Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.3 Texcoco Abr./Mai. 2017
https://doi.org/10.29312/remexca.v8i3.25
Articles
Effect of salinity on calli of wheat varieties during in vitro culture
1Universidad de Granma. Carretera a Manzanillo, km 17 ½. Peralejo, Bayamo, Cuba. CP. 85100. (oleinismora@gmail.com, idalmisfr@gmail.com).
2Instituto Tecnológico de Sonora. 5 de febrero 818 sur, Col. Centro, Cd. Obregón, Sonora, México. CP. 85000. (garatuza@itson.edu.mx, yepezglz@gmail.com).
3Embrapa Trigo. Rodovia BR 285, km 294. 99001-970 Passo Fundo - RS, Brasil. CP. 45. (j51173@yahoo.com).
The effect of salinity on the stress tolerance degree and the radioinduction effectiveness of callus mutations of three common wheat varieties during in vitro culture was studied. The researcg was developed at the Center for Genetic Engineering Biotechnology (CIGB) of Havana, Cuba in the period from November 2015 to February 2016 in a genetic improvement program for tolerance to salt stress in in vitro culture phase. The variables cell permeability, peroxidase activity, soluble protein concentration and abscisic acid synthesis were evaluated at 24 h, 30 days and 70 days of callus exposure to treatments, which consisted of electrical conductivities (EC) of 0.02, 5, 7, 9 dS m-1 in the culture medium. An increase in PMC was observed at 24 h as the initial salinity stimulating effect, values that decreased as the EC and the time of exposure to stress increased. Peroxidase activity increased to 7 dS m-1 in all three varieties but the trend was to be maintained or decreased when CE increased. Similar response showed total soluble proteins. The concentration of ABA increased by more than 25 units in the varieties and the hypothesis of its synthesis was ruled out from photosynthetic and protective pigments.The evaluation showed the positive effect of radioinduction on wheat tolerance to saline stress.
Keywords: Triticum aestivum L.; ABA; callus; salinity; wheat
Se estudió el efecto de la salinidad en el grado de tolerancia al estrés y la efectividad de la radioinducción de mutaciones en callos de tres variedades de trigo harinero durante el cultivo in vitro. El estudio se desarrolló en el centro de Ingeniería Genética Biotecnología (CIGB) de la Habana, Cuba en el período comprendido entre noviembre de 2015 a febrero de 2016 en un programa de mejora genética para tolerancia a estrés salino en fase de cultivo in vitro. Se evaluaron las variables permeabilidad de las membranas celulares, actividad peroxidasa, concentración de proteínas solubles y síntesis de ácido abscísico a las 24 h, 30 días y 70 días de exposición de los callos a los tratamientos, los cuales consistieron en conductividades eléctricas (CE) de 0.02, 5 , 7, 9 dS m-1 en el medio de cultivo. Se observó a las 24 h, un incremento de la PMC como efecto estimulante inicial de la salinidad, valores que disminuyeron conforme se incrementó la CE y el tiempo de exposición al estrés. La actividad peroxidasa se incrementó hasta los 7 dS m-1 en las tres variedades pero la tendencia fue a mantenerse o disminuir cuando se incrementó CE. Respuesta similar mostraron las proteínas solubles totales. La concentración de ABA aumentó en más de 25 unidades en las variedades quedando descartada la hipótesis de su síntesis a partir de pigmentos fotosintéticos y protectores.La evaluación realizada demostró el efecto positivo de la radioinducción en la tolerancia del trigo al estrés salino.
Palabras clave: Triticum aestivum L.; ABA; callos; salinidad; trigo
Introduction
Among the adverse conditions of the worldʼs agricultural systems, soil salinity has become the abiotic factor with the greatest impact on plant productivity (Setter et al., 2016). At present, more than 74% of agricultural soils worldwide present salinity problems (Zaki and Yokoi, 2016) and in many cases the electrical conductivity of the soil exceeds the tolerance index of important economic species (Zhao et al., 2016) and wheat (Triticum aestivum L.), which is the feeding basis of more than 96.4% of the world population (Argentel et al., 2016).
The evaluation of the effect of saline stress on economic species such as wheat is of vital importance in order to know and understand the possible mechanisms that these develop in its adaptation to this abiotic factor. However, for the final evaluation and recommendation of tolerant varieties, the main task is to identify the main characters associated to this tolerance (Argentel et al., 2013), which, once identified and evaluated, simplify and accelerate experimental work in genetic improvement for the tolerance character to saline stress (Lamz et al., 2013).
Numerous methods are used to simulate salinity tolerance under in vitro conditions once the genetic variability of available germplasm has been identified. Occasionally, solutions of certain substances such as mannitol, polyethylene glycol (González and Argentel, 2005) and sodium chloride have been added to the culture medium to evaluate the response to salinity (Munss et al., 2016). It is precisely through the in vitro culture of plant tissues that it is physically possible to determine the response of important variables that determine the tolerance to stress as the selectivity of membranes (García et al., 2003), the synthesis and concentration of hormones such as abscisic acid (ABA), which regulates the induction of structural genes encoding enzymes and proteins involved in such tolerance (Kadri et al., 2014).
Therefore, its determination and evaluation during tissue culture has important practical significance (Zaidi et al., 2016). It was the objective of this paper to evaluate the effect of different saline concentrations and stress exposure times on cell membrane permeability, peroxidase enzyme activity, soluble protein concentration and abscisic acid synthesis in three common wheat varieties: a progenitor and two radiomutants during callus culture for possible recommendation in the process of genetic improvement through the technique of induction of mutations for tolerance to saline stress.
Materials and methods
Development of the experimentand varieties used
The experiments were carried out at the Plant Biotechnology Center of the Center for Genetic Engineering and Biotechnology, Havana, Cuba. Three varieties of common wheat were used. The variety Cuba-C-204 (progenitor), the first variety of wheat obtained in Cuba from the Brazilian wheat variety BH-11 (Gutiérrez et al., 2005) and two radiomutant varieties INIFAT RM 26; INIFAT RM 29 obtained from Cuba-C-204 (Argentel et al., 2012).
Callus formation stage
Callus formation was based on mature seeds previously disinfected (Labrada, 1999). Of each variety studied, 80 seeds were used and grown in MS-based culture medium. Growth regulators 2,4-dichlorophenoxyacetic (2,4-D; 2 mg L-1) and 6-benzylaminopurine (BAP; 2 mg L-1) and NaCl concentrations representing electrical conductivities (EC) of 0.002 dS m-1 (control treatment); 5 dS m-1, 7 dS m-1 and 9 dS m-1. The culture medium was adjusted to pH 5.8, it was solidified with Gelrite agar (2 g L-1) and autoclaved at 120 °C and 1.5 atmospheres of pressure for 20 min.
Seeding and disinfection of seeds were performed under asepsis conditions in a laminar flow chamber. Two seeds were seeded per test tube (15.5 cm * 2.3 cm), and eight tubes were used per treatment. The seed tubes were placed in a growth chamber under dark conditions and a temperature of 25±2 °C. Three replicates were established for each experimental variant. For each variety in the electrical conductivities, evaluations were performed at 24 h of stress exposure, at 30 days and 70 days in order to evaluate the variability degree of responses as a function of time. The evaluated variables were as follows.
Permeability of cell membranes (PMC)
PMC was quantified in the callus obtained at 24 hours, 30 days and 70 days of stress exposure. 0.05 g of callus was weighed from each treatment in six replicates and washed four times with deionized water. The calluses were then placed in 5 mL of deionized water for 20 h at 18 °C in the dark. The electrolyte output of the samples subjected to different stress treatments was evaluated by the electrical conductivity of the solution after 24 h. A conductivity meter (DD-IIA, China) was used. PMC was estimated with respect to control following the methodology proposed by Blum (1981).
Peroxidase activity
0.25 g of plant tissue were homogenized in a mortar with 5 mL of tris-HCl buffer pH 7.4. The homogenate was centrifuged at 20 000 g for 15 min. The supernatant was used to determine the peroxidase activity and was defined as unit of enzyme activity (UAE), a change of 0.01 at absorbance (420 nm) in 1 min per gram of fresh mass. The determination, properly speaking, of the peroxidase activity was performed according to the continuous method described by Martínez (2013).
Total soluble protein content
The total soluble protein content was determined by the photometric method. A sample of 1 g of fresh material was deposited in a mortar and macerated. The extraction was performed with 40 mL of a phosphate buffer solution at pH 6.86 prepared from potassium phosphate, a solution which was added slowly while the extraction was being carried out. The extract was decanted in 50 mL centrifuge tubes and centrifuged at 10 000 revolutions per minute for 7 min. Finally the separated supernatant solution was transferred to 50 ml volumetric flasks. Total soluble protein content was quantified by absorption photometry on a Hewlett Packard 8452 spectrophotometer, at a wavelength of 750 nm (Galvani and Gaertner, 2006).
Abscisic acid content
A mass of 5 g of calli of the varieties was taken at each concentration and time of incubation to the stress and these were macerated to make the determination (liquid-liquid extraction) of ABA. The quantification of the phytohormone was performed by an ABA calibration curve and its final concentration was expressed in µm g mf-1 using a mass spectrometer (Bautista and Gallardo, 2008).
Statistical analysis
The data were processed through analysis of variance of simple classification based on linear models of fixed effects (Fischer, 1935) without interaction between the factors conductivity/variety/time of exposure to saline stress. Independently the response of each variable was evaluated between electrical conductivities, means that were compared by Tukey's multiple comparisons test (Tukey, 1960) for significance levels of 5% and 1%. The comparisons between exposure times to 30 and 70 days saline stress were performed intravarietally at each level of electrical conductivity using the theoretical distribution of probabilities of t-student (Gosset, 1917) for significance levels of 5% and 1%. The Statistical Professional Statistical Package, version 8.0 for Windows was used for all analyzes.
Results and discussion
Permeability of cell membranes
The permeability of cell membranes (PMC) was not significantly affected, although the tendency was to increase when the calli of the three varieties were incubated for 24 h in the saline solutions (Figure 1).

Figure 1 Permeability of the cell membrane (PMC) in calluses of three varieties of common wheat formed in saline medium for 24 h. R2= 0.822; 0.824; 0.831 for Cuba-C-204, INIFAT RM 26, INIFAT RM 29 respectively. ESx= 0.001 ns NS= not significant.
Several studies try to explain that initially under conditions of saline stress there is a stimulation of the enzymatic activity and the permeability of membranes, such was the result obtained here, an aspect that is known as salinity stimulating effect or euestres (Bakry et al., 2012).
Changes in cell membrane permeability (PMC) generally vary depending on the exposure time of tissues to stress and degree of tolerance of the varieties and has variation between the types of water and saline stress (Chávez et al. 2012). In this paper it was observed that as the electrical conductivity of the culture medium increased, the permeability of cell membranes decreased significantly after 30 days of exposure to stress, perhaps to avoid adverse effects such as ionic toxicity. The largest decrease in PMC was obtained in radiomutants (Table 1). The progenitor variety showed the most marked differences in the response to imposed saline stress and in the times of stress exposure and the highest PMC values at 30 days, which indicates that as the stress exposure period increased, PMC decreased, although to a lesser extent than the mutants. This response has important practical significance because it offers the extent to which membranes become less permeable to ions such as sodium and chlorine that could lead to toxicity.
Table 1 Permeability of cell membranes in callus of three wheat varieties subjected to different electrical conductivities at 30 and 70 days of saline exposure.

Medias con letras iguales no difieren según la prueba de comparación múltiple de Tukey HSD. ** y *= diferencias para el 5% y 1% respectivamente por t-student; Es(x)=error estándar de la media.
Another important result in the radiomutants was that even in 9 dS m-1 the PMC values were similar to when the stress was imposed during the 24 h. Perhaps this element is due to the effect of the radioinduction where it shows superdominance of desired characters as the tolerance to the saline stress. In this sense it has been reported that mutation induction has been proposed as an effective biotechnological technique for such purposes in other plant species (Oladosu et al., 2016). The evaluation of PMC in foliar tissues has been an efficient indicator to detect variations in the tolerance levels to saline and water stress in different cereal genotypes (Munss et al., 2012).
Peroxidase activity
The peroxidase activity experienced significant differences with respect to the control at the two evaluation times in the three varieties and the initial tendency was to increase, except for the progenitor variety (Cuba-C-204) in which at 9 dS m-1 the same value of enzymatic activity as the control. The INIFAT RM-26 variety showed a similar response in the different saline concentrations and in the time of exposure to stress and their values were the highest among the three varieties, although at 9 dS m-1 the value of the indicator did not show significant differences When compared to that obtained for EC of 5 dS m-1. In the parent variety the peroxidase activity did not exceed 100 UAE min-1 at 30 days of exposure while at 70 days it increased to more than twice that at 30 days. In general, the peroxidase activity was higher in the radiomutant varieties than in the progenitor (Table 2). The increase in peroxidase activity in this paper demonstrates the protective ability of the enzyme to prevent oxidative damage caused directly or indirectly by salinity.
Table 2 Activity of the peroxidase enzyme, in calluses of three wheat varieties subjected to different electrical conductivities at 30 and 70 days of exposure to saline medium.
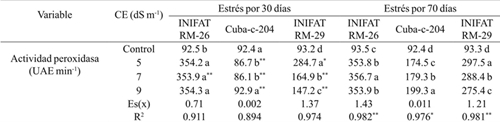
Medias con letras iguales en una misma columna no difieren según la prueba de comparación múltiple de Tukey HSD. ** y *= representan los tiempos de estrés, diferencias para el 5% y 1% respectivamente por t-student; Es(x)= error estándar de la media; R2= coeficiente de determinación sin ajustar.
When analyzing the values of the determination coefficient for each variety in the two times of exposure to stress evaluated, it was observed that by EC effect explained more than 89% of the variability found.
There is evidence that in maize exposed to water and saline stress the peroxidase activity increases significantly causing the formation of superoxide radicals (O2 -) (Ahmad et al., 2016). The superoxide radical and its reducing agent hydrogen peroxide (H2O2) are potentially toxic compounds that when combined form a radical known as hydroxyl radical (OH) and are known as reactive oxygen species (ROS) (Zhang et al., 2016). Stress-tolerant genotypes such as drought and salinity present an efficient active system to prevent oxidative damage as a function of peroxidase activity (Young et al., 2012), perhaps this process was evidenced in this paper.
Concentration of soluble proteins
The concentration of total soluble proteins was similar in the control treatment in the three varieties; however, a significant increase was observed when saline concentration was increased in the two radiomutant varieties but its increase was only up to the EC of 5 dS m-1 when it was evaluated at 30 days while the parent increased the value of the indicator until 9 dS m-1. The increase obtained was at least 0.92 mg g mf-1 of proteins when the incubation period of callus was of 70 days, aspect that denotes the biochemical transformations significant to tolerate the condition of stress. The highest concentrations of soluble proteins were obtained in the INIFAT RM-26 mutant, although the value of the evaluated indicator decreased (at 30 days of stress exposure) from CE= 7 dS m-1 (Table 3).
One way to maintain the stability and integrity of cell membranes is through the increase in structural protein synthesis. This increase could be associated with the synthesis of new proteins under stress conditions (Valifard et al., 2012). Such an assertion demonstrates that the varieties studied synthesize new proteins in response to the imposed stress.
Table 3 Activity of the peroxidase enzyme, in calluses of three wheat varieties subjected to different electrical conductivities at 30 and 70 days of exposure to saline medium.
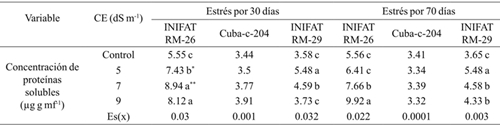
Medias con letras iguales no difieren según la prueba de comparación múltiple de Tukey HSD. ** y *= representan diferencias para el 5% y 1% respectivamente por t-student; Es(x)= error estándar de la media.
In salinity conditions, in many species of cereals, there is an increase in the content of total soluble proteins, but these values are decreasing due to the activity of the proteases to use the amino acids for protein exchange, as energy source, as a carbon or nitrogen source (Mollasadeghi et al., 2011), as well as using amino acids in the first step to increase the concentration of osmotically active compounds, among others, proline and glycine, glycine-betaine, and thus to ensure a decrease in the osmotic potential and consequently the cellular water potential (Argentel et al., 2013).
Abscisic acid content (ABA)
The concentration of ABA was significantly increased in calli of all varieties under salinity conditions of the incubation medium. In addition, differences between their concentrations were observed in the two incubation times evaluated, being the highest concentration at 70 days of stress. Increases in ABA concentration were found in more than 25 units in established electrical conductivities. The ABA concentration was similar for each of the three varieties studied when the exposure time to the salinity was 70 days to the electrical conductivities of 7 dS m-1 and 9 dS m-1 (Figure 2).
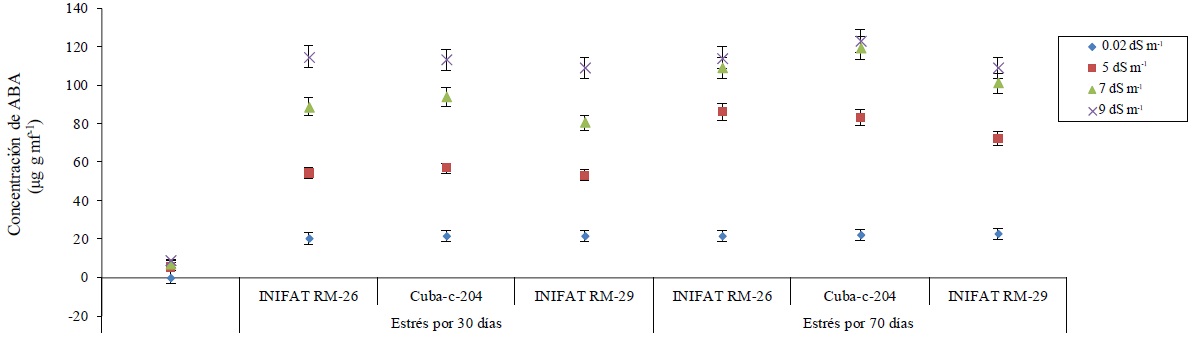
Figure 2 Concentration of ABA in callus of three wheat varieties subjected to different electrical conductivities at 30 and 70 days of saline exposure.
In most of the higher plants, the ABA biosynthesis is indirectly carried out by degradation of some carotenoids (C40) present in chloroplasts (Wang et al., 2016); however, the results shown here demonstrate that its synthesis occurred fully in callus, not in vascular cells or from carotenoids, perhaps from non-pigmented precursors, showing the ability to synthesize ABA by any plant cell under saline stress conditions.
Among the effects of ABA on plants in addition to stomatal regulation and opening (Fujita et al., 2011), bud dormancy (Nakashima and Yamaguchi-Shinozaki, 2013) and seeds (Kadri et al., 2014) the inhibition of RNA synthesis and some proteins is shown (Alhasnawi et al., 2016). Therefore, it would be interesting to test if from the callus with high concentrations of endogenous ABA, as in this paper, the capacity of regeneration of plants is affected.
The results of the evaluated biochemical variables in this paper show the effect of the induction of mutations to create response variability and superdominance from varieties considered as tolerant in agricultural production under conditions of stress such as saline (Jimenez et al. 2016). Genetic improvement programs are currently involved in the creation, identification and characterization of such variability by rapid and precise techniques such as mutation induction, since breeding for abiotic stress conditions in general is a long process (Oyiga et al., 2016), and sometimes when a promising variety is obtained the edaphic conditions have changed and the new variety does not express its productive genetic potential (Al-Mashhadani et al., 2016).
The research was a feasible and efficient way to determine the effect of saline stress on these tissues and at the same time to define the variability of the response of mutants regarding to their parent, which showed greater tolerance. The characterization of these radiomutants will allow their recommendation as future parents for the genetic improvement since their permeability decreased even when the saline concentrations were high, at the same time they demonstrated to develop mechanisms to mitigate the saline stress through the synthesis of osmotically active compounds to ensure a good water status and prevent the physiological drought that occurs under conditions of saline stress.
Conclusions
The salinity caused significant variations in the evaluated variables, being the permeability of the cellular membranes and the concentration of abscisic acid the most affected. Such characteristics demonstrate tolerance, and can be used in breeding for the ability to avoid ionic toxicity by increasing their selectivity.
The callus of the evaluated radiomutants presented better response to the saline stress imposed on the culture medium and the most favorable results were obtained up to the electrical conductivity of 7dS m-1 at both exposure times, demonstrating the tolerance to this type of stress in the short and medium term exposure to this level of EC and the positive effect of mutations induction to mitigate the adverse effects of saline stress.
Literatura citada
Argentel, L. 2013. Efectos de la salinidad en las variables hídricas potenciales hídrico y osmótico y ajuste osmótico en cultivares cubanos de trigo (Triticum aestivum L. y T. durum L.). Cultivos Tropicales. 34(4):43-48. [ Links ]
Argentel, L. 2012. Tolerancia del trigo a la salinidad. Caso de estudio de variedades cubanas, Número 12479 e ISBN 978-3-659-02109-1. 2012. EAE. [ Links ]
Bakry, A. B.; Abdelraouf, M. A.; Ahmed, M. F. 2012. Effect of drought stress and ascorbic acid foliar application on productivity and irrigation water use efficiency of wheat under newly reclaimed sandy soil .J. Appl. Sci. Res. 8(8):4552-4558. [ Links ]
Bautista, L. X. C., y Gallardo, I. R. 2008. Estandarización de métodos de detección para promotores de crecimiento vegetal (AIA, ABA y GB) en cultivos microbianos. Pontificia Universidad Javeriana. [ Links ]
Blum, A. 1981. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Science. 21(1):43-47. [ Links ]
Borges, M.; Estrada, E.; Pérez, I. y Meneses, S. (2012). El cultivo in vitro del ñame. Rev. Colomb. Biotecnol. 11(2):127-135. [ Links ]
Broertjes, C. E. 2012. Application of mutation breeding methods in the improvement of vegetatively propagated crops.Vol. 2. Elsevier [ Links ]
Chávez, L.; Fonseca, A. y Ramírez, R. 2012. Apuntes sobre algunos reguladores del crecimiento vegetal que participan en la respuesta de las plantas frente al estrés abiótico. Cultivos Tropicales. 33(3):47-56. [ Links ]
Fisher, R. A. 1935. The design of experiments. Oliver & Boyd. Londres. [ Links ]
Fujita, Y.; Fujita, M.; Shinozaki, K. y Yamaguchi-Shinozaki, K. (2011).ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 124:509-525. DOI 10.1007/s10265-011-0412-3 [ Links ]
Galvani, F. & Gaertner, E. 2006. Adequação da metodologia Kjeldahl para determinação de nitrogênio total e proteína soluble totale e bruta. XI MET, 34. [ Links ]
García, A.; Florido, M. y Lara, R. M. 2003. Estudios bioquímicos para la selección in vitro de variedades de arroz con tolerancia a estrés hídrico y salino. Biotecnología Vegetal, 3(3):181-186. [ Links ]
González, L. M. y Argentel, L. 2005. Efectos de la sequía simulada con PEG-600 en la germinación y el crecimiento de las plántulas de dos variedades de trigo. Cultivos Tropicales. 26(3):65-69. [ Links ]
Gosset, E. 1917. An other diferences calculus based on standar desviation and confidence interval. Statistical References. 26:66-72. [ Links ]
Gutiérrez, L.; Pérez, S.; Cabrera, M. y Villasana, R. 2005. Algunas experiencias en la producción de trigo cubano. Memorias VI taller internacional de recursos fitogenéticos. Sancti Spíritus,Cuba. 180 p. [ Links ]
Kadri, K.; Abdellaoui, R.; Mhamed, H. C. and da Silva, J. A. T. 2014.Analysis of salt-induced mRNA transcripts in tunisian local barley (Hordeum vulgare) leaves identified by differential display RT-PCR. Biochemical genetics. 52(1-2):106-115. [ Links ]
Labrada, M. 1999. Selección in vitro en condiciones salinas de líneas de arroz obtenidas a través del uso combinado de técnicas biotecnológicas y nucleares. [ Links ]
Lamz, P. A.; Reyes, G. Y. y González, C. M. C. 2013. Indicadores bioquímicos para la selección de genotipos de arroz (Oryza sativa L.) con tolerancia a la salinidad. Cultivos Tropicales.34(1):11-17. [ Links ]
Martínez C. T. 2013. Estudio y purificación de peroxidasas implicadas en la lignificación de plantas basales. [ Links ]
Mollasadeghi, V.; Valizadeh, M.; Reza, S. R. and Imani, A. A. 2011.Evaluation of end drought tolerance of 12 wheat genotypes by stress indices. Middle-East J. Sci. Res. 7(2):241-247. [ Links ]
Munss, R.; James, R. A.; Xu, B.; Athman, A. and Conn, S. J. 2012.Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nature Biotechnology. 30(4):360-364. [ Links ]
Nakashima, K. and Yamaguchi-Shinozaki, K. 2013. ABA signaling in stress-response and seed development. Plant cell reports.32(7):959-970. [ Links ]
Valifard, M.; Moradshahi, A. and Kholdebarin, B. 2012. Biochemical and physiological responses of two wheat (Triticum aestivum L.) cultivars to drought stress applied at seedling stage. J. Agri.Sci. Tech. 14:1567-1578. [ Links ]
Young, J.; Ye, J.; Chae, G. y Hunseung, G. 2012. Aquaporin as a membrane transporter of hydrogen peroxide in plant response to stresses.Plant Signaling & Behavior. 7(9):1180-1181. [ Links ]
Tukey, J. W. 1960. “A survey of sampling. Post-hoc comparations”.Contribution to probability and statisties. Essays in honor to Harold Hotelling. Sandford: Sandford University Press.448-485 pp. [ Links ]
Received: January 2017; Accepted: March 2017











 texto em
texto em 


