Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.2 Texcoco feb./mar. 2017
https://doi.org/10.29312/remexca.v8i2.58
Articles
Leaf senescence in micropropagated plants of Agave americana during acclimation
1Posgrado en Ciencias en Productividad de Agroecosistemas-Instituto Tecnológico del Valle de Oaxaca. Ex hacienda de Nazareno, Xoxocotlán, Oaxaca. CP. 71230. Tel. (01) 951 5170444.
2Posgrado e Investigación-Instituto Tecnológico del Valle de Oaxaca (ITVO). Ex hacienda de Nazareno, Xoxocotlán, Oaxaca. CP. 71230. Tel. (01) 951 5170444.
In the state of Oaxaca, Agave americana var. oaxacensis is one of the agavaceous species of wild collection that are used for the artisan elaboration of mezcal. There is little information on its propagation and cultivation, recently the propagation of agaves has been increased, through the technique of plant tissue culture, for conservation purposes. However, there is little information on morphological and functional changes by plants when they are transferred to ex vitro environment, among them is senescence or gradual loss of leaves formed in the in vitro environment, where there is manipulation of conditions to control certain physical and biological factors. This study aimed to evaluate the foliar behavior of micropropagated plant Agave americana var. oaxacensis in response to different substrates and fertilization doses during their acclimatization. The results showed that the substrate and fertilization dose influenced leaf senescence or loss. The conserving leaves formed in vitro for longer, he influenced the formation of new leaves in ex vitro conditions. Generally, the plants replaced all its leaves formed in vitro in a span of 240 days.
Keywords: fertigation; growth; leaf blade; nutrients; substrates
En el estado de Oaxaca, Agave americana var. oaxacensis es una de las especies agaváceas de recolección silvestre que son utilizadas para la elaboración artesanal de mezcal. Existe poca información sobre su propagación y cultivo, recientemente se ha incrementado la propagación de agaves, mediante la técnica de cultivo de tejidos vegetales, con fines de conservación. Sin embargo, hay poca información sobre los cambios morfológicos y funcionales que realizan las plantas cuando son transferidas al ambiente ex vitro, entre ellos se encuentra la senescencia o pérdida gradual de las hojas formadas en el ambiente in vitro, en donde hay manipulación de las condiciones para controlar ciertos factores físicos y biológicos. El presente estudio tuvo como objetivo evaluar el comportamiento foliar de plantas micropropagadas de Agave americana var. oaxacensis como respuesta a diferentes sustratos y dosis de fertilización durante su aclimatación. Los resultados mostraron que el sustrato y la dosis de fertilización influyeron en la senescencia o pérdida de las hojas. La conservación de las hojas formadas in vitro por más tiempo, influyó en la formación de hojas nuevas en condiciones ex vitro. En general las plantas sustituyeron todas sus hojas formadas in vitro en un lapso de 240 días.
Palabras claves: crecimiento; fertirrigación; lámina foliar; nutrimentos; sustratos
Introduction
In 2011, agave plants represented a source of income for a total of 4 407 magueyeros producers in the “mezcal region”, which covers seven political districts (Antonio and Teran, 2008; Snidrus and Oeidrus 2011). About nine species of agave of which Agave angustifolia (sprat maguey) is the most in demand and the only grown significantly used (Cruz et al., 2013), the remaining eight are collected from wild or semi-cultivated mainly in living fences with little or no cultural management. Such is the case of Agave americana var. oaxacensis commonly known as maguey “arroqueño”.
The individuals in this species population are declining more and more due to various factors including their very slow growth rate (it takes 15 to 20 years to reach maturity for harvest); the change of soil use and the difficulty of its reproduction, since peasants eliminate the floral scape when it begins its growth, to avoid that the plant consumes the accumulated sugars in the stem or pineapple; but, this prevents seed production (Enríquez-del Valle, 2008; Ángeles, 2010).
From any point of view, it is very important to generate alternatives of reproduction. Most agaves can be propagated by germination of seeds, rhizome stems, inflorescence bulbiles, and by non-conventional methods such as micropropagation through the plant tissue culture technique, which is efficient to achieve multiplication of a large number of plants from somatic cells and tissues that are established in a culture medium consisting of carbohydrates, vitamins, aminoacids, growth hormones, inorganic salts, both macronutrients and micronutrients, physical substances and water (Hartman and Kester, 1994; Enríquez-del Valle, 2008).The micropropagation process consists of several stages: 1) establishment of aseptic crops; 2) propagation multiplication; 3) rooted shoots; and 4) acclimatization of seedlings (George and Debergh, 2008, Enríquez-del Valle, 2008). This last mentioned stage is considered critical for the majority of the species; And in the case of micropropagated agave plants, high percentages of survival have been reported. Abreu et al. (2007) report 90% survival in A. fourcroydes; Enríquez-del Valle and Cruz-Valdez (2012) 100% for Agave angustifolia Haw.
In order to obtain good quality plants, special attention has been given to the type of substrate and the availability of nutrients required by the plants during their acclimatization process, during which they must assume changes in their morphological and physiological characteristics that allow them to continue their growth and development when setting them in the field, where environmental conditions are unfavorable (Enríquez-del Valle et al., 2009). Among the morphological changes during the acclimation process is the replacement of the leaves formed in the in vitro environment, by new leaves (Zimmerman, 1991; Pospisilova et al., 1999), because the sheets formed in the in vitro environment have morphological and physiological deficiencies, including mentioned that are very fragile, thin, stomata little functional, cuticle very thin and poorly developed, high transpiration rate, low photosynthetic rate (Pierik, 1990; Zimmerman, 1991).
However, the information that exists on this process of replacement of sheets formed in vitro, as well as the factors influencing during acclimatization of micropropagated plants, is scarce. Because the response or acclimatization of plants depends on the management to be provided, in this study was evaluated the effect of substrate type and dose of fertilization on foliar behavior occurring in micropropagated plants Agave americana var. oaxacensis during its acclimatization.
Materials and methods
The research was carried out during the period from September 2011 To August 2013, in the plant tissue culture and greenhouse laboratory of the Technological Institute of the Valley of Oaxaca, located in Nazareno, Xoxocotlan, Oaxaca. By micropropagation, buds from stem tissue, which were subjected to processes of multiplication and rooting later, plants were obtained Agave americana var. Oaxacensis, which had on average 6.75 leaves, 5 roots, 9.78 cm of longest leaf length with 0.64 cm wide. It were transplanted to polyethylene pots with a c apacity of 171 cm3, the substrates were produced with peat and sand in different proportions (1:2, 2:1, 3:1) in relation to its volume.
The acclimatization of the plants was carried out in a greenhouse with solar radiation reduced to 50%, temperature from 24 to 28 °C, high relative humidity 80-90% and received once a week 10 mL of the Universal Steiner Solution (1984) a 10% of nutrient concentration, under these conditions were maintained for 45 days, later they were transferred to a second greenhouse with higher solar radiation, temperature of 20 to 38 °C, low relative humidity and the contribution of the Steiner solution at the substrate level, was applied three times per week according to established treatments, until the end of the experiment.
During the course of the experiment will be followed up sheets formed in condition in vitro and ex vitro leaves formed in condition. To differentiate them were marked with hemp yarn red and yellow. Growth data on leaf length and width were recorded every 30 days from the beginning of the acclimatization of the greenhouse plants. Other variables evaluated were the replacement of sheets, i.e. sheets formed in number of in vitro condition and number of senescent leaves or lost formed in vitro condition.
The data recorded for each study variable were subjected to an analysis of variance. Tukey’s mean comparison was performed with a level of significance at 95% (α= 0.05) using the SAS statistical package (The SAS System for Windows 9.0). With the data obtained tables, graphs and figures were elaborated.
Results and discussion
Senescence or gradual loss of leaves formed in vitro environment
During the acclimatization process, the plants move from a low-sweeping environment to one that demands increased water demand. However, in plants grown in vitro the root it has little connection to the stem and are very fragile, which according Fila et al. (1998); Hazarika (2006) causes that when establishing them in the substrates are broken, therefore it prevents the efficient transport of water and nutrients. The low assimilation of water and nutrients, coupled with the morphology of leaves that are very thin, little development of the cuticle and little or no accumulation of waxes, influence their behavior during their acclimatization. Pospíšilová et al. (1999), mention that the sheets forming the in vitro plant condition, are unable to continue their development when are transferred to ex vitro environment. Fuentes et al. (2005) mention that the leaves produced in vitro storing structures act as nutrients, supporting the growth of new leaves developed ex vitro. Quesada and Valpuesta (2004) indicate that senescence and subsequent leaf loss is not only a degenerative process but also a recycling process in which nutrients are transported from aging cells to young leaves.
The plants Agave americana var. oaxacensis studied showed changes in their morphology when exposed to higher solar radiation; this occurred after 45 days of acclimatization. One of the first signs that acquired plants in response to the onset of the loss of their leaves formed in vitro, was the change from green to purple (Figure 1). This coloration was first presented in the lower leaves of older age, manifesting in the distal part and later expanding throughout the leaf blade. The purple color of the leaves was due to the presence of anthocyanins. The accumulation of anthocyanins in the leaves may be due to multiple stressors, stands out the high irradiation and nutrient deficiency (Peng et al., 2008). In senescent leaves where nutrient mobilization to other organs increases, anthocyanin accumulation would minimize the potential for photo-oxidative damage (Hoct et al., 2003).

Figure 1 Changes in the morphology of plants A. Americana var. oaxacensis, for the loss and formation of new leaves during their acclimatization in greenhouse.
Among the most important changes that have to adapt plants produced in vitro, is the gradual increase in light intensity. Once micropropagated plants are transferred to ex vitro environment, these are highly susceptible to photoinhibition (Osorio et al., 2010).
The loss of leaves formed in vitro condition was gradual, with the lower older than died first. Generally plants replaced all its leaves formed in vitro in a span of 240 days. This refractivity as mentioned by Pyung et al. (2007), who mention that the degeneration of cells in the tissue is slow, because the plant will ensure the translocation of the macromolecules to the sites of demand such as formation of new leaves, fruits or growth buds. It could also be due to the type of species, since the agaves have low growth rates, in addition their leaves are hard, fleshy, fibrous etc., which slows the degeneration of their tissues, as well as the capacity of renewal of the same.
In this study, the time it took the plants to lose their leaves was different. The plants that settled on the substrate that had a portion of peat combined with two of sand and that also received different fertilization, lost their leaves faster in a time of 180 days, compared to the plants that settled in the substrate with two to three servings of peat combined with sand and also received fertilizer, which lost their leaves formed in vitro condition, in a time of 240 days. At 90 days, the plants established in the substrate with a portion of peat combined with two of sand and that also received different fertilization, had lost 50% of their total leaves, whereas the plants established in the substrates with two and three portions of peat and also received different fertilization, had lost 30.2 and 33.15% of its leaves formed in ex vitro condition, respectively (Table 1).
Table 1 Effect of substrate and fertilization on leaf senescence of A. americana during acclimatization greenhouse.
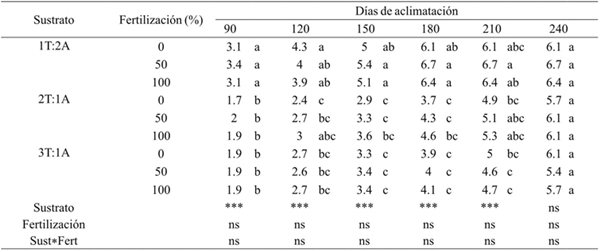
T= turba; A= arena; ***= valor de F altamente significativo (p≤ 0.01); ns= valor de F no significativo (p≥ 0.05). Medias con letras iguales no son estadísticamente diferentes (Tukey, 0.05).
Furthermore, the doubling time of acclimatization i.e. to the 180 days was observed the loss of 100% of the sheets formed in vitro plants were established in the substrate with a portion of combined peat with two sand and which also received different fertilization. But for established plants in substrates with two and three parts peat and also received different fertilization, had lost an average of 0.46 and 68.8% of its leaves formed in vitro respectively.
According to the analysis of variance, the substrate factor had significant effects (p≤ 0.01) on the decrease or loss of leaves formed in vitro during acclimatization at 90, 120, 150, 180 and 210 days. Instead the fertilization factor did not influence the loss of leaves formed in vitro during the days tested, or interaction substrate-fertilization.
The mean comparison analyzes showed that there was a significant difference in the number of leaves lost by the plants established in the different treatments. At 90 days of acclimatization, the plants that had lost the largest average amount of 3.2 leaves were those that settled on the substrates with a portion of peat combined with two of sand and that received different fertilization, while the plants that were established on substrates with two and three portions of peat, had lost an average of 1.8 leaves. This behavior of leaf loss in plants according to treatments was similar to the other days evaluated during acclimatization. The substrates in which the plants lost faster leaves formed in vitro, were compounds by a portion of peat combined with two sand and also received different fertilization (Table 1).
The plants were established on substrates with two and three servings of combined peat with two sand and we applied different fertilization, were favored as the total of their leaves that had formed during culture in vitro replaced by leaves new, 60 days later compared to plants established on substrates with a portion of peat combined with two of sand.
This indicates that senescence and subsequent loss of leaves formed in vitro was slower in plants that were established in a substrate with a higher proportion of peat combined with sand. The permanence of the sheets formed in vitro longer favored the generation of new leaves in the plant.
Formation of new leaves in ex vitro environment
During acclimatization, micropropagated plants generate new leaves with characteristics similar to plants growing in a natural environment.In the case of plants Agave americana var. oaxacensis, itwas observed that the first leaves that were generatedex vitro, showed a light coat of wax, which gave them a grayish hue; also began to acquire hardness. However, these leaves had not yet acquired the characteristics of a plant growing under natural environmental conditions. In this research, it was observed that from the second or third new sheet that formed ex vitro, showed a layer denser waxes, fleshy, rigid, hard and presence of spines on the edges of the leaves (Figure 2), which differs from the first leaves that the plants generated.

Figure 2 Morphology of new A. americana plants generated during acclimatization in greenhouse leaves
This indicates that the change is gradual and as the days of acclimatization advance, the plant presents changes in its leaves in response to the adaptation process. The agaves have morphological, anatomical and physiological characteristics such as the development of succulence or thickening in leaves, thick cuticle and deposit of different layers of waxes on the epidermal surface and CAM metabolism, which have allowed them to grow and develop in unfavorable environments (García-Mendoza, 2007).
The growth and development of the leaves will depend on the type of substrate and availability of nutrients. Based on the analysis of variance, the substrate factor had significant effects (p≤ 0.01) on the number of new leaves formed at 90, 120, 150, 180, 210 and 240 days of acclimatization. While the fertilization factor showed statistical significance in its effect on the mentioned characteristic, from the day 180 to the 240 days of acclimatization. In contrast, the substrate-fertilization interaction showed significant effects on the number of new leaves formed at 150, 180 and 240 days of acclimatization (Table 2).
Table 2 Effect of substrate and fertilization on the formation of new leaves of A american during acclimatization greenhouse.
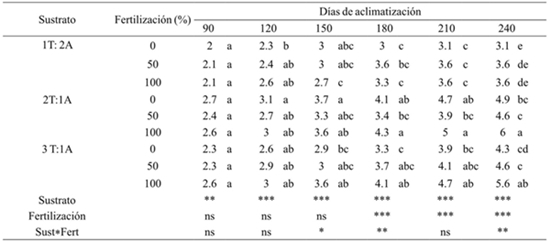
T= turba; A= arena; ***= valor de F altamente significativo (p≤ 0.01); ns= valor de F no significativo (p≥ 0.05). Medias con letras iguales no son estadísticamente diferentes (Tukey, 0.05).
The mean comparison analyzes showed that there was a significant difference in the number of new leaves generated in the plants established in the different treatments. At 90 days of acclimatization the plants established in the different treatments had generated the same amount of leaves on average (2.18). However, at 180 days the difference between plants was more noticeable. Those that were established in the substrate with two portions of peat combined with two of sand and that also received 100% fertilization had formed the largest number of leaves (4.3), while the smaller number of leaves (3), the plants established in the substrates with a portion of peat combined with two of sand that did not receive fertilization.
At 240 days of acclimatization, the plants established in the substrates with two and three portions of peat combined with a potion of sand and that also received different fertilization, had formed 32% more leaves with respect to the plants established in the substrates with a portion of peat combined with two of sand and which received different fertilization.
In general, it was observed that the plants generated more leaves when the nutrient solution with 100% concentration of nutrients was applied to them at the substrate level. This indicates that the substrate does not cover nutritional needs and that when a nutrient solution is added, the plants improve their condition. This is consistent with Enriquez-del Valle et al. (2012), who mentions that at the end of the stage of acclimatization of A. angustifolia, the level of plant growth and accumulation of N, P and K increased in direct relation to the concentration of nutrients in the nutrient solution, to the optimum at 66 and 100%.
Conclusions
The micropropagated plants supersede or replace the sheets formed in vitro as a physiological adaptation strategy for growth and development, as saying the new leaves formed in ex vitro conditions acquire phenotypic features that characterize the species of agave. The availability of nutrients determines the replacement of the leaves, in this case the contribution of organic matter in greater quantity, through a substrate such as peat and additional fertilization, favors the relationship between loss and formation of leaves making it gradual.
Literatura citada
Abreu, E.; González, G.; Ortiz, R.; Rodríguez, P.; Domech, R. y Garriga, M. 2007. Evaluación de vitroplantas de henequén (Agave fourcroydes Lem.) durante la fase de aclimatización. Cultivos Tropicales. 28(1):5-11. [ Links ]
Ángeles, C. G. C. 2010. De la biodiversidad al monocultivo: efectos del monocultivo de Agave angustifolia en el estado de Oaxaca. In: patrimonio natural y territorio. Ávila, R. L. E. y Pardini, G. (Coords). 1. Valle de Jovel, Chiapas. 96-138 pp. [ Links ]
Antonio, B. J. y Terán, M. E. 2008. Estrategias de producción y mercadotecnia del mezcal en Oaxaca. In: El cotidiano revista de la realidad mexicana actual. División de Ciencias Sociales y Humanidades. Universidad Autónoma Metropolitana- Unidad Azcapotzalco. Marzo- abril. México, D. F. 128:113-121. [ Links ]
Cruz, G. H.; Enríquez, del V. J. R.; Velasco, V. V. A.; Ruiz, L. J.; Campos, A. G. V y Aquino, G. D. E. 2013. Nutrimentos y carbohidratos en plantas de Agave angustifolia Haw y Agave karwinskii Zucc. Revi. Mex. Cienc. Agríc. 6:1161-1173. [ Links ]
Enríquez, del V. J. R. 2008. La propagación y crecimiento de agaves. Fundación Produce Oaxaca, A. C. Instituto Tecnológico del Valle de Oaxaca. Oaxaca. México. 46. p. [ Links ]
Enríquez, V. J. R.; Velasco, V. V. A.; Campos, A. G. V.; Hernández, G. E. and Rodríguez, M. M. N. 2009. Agave angustifolia plants grown with different fertigation doses and organic substrates. Acta Hortic. 843:49-55. [ Links ]
Enríquez, del V. J. R. y Cruz-Valdez, I. 2012. Acclimatization of Agave angustifolia Haw. vitroplants in inert substrates and fertirrigated with different nutrimental dose. In: Proceedings of the Second International Symposium on Soilless Culture and Hydroponics. Gómez-Merino, F. C.; Trejo-Téllez, L. I. y Rodríguez-Mendoza, M. N. (Eds). Acta Hortic. 947:101-104 [ Links ]
Fila, G.; Ghashghaie, J.; Hoarau, J. and Cornic, G. 1998. Photosynthesis leaf conductance and water relations of in vitro cultured grapevine rootstock in relation to acclimatization. Physiol. Plant. 102:411-418. [ Links ]
Fuentes, G.; Talavera, C.; Oropeza, C.: Desjardins,Y. and Santamaria, J. 2005. Exogenous sucrose can decrease in vitro photosynthesis but improve field survival and growth of coconut in vitro plantlets. In vitro Cell. Dev. Biol. Plant. 41:69-76. [ Links ]
García-Mendoza, A. 2007. Los agaves de México. Ciencias. (87):14-23. [ Links ]
George, E. F. y Debergh, P. C. 2008. Micropropagation: uses and methods. In: George, E. F.; Hall, M. and De Klerk, G. Plant propagation by tissue culture. 3er edition The Bacground.. Springer. 29-64 pp. [ Links ]
Hazarika, B. N. 2006. Morpho-physiological disorders in vitro culture of plants. Scientia Hortic. 108:105-120. [ Links ]
Hartmann, H.T. y Kester, D. E. 1994. Propagación de plantas. Principios y prácticas. 2da edición. Editorial CECSA. México. 760 p. [ Links ]
Hoch, W. A.; Singsaas, E. L. and Mc Cown, B. H. 2003. Resorption protection. Anthocyanins facilitate nutrient recovery in Autumn by shielding leaves from potentially damaging light levels. Plant Physiol. 133:1296-1305. [ Links ]
Osório, M.; Osório, J. and Romano, A. 2010. Chlorophyll fluorescence in micropropagated Rhododendron ponticum subsp. baeticum plants in response to different irradiances. Biol. Plant. 54(3):415-422. [ Links ]
Peng, M.; Hudson, D.; Schofield, A.; Tsao, R.; Yang, R.; Gu, H.; Bi, Y. and Rothstein, S. J. 2008. Adaptation of arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J. Exp. Bot. 59:2933-2944. [ Links ]
Pierik, R. L. M. 1990. Cultivo in vitro de las plantas superiores. Ed. Mundi Prensa. Madrid, España. 326 p. [ Links ]
Pospíšilová, J.; Tichá, I.; Kadleček, P.; Haisel, D. and Plzáková, Š. 1999. Acclimatization of micropropagated plants to ex vitro conditions. Biol. Plantarum. 42:481-497. [ Links ]
Pyung, O. L; Kim, H J and Nam, H. G. 2007. Leaf senescence. Annu. Rev. Plant Biol. 58:115-136. [ Links ]
Quesada, M. A y Valpuesta, V. 2004. Juvenibilidad, senescencia y abscisión. In: la ecofisiología vegetal. Una ciencia de síntesis. Reigosa M. J.; Pedrol, N. y Sánchez, A. (Coord.). Editorial Paraninfo, S.A. Madrid, España. 451-464 pp. [ Links ]
Sistema Nacional de Información para el Desarrollo Rural Sustentable (SNIDRUS) y Oficina Estatal de Información para el Desarrollo Rural Sustentable del estado de Oaxaca (OEIDRUS). 2011. Maguey mezcal. Regiones productoras del estado de Oaxaca 2011. San Bartolo Coyotepec, Oaxaca. 79 p. [ Links ]
Zimmerman, R. H. 1991. Micropropagation: technology and application. Ediciones Dordrecht. Kluwer Academic. 263 p. [ Links ]
Received: February 2017; Accepted: March 2017











 texto en
texto en 


