolong shelf life, the effect of high relative humidity and low storage temperature on the physical properties of huitlacoche (whole and in galls) and the presence of phytopathogenic fungi were evaluated. The results showed that at 3 ºC the dehydration rate decreased, maintaining the visual quality for longer, both the whole huitlacoche and in galls. Storage with high relative humidity at room temperature favored the proliferation of phytopathogenic host species such as Fusarium oxysporum, Rhizopus stolonifer and Penicillium expansum. The highest incidence and severity was shown by F. oxysporum inoculated with a wound in the gall, while R. stolonifer, although with a lower incidence than the other two species, was able to survive up to 10 ºC. The results demonstrate that the management of huitlacoche at 3 °C and high relative humidity in ambient air is feasible, as it counteracts the rate of dehydration and eliminates the risk of proliferation of host phytopathogenic fungi.
Keywords: Ustilago maydis; phytopathogenic fungi; relative humidity; refrigeration; shelf life
El huitlacoche (Ustilago maydis (DC) Corda) es una buena fuente de aporte de nutrientes en la alimentación. Durante el manejo poscosecha, el producto muestra una rápida senescencia, atribuida a las altas tasas de deshidratación y respiración del producto, además de la composición del mismo. Con la finalidad de prolongar la vida de anaquel, se evaluó el efecto de alta humedad relativa y baja temperatura de almacenamiento sobre las propiedades físicas del huitlacoche (entero y agalla) y la presencia de los hongos fitopatógenos. Los resultados mostraron que a 3 ºC la tasa de deshidratación disminuye, manteniendo la calidad visual por más tiempo, tanto del huitlacoche entero como en agalla. El almacenamiento con alta humedad relativa a temperatura ambiente favoreció la proliferación de especies fitopatógenas hospederas como Fusarium oxysporum, Rhizopus stolonifer y Penicillium expansum. La mayor incidencia y severidad la presentó F. oxysporum inoculada con herida en agalla, mientras que R. stolonifer, aunque con menor incidencia que las otras dos especies, fue capaz de sobrevivir hasta 10 ºC. Los resultados demuestran que es viable el manejo del huitlacoche a 3 °C y alta humedad relativa en el aire del ambiente, ya que contrarresta la velocidad de deshidratación y elimina el riesgo de proliferación de hongos fitopatógenos hospederos de la superficie.
Palabras clave: Ustilago maydis; hongos fitopatógenos; humedad relativa; refrigeración; vida de anaquel
Introduction
Ustilago maydis (DC) Corda is a biotrophic pathogenic basidiomycete fungus, which causes charcoal cob or huitlacoche. Its development is due to the infection caused by U. maydis in the meristematic region of the immature corn cob (Zea mays) and induces the formation of tumors full of diploid teliospores, also known as galls (Valverde el at., 1995; Salmerón-Santiago, 2011). Galls represent an interesting source of food, with good content of proteins, fiber and fatty acids such as linoleic, linolenic and palmitic (Valverde el at., 1995). Martínez-Flores el at. (2008) reported that the most abundant amino acid in huitlacoche is lysine with 27.2% of the total essential amino acids. In fact, it has been considered as a high quality functional food (Valverde el at., 2012).
Nowadays, it is sought to improve the production and conservation of huitlacoche for fresh consumption (Madrigal-Rodríguez el at., 2010; Aydogdu, 2015). However, the main disadvantage is its short shelf life, three or four days, due to its high metabolic activity, lack of cuticle and accelerated water loss, which is why it is consumed fresh and almost immediately to its collection or harvest. According to previous research, the respiratory metabolism of whole huitlacoche is high (> 300 ml CO2 kg-1 h-1) under normal storage conditions (20 ±2 ºC) and in refrigerated environments it can be reduced up to 38% (10 °C) or up to 22% (3 °C) compared to its normal environment (Martínez- Flores el at., 2008; Monroy-Gutiérrez el at., 2012).
The product is usually marketed threshed out (galls) in the local markets. During the threshing activity, the outer shell (peridium) receives compressive stresses that exceed the elastic limit of the cover, causing mechanical damage, besides exposing the individual galls to the ambient air conditions with a larger contact surface. This situation generates significant changes in respiration rate and weight loss due to dehydration. Martínez-Flores el at. (2008) report weight losses of nearly 30% in whole huitlacoche (cob) and up to 65% in galls after three days storage at room temperature. The most evident visual change in dehydrated galls is the external color change due to the rupture of the peridium and the consequent exposure of the fungus mycelium (dark color). These significant changes in dehydration suggest that the air in the storage environment is maintained at high relative humidity to minimize the gradient of moisture concentration between the air and the peridium surface, as the water diffusion rate through air depends mostly on it.
The storage of huitlacoche in high humidity chambers can be an alternative to solve the problem of dehydration; however, this condition may favor the proliferation of pathogenic flora that may be present on the fungus surface, in addition to the fact that huitlacoche contains high levels of free sugars (56.2-268 mg g-1, dry basis) (Valdez-Morales el at., 2010), such as glucose and fructose, which are a natural growth medium for microorganisms. The free sugars concentration is higher than that reported for normal and extra-sweet hybrid maize (Reyes el at., 1982) and other species such as papaya and banana (Torija el at., 1998). Therefore, in the searching for new alternatives to prolong shelf life it is important to identify the physical and microbiological factors that contribute to its rapid senescence. In this context, the objective of this paper was to evaluate the influence of conditions of high relative humidity and low storage temperature on the physical properties of huitlacoche and the presence of phytopathogenic fungi.
Materials and methods
Vegetal material
Whole corn cobs infected with the huitlacoche fungus were purchased at a Mexico City market approximately 12 hours after harvesting. In the experimental laboratory, located in the Department of Agroindustrial Engineering of the Universidad Autónoma Chapingo, a selection was made to eliminate the damaged cobs. The complete corn cob covered of the Ustilago maydis fungus and groups formed by 10 galls of huitlacoche, both randomly selected, were managed as experimental units.
Post-harvest characterization
The following treatments according with ambient air temperatura were established: a) 3 ±2 °C, b) 10 ±2 °C and c) 20 ±2 °C. For each treatment the following variables were measured over a period of 20 days. The weight was measured on an Ohaus digital electronic scale (Adventurer™ Pro AV8101, Ohaus Corporation, Pine Brook, NJ, USA). With these data the percentage of daily weight loss was calculated. Color was measured with the Hunter Lab® color gauge (Mini Scan XE Plus núm. 45/0-L, Reston, VA, USA) on the galls surface and reported on CIELab scale. Firmness of the gill peridium was measured with a Stable Micro System texture analyzer (Mod. TA-XT2i, Stable Micro Systems Ltd., Godalming, Surrey, UK) it was fitted with a rounded-tipped conical fixture of 2.6 cm diameter and 75° angle. The compression force, with a deformation distance of 5 mm and a test speed of 5 mm s-1 was measured. The results are expressed in newtons (N) (Monroy-Gutiérrez el at., 2012). Photographs were taken daily and annotations were made on visually observed changes in the gills and cobs to assess the appearance.
Diagnosis and identification of pathogenic fungi
In order to induce growth of microorganisms harboring on the surface of huitlacoche galls and cobs, samples were respectively taken and placed in moist chambers that were placed at different temperatures (3, 10 and 20 °C) until reaching the decomposition degree (loss of firmness and appearance of dark color and strange odors). The wet chamber consisted of placing a moistened paper towel with sterilized distilled water on an expanded polystyrene tray and placing the huitlacoche samples on the wet towel. Immediately, the tray was placed inside a polyethylene bag and sealed. The relative humidity in the internal environment of the bag was maintained at approximately 95%.
In the isolation stage of the microorganisms rectangular portions of the healthy and diseased part of the gills were taken 1 cm*0.5 cm each. The sections were disinfested with 1.5% sodium hypochlorite for 1.5 min. Subsequently, they were rinsed with sterilized distilled water and four cuts were seeded in each petri dish with potato agar and dextrose (PDA) culture medium. Three days after planting different growth forms and fungal, stains were observed, which were isolated and purified in Petri dishes with PDA. Isolation of the fungi was done using the hyphal tip technique using the same culture medium, where riphampicin and chloramphenicol (500 ppm) were previously added to inhibit bacterial growth (Rodríguez-Mejía, 2010). The boxes were incubated for 72 h at 25 °C. After eight days of sowing, observations were made under a microscope and monoconidial cultures were obtained. The obtained isolates were seeded in PDA and incubated at 24 °C in white light for 15 days to increase the inoculum. The identification of the isolated fungi was carried out by means of their morphological structures and cultural characteristics using Barnett and Hunter keys (1998).
Also, fungi were identified by the PCR-ITS technique (the polymerase chain reaction-internal transcribed spacer), which gave greater reliability to identification due to the accuracy of the technique. In order to do this, the microorganisms DNA extraction was performed eight days after growth, according to the methods of Ahrens and Seemüller (1992) and Lee el at. (1993). The quality of the DNA was verified on a 1% agarose gel by electrophoresis. The gel was observed in a Bio Rad® transilluminator (Bio Rad Laboratories Inc., CA, USA). The internal ITS region (ITS1 and ITS2) of the ribosomal RNA genes 18S-5.8S-28S was amplified by PCR. The primers used were ITS4 (TCCTCCGCTTATTGATATGC) and ITS5 (GGAAGTAAAACTCGTAACAAGG) (White el at., 1990).
The PCR reaction mixture consisted of 1X buffer, 1.5 mM (millimolar) MgCl2, 1 U (unit) DNA polymerase, 200 mM dNTP’S, 10 pm of primers, 80 ng of DNA sample, and sterilized deionized water To set a final volume of 50 μL. The reaction was conducted in a Perkin Elmer thermocycler (Mod. 2400, PerkinElmer Inc., Waltham, Massachusetts, USA) with the following amplification program: an initial denaturation cycle (95 °C for 2 min); 30 denaturation cycles (95 °C for 1 min), alignment (50 °C for 30 s) and extension (72 °C for 2 min); and a final extension (72 °C for 10 minutes) (White el at., 1990). The amplification quality of the fragments was visualized by electrophoresis on a 1% agarose gel in the Bio Rad® transilluminator (Bio Rad Laboratories Inc., CA, USA). Amplification was purified with the QIAquick PCR purification kit (QIAGEN® N.V, Venlo, The Netherlands). Genes were sequenced in two directions 5’-3’ and 3’-5’ with the ITS4 and ITS5 primers (White el at., 1990).The sequencer used was an ABI PRISM 3700 (Macrogen Inc., Seoul, Republic of Korea). Finally, the sequences were analyzed in Lasergene 2001 software version 5 (DNAStar® Inc, Madison, Wisconsin, USA). The alignment was performed in the GenBank NCBI (National Center for Biotechnology Information) database.
Pathogenicity tests
The tests consisted in inoculating the fungi identified at known concentrations on the harvested huitlacoche (10 galls) and on the cobs. Prior to inoculation, galls and cob were disinfested by immersing them in a solution of sodium hypochlorite at 1.5% for 3 min. And subsequently, rinsed with sterile distilled water. The inoculum was prepared from the monoconidial cultures of each fungi identified in the previous phase (Fusarium oxysporum, Penicilium expansum and Rhizopus stolonifer), with which stock solutions were prepared. Subsequently, the conidia concentration was adjusted to 8*106 conidia mL-1 with the help of the Neubauer chamber (Marienfield, Laboratory Glassware, Konigshofen, Germany).
The fungi inoculation was made by wound deposition and by deposition without wounding. In the first, 2 mm deep incisions were made on the galls and cobs, and 0.1 mL of the inoculum was deposited with a sterilized needle. The woundless deposition was done directly on the surface of the product by inoculating them with the same amount. Cobs and galls were placed in humid chambers at 3, 10 and 20 °C, at 95% relative humidity for eight days. The wet chamber consisted of placing the material in expanded polystyrene trays with a paper towel moistened with sterile distilled water inside a closed polyethylene bag. The variables of incidence and severity of the fungi were evaluated.
Statistic analysis
A completely randomized design was used to study the changes in weight loss, firmness and color in the peritoneum of huitlacoche, as well as the percentage incidence and severity of the fungi. A simple classification variance analysis was used in the variable weight loss and L* to determine differences between the manipulation degree and storage temperature. The results are shown in the respective graph as the mean of the observations with the respective standard error bar. A multivariate analysis of variance was applied to determine the effect of the factor (temperature, manipulation and inoculation method) on the percentage of incidence and severity of fungi on huitlacoche. The Tukey test (p< 0.001) was used to determine significant differences with Statistical Analysis System for Windows 9.2 (SAS Institute Inc., Cary, NC, USA).
Results and discussion
Postharvest characterization
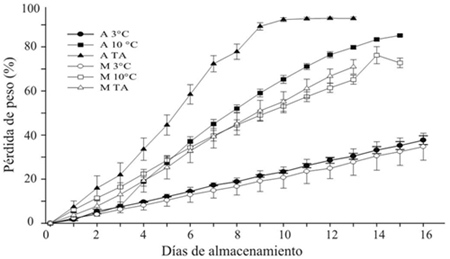
The storage temperature showed influence on weight loss in the galls and cob of huitlacoche. A higher rate of weight loss was observed in the galls compared to the whole cob (Figure 1). Technically, in fresh vegetables, a 5-10% weight loss is considered unfit for sale as a fresh product (Kays, 1991). At ambient temperature, both the gall (ATA) and the cob (MTA) lost approximately 8% of their weight after one and two days of storage, respectively. Similar weight loss values were observed at 10 °C compared to the previous treatment at similar time periods. At 3 °C, both in gall and cob, the loss was kept below 10% for up to 4 days of storage.
On subsequent storage days, the trend continued in ascending order as follows: 20 °C> 10 °C> 3 °C and gall (A)> cob (M) (Figure 1). This behavior reflects that in addition to the environmental factors, the manipulation of the product in the shredded gall and surface area that is exposed to the environment are important factors in the dehydration rate and that lead to the product senescence (Whitelock el at., 1999; Martínez-Flores el at., 2008). The storage of huitlacoche at 3 °C was the best condition to keep the dehydration rate at a low rate and maintain the visual quality of the product for a longer period of time.
On the other hand, the firmness of the galls and the cobs was reduced during the post-harvest storage time. Treatments stored at room temperature showed a greater reduction in peridium compression force, which indicates that the loss of firmness was greater compared to the refrigerated material. Treatments stored at 3 and 10 °C did not show significant differences (p≤ 0.05).
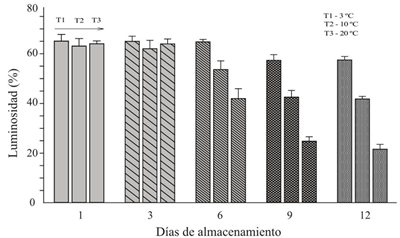
The changes of color and flavor in the huitlacoche, during the period of storage, constitute a parameter of quality measurement (Tracy el at., 2007). In this sense, Martínez-Flores el at. (2008) concluded that the most significant changes in color were the brightness (L*) of the peridium surface. Figure 2 shows a decrease in luminosity during storage days, with a significant difference (p≤ 0.05) between storage temperatures, from day six whose decrease was in accordance with the following relation 20 ºC> 10 ºC> 3 °C.
The peritoneum of huitlacoche showed a rough appearance after three days of storage at room temperature, which coincides with the manifestation of a high moisture loss (> 10%) in the product. After four days the peridium presented a rupture and as a consequence teleospores were released, although there was no manifestation of unpleasant odor that was indicative of rot (Figure 3). This visual appearance is indicative of rapid senescence in the product, as reported by Martínez-Flores el at. (2008).
Visual appearance
10 °C storage showed a similar behavior as the previous treatment; while at 3 °C, the peridium of the galls showed an acceptable visual appearance for a longer storage time (greater than 12 days) than the whole cob (Figure 3).
The prolongation of shelf life at low temperatures, despite the manipulation and greater exposed surface area, suggests that the low compaction of galls of the whole cob leaves spaces that function as reservoir of moisture and phytopathogenic microorganisms that are responsible for initiating decomposition processes.
Diagnosis and identification of pathogenic fungi
Galls were kept in moist chambers at 3, 10 and 20 ±2 °C to induce the growth of microorganisms. Three days after the establishment of the experiment, growth of Fusarium sp., Penicilium sp. and Rhizopus sp., were identified by their cultural and morphological characteristics with the help of the Barnett and Hunter (1998) keys and later corroborated by the PCR-ITS technique. Table 1 shows the molecular characterization of the identified fungi, the genus morphologically classified as Fusarium sp. corresponds to F. oxysporum with a similarity of 97%, the genus Penicillium sp. to P. expansum with a similarity of 98% and the genus Rhizopus sp. to R. stolonifer with 97% similarity (%) due to its molecular features.
Pathogenicity tests
Fungus species previously identified as F. oxysporum, P. expansum and R. stolonifer were inoculated in the galls and cobs of huitlacoche, using puncture and deposition techniques, in the three storage temperatures. It was observed that fungi did not develop at refrigeration temperatures (3 and 10 °C), except Rhizopus that at 10 °C had an incidence of 46% (Table 2). The result indicates that the risk of phytopathogenic fungi in huitlacoche is lower in environments below 10 ºC and high relative humidity. This condition of high humidity in the refrigeration environment would be beneficial for huitlacoche, as it would counteract its accelerated rate of dehydration (Whitelock el at., 1999).
Table 2 Percentage of incidence of Fusarium oxysporum, Rhizopus stolonifer and Penicillium expansum inoculated by wound (puncture) and without wound (deposition) on shredded galls and on huitlacoche cobs stored at three post-harvest temperatures.
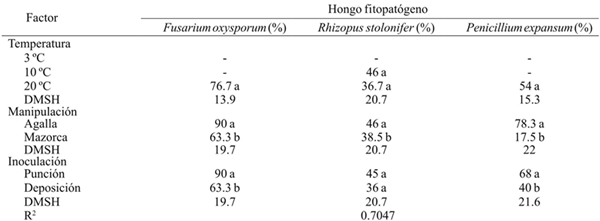
-= no se presentaron síntomas del hongo en el periodo de evaluación; DMSH= Diferencia mínima significativa honesta. Letras diferentes dentro de la misma columna corresponde a un factor indica que la media difiere significativamente (p≤ 0.001).
At ambient temperature, the three inoculated species developed favorably with decreasing percentage incidence as follows: R. stolonifer< P. expansum< F. oxysporum. There was no significant difference in the percentage incidence of R. stolonifer at 10 °C and 20 °C. The manipulation degree showed that the incidence of the three fungus species was higher (p≤ 0.05) in the galls than in the cob, with the highest incidence being F. oxysporum species (90%), followed by P. expansum (78.3 %) (Table 2). Likewise, the effect of the puncture inoculation method was higher (p≤ 0.05) than the deposition method, except in R. stolonifer. Thompson el at. (2013) reported that corn cob rot (Zea mays L.) is largely attributed to fungi of the species F. oxysporum, which explains the high incidence rate of this species, on the host of Ustilago Maydis, in the three factors studied. The species F. oxysporum survives in the soil under severe conditions, which makes it a very aggressive pathogen when infesting host plants (Momma el at., 2010), although the invasion can be completed at any post-harvest stage with the consequent production of toxins (Logrieco el at., 2002; Agrios, 2005).
In other plant species it has been observed that the pathogenicity of Penicillium and Fusarium is not as severe when inoculation is performed without injury (Hernández el at., 2006), which highlights the importance of the manipulation degree of huitlacoche, since the galls presented the highest incidence percentage.
The percentage severity of P. expansum< R. stolonifer< F. oxysporum at room temperature (Table 3). At cooling temperatures there was no effect of the fungus except R. stolonifer with 8.3% severity at 10 °C. The manipulation of huitlacoche influenced the severity of the phytopathogenic fungi invasion, being that in the F. oxysporum species there was significant difference (p≤ 0.001) with respect to the whole cob. This suggests that the stress applied to the galls during its manual shred from the corn cob causes damage to the peridium surface that favors the pathogen entrance (Pérez-López el at., 2012).
Table 3 Severity of Fusarium oxysporum, Rhizopus stolonifer and Penicillium expansum invasion inoculated by wound (puncture) and without wound (deposition) on shredded galls and on cob of huitlacoche stored at three postharvest temperatures.
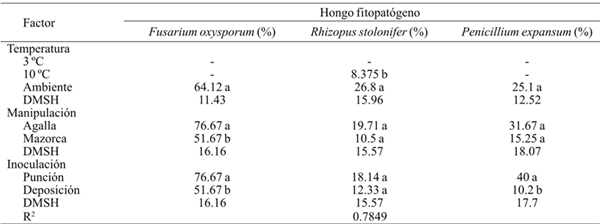
-= no se presentaron síntomas del hongo en el periodo de evaluación; DMSH= diferencia mínima significativa honesta. Letras diferentes dentro de la misma columna correspondientes a un factor indica que la media difiere significativamente (p≤ 0.001).
The severity was significantly higher (p< 0.001) in the puncture inoculation method in the species F. oxysporum and P. expansum than in inoculation by deposition. The results indicate that the incidence and severity of the phytopathogenic fungi invasion increases as much as the damage shown by the peridium, such that the species P. expansum and R. stolonifer, which are considered as wound pathogens, increase their severity level (Agrios, 2005).
The presence of pathogens in fresh products for consumption is undesirable. Nowadays, there are post-harvest control alternatives such as biological control that help reduce the incidence and severity of pathogens. Guevara-Vázquez el at. (2009) isolated and identified, by molecular techniques, yeast associated with huitlacoche belonging to the species Candida railenensis, C. quercitrusa and Pichia guilliermondii. These species have shown an antagonistic effect against some pathogens, especially P. guilliermondii which has been evaluated against Rhizopus species (Zhang el at., 2007; Zhao el at., 2010), Fusarium (Laitila el at., 2007) and P. Expansum (Pacheco el at., 2008). The evaluation of the yeast associated with huitlacoche for the control of the pathogens found could represent an opportunity for its effective control.
Conclusions
The huitlacoche shred factors and storage temperature ≥10 °C significantly affect huitlacoche weight loss, while the 3 °C temperature helps to keep the dehydration rate low (<10%) and is favorable for maintaining quality visual appearance of the product over a longer period of time. The identification techniques employed revealed, with a similarity of 98, 97 and 97% respectively, that P. expansum, R. stolonifer and F. oxysporum are the pathogens that are hosted on the surface of the post-harvest huitlacoche fungus. Pathogenicity tests confirmed the severity of the damage of these species on the quality and shelf life of huitlacoche. The fungus F. oxysporum showed the highest percentage of incidence and severity in galls stored at normal ambient air temperature. The R. stolonifer species showed a high incidence in refrigeration environments ≥10 ºC, which suggests that the most suitable environments for the storage of huitlacoche are those close to 3 °C with a high percentage of relative humidity (95%). This results in a lower rate of dehydration in the huitlacoche, in addition to providing conditions that reduce the risks of development of phytopathogenic fungi on the surface.
Literatura citada
Agrios, G. N. 2005. Fitopatología. 5th. (Ed.). México: Ed. Limusa. [ Links ]
Ahrens, U. and Seemüller, E. 1992. Detection of DNA of plant pathogenic mycoplasmalike organisms by polymerase chain reaction that amplifies a sequenceof the 16S rRNA gene.Phytopathology. 82:828-832. [ Links ]
Aydogdu, M. 2015. Huitlacoche yield in some maize varieties in the mediterranean region of Turkey. Food Sci. Technol. 35(2):386-390. [ Links ]
Barnett, H. L. and Hunter, B. B. 1998. Illustred genera of imperfect fungi 4th. (Ed.) Minnesota: APS Press. [ Links ]
Guevara, V. E.; Valadez, M. E.; Acosta, R. E.; Espinosa, S. T. y Villanueva, V. C. 2009. Identificación de levaduras asociadas al huitlacoche. Rev. Chapingo Ser. Hortic. 15:225-230. [ Links ]
Hernández, A. A. Ma.; Juárez, L. G.; Fucikovsky, Z. L.; Zavaleta, M. E. y González, H. V. A. 2006. Impacto del almacenamiento en la brotación de bulbos de ajo y especies patogénicas de Penicillium y Erwinia asociadas. Rev. Fitotec. Mex. 29(4):283-290. [ Links ]
Kays, S. J. 1991. Postharvest physiology of perishable plant products 1th. (Ed.). New York: Van Nostrand Reinhold. [ Links ]
Laitila, A.; Sarlin, T.; Kotaviita, E.; Huttunen, T.; Home, S. and Wilhelmson, A. 2007. Yeasts isolated from industrial maltings can suppress Fusarium growth and formation of gushing factors. J. Industrial Microbiol. Biotechnol. 34:701-713. [ Links ]
Lasergene. 2001. Expert Sequence Analysis Software. Version 5.DNASTAR, Inc. Madison, Wisconsin, USA. [ Links ]
Lee, I. M.; Hammond, R. R.; Davis, E. and Gundersen, D. E. 1993.Universal amplification and analysis of pathogen 16s rDNA for classification and identification of mycoplasma-like organisms. Phytopatology. 83:834-842. [ Links ]
Logrieco, A.; Mulé, G.; Moretti, A. and Bottalico, A. 2002. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 108:597-609. [ Links ]
Madrigal, R. J.; Villanueva, V. C.; Sahagún, C. J.; Acosta, R. M.; Martínez,M. L. y Espinosa, S. T. 2010. Ensayos de producción de huitlacoche (Ustilago maydis Cda.) hidropónico en invernadero.[Production test of green house hydroponic huitlacoche (Ustilago maydis Cda.)]. Rev. Chapingo Ser. Hortic. 16(3):177-182. [ Links ]
Martínez, F. A.; Corrales, G. J. J.; Espinosa, S. T.; García, G. P. G. y Villanueva, V. C. 2008. Cambios postcosecha del hongo comestible huitlacoche (Ustilago maydis (DC) Corda). Rev.Chapingo Ser. Hortic. 14(3):339-346. [ Links ]
Monroy, G. T.; Valle, G. S.; Espinosa, S. T.; Martínez, D. M. T. and Pérez,L. A. 2012. Effect of microperforation and temperature on quality of modified atmosphere packaged huitlacoche (Ustilago maydis). CyTA. J. Food. 11(4):309-317. [ Links ]
Momma, N.; Momma, M. and Kobara, Y. 2010. Biological soil disinfestation using ethanol: effect on Fusarium oxysporum f.sp. lycopersici and soil microorganisms. J. Gen. Plant Pathol. 76(5):336-344. [ Links ]
Pacheco, N.; Larralde, C. C. P.; Sepulveda, J.; Trombotto, S.; Domardc,A. and Shirai, K. 2008. Evaluation of chitosans and Pichia guillermondii as growth inhibitors of Penicillium digitatum. Int. J. Biol. Macromol. 43:20-26; 76(5):336-344. [ Links ]
Pérez, L. A., Villaseñor, P. C. A., Espinosa, S. T., Ortega, H. C. y Reyes,V. M. 2012. Caracterización física, geométrica y mecánica de huitlacoche (Ustilago maydis (D.C.) Corda). Ing. Agrí Bio. 4(1):41-45. [ Links ]
Reyes, F. G. R.; Varseveld, G. W. and Kuhn, M. C. 1982. Sugar composition and flavor quality of high sugar (Shrunken) and normal sweet corn. J. Food Sci. 47(3):753-755. [ Links ]
Rodríguez, M. M. L. 2010. Manual de identificación de bacterias fitopatógenas. México: UACH. [ Links ]
Salmerón, S. K. G.; Pardo, J. P. Flores, O. H. G.; Mendoza, H. M.; Miranda, A.and G. Guerra, S. 2011. Response to osmotic stress and temperatura of the fungus Ustilago maydis. Arch. Microbiol, 193:701-709. [ Links ]
SAS Institute Inc. 2008. SAS/STAT® 9.2 Software. Cary, NC: SAS Institute Inc. [ Links ]
Thompson, R. S.; Avelinga, T. A. S. and Blanco, P. R. 2013. New semiselective medium for Fusarium graminearum, F. proliferatum,F. subglutinans and F. verticillioides in maize seed. South Afr.J. Bot. 84:94-101. [ Links ]
Torija, E.; Díez, C.; Matallana, C.; Camara, M.; Camacho, E. and Mazarío,P. 1998. Influence of freezing process on free sugars content of papaya and banana fruits. J. Sci. Food Agric. 76(3):315-319. [ Links ]
Tracy, W. F.; Vargas, C.; Zepeda, L.; Pataky, J. K. and Chandler, M. A.2007. Production and marketing of huitlacoche. Issues in new uses. Alexandria, VA: ASHS Press. [ Links ]
Valdéz, M. M.; Barry, K.; Fahey, J. G. C.; Domínguez, J.; González, M.E.; Valverde, M. E. and Paredes, L. O. 2010. Effect of maize genotype, developmental stage, and cooking process on the nutraceutical potential of huitlacoche (Ustilago maydis). Food Chem. 119:689-697. [ Links ]
Valverde, M. E.; Paredes, L. O.; Pataky, J. K. and Guevara, L. F. 1995.Huitlacoche (Ustilago maydis) as a food source - biology,composition, and production. Critical Rev. Food Sci. Nutr. 35(3):191-229. [ Links ]
Valverde, M. E.; Hernandez, P. T. and Paredes, L. O. 2012. Huitlacoche- a 21st century culinary delight originated in the aztec times. In: Tunick, M. H. and De Mejia, E. G. (Eds.). Hispanic Foods:chemistry and bioactive compounds. Washington: Amer Chemical Soc. 1109:83-100. [ Links ]
White, T. J.; Bruns, S.; Lee, T. and Taylor, J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics.In: Innis, M. (Ed). PCR Protocols: a guide to methods and applications. San Diego, CA: Academic Press. [ Links ]
Whitelock, D. P.; Brusewits, G. H. and Ghajar, A. J. 1999. Thermal/physical properties affect predicted weight loss of fresh peaches. Trans. ASAE. 42(4):1047-1053. [ Links ]
Zhang, H.; Zheng, X.; Wang, L.; Li, S. and Liu, R. 2007. Effect of yeast antagonist in combination with hot water dips on postharvest Rhizopus rot of strawberries. J. Food Eng. 78:281-287. [ Links ]
Zhao, Y.; Tu, K.; Tu, S.; Liu, M.; Su, J. and Hou, Y. 2010. Acombination of heat treatment and Pichia guilliermondii prevents cherry tomato spoilage by fungi. Int. J. Food Microbiol. 137:106-110. [ Links ]
Received: February 2017; Accepted: March 2017











 texto em
texto em