Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.2 Texcoco Fev./Mar. 2017
https://doi.org/10.29312/remexca.v8i2.50
Articles
Diagnosis of symptoms and pathogens associated with pepper wilt in Central Valleys of Oaxaca
1Instituto Tecnológico del Valle de Oaxaca. Ex Hacienda de Nazareno, Xoxocotlán, Oaxaca. CP. 71230. Tel. (01) 951 70444.
2CIIDIR-IPN-OAXACA. Calle Hornos, núm. 1 003. Santa Cruz, Xoxocotlán, Oaxaca, México. CP. 71230. Tel. y fax. (01) 951 5170610.
3Instituto Tecnológico El Llano. Aguascalientes, Aguascalientes, México. Tel. (01) 449 9161251. Fax. (01) 449 9162094.
In Central Valleys of Oaxaca growing pepper (Capsicum annuum L.) it is of great economic, social and nutritional importance. However, production is affected by ‛pepper wilt’ (MCH) because it generates significant economic losses. In this sense, a rapid diagnosis was made of the pepper production system, management, symptoms and pathogens related to MCH, from June 2013 to March 2014, through field trips, interviews with 63 farmers and sampling of field in 27 communities. The agrosystems of pepper production are diverse in infrastructure used, acreage, crop management, pests and diseases. The water pepper is the most cultivated and producers get their own seed, use chemical fertilizers and pesticides to control pests and diseases. The different symptoms were observed and listed as pepper wilt various production sites in Central Valleys, and pathogens were related symptoms, Rhizoctonia and Fusarium, and two oomycetes: Phytophthora and Pythium. Also determined a significant relationship (X2, p< 0.05) between subregions visited and frequency of distribution of pathogens: in the regions of Coatlan-Sola of Vega and Ejutla-Ocotlan had higher incidence of Rhizoctonia and Fusarium, but Zimatlán -Tacolula and Etla also presented Phytophthora and Pythium. The results show the presence of two or more pathogens in the same site, and consequently higher incidence in the field of the disease.
Keywords: Capsicum annuum; Phytophthora capsici; Rhizoctonia solani; host-pathogen; pathogenicity
En Valles Centrales de Oaxaca el cultivo de chile (Capsicum annuum L.) es de gran importancia económica, social y alimenticia. No obstante, la producción es afectada por ‘marchitez del chile’ (MCH) porque genera pérdidas económicas importantes. En este sentido, se realizó un diagnóstico rápido del sistema de producción del chile, manejo, síntomas y patógenos relacionados con MCH, de junio de 2013 a marzo de 2014, mediante recorridos de campo, entrevistas a 63 agricultores y toma de muestreos de campo en 27 comunidades. Los agrosistemas de producción de chile son diversos en infraestructura utilizada, superficie sembrada, manejo del cultivo, plagas y enfermedades. El chile de agua es el más cultivado y los productores obtienen su propia semilla, utilizan fertilizantes químicos y pesticidas para el control de plagas y enfermedades. Se observaron diferentes síntomas catalogados como marchitez del chile en diversos sitios de producción de Valles Centrales, y los patógenos relacionados con el síntoma fueron, Rhizoctonia y Fusarium, y dos oomycetos: Phytophthora y Pythium. También, se determinó una relación significativa (X2, p< 0.05) entre subregiones visitadas y frecuencia de la distribución de patógenos: en las regiones de Coatlán-Sola de Vega y Ejutla-Ocotlán hubo mayor incidencia de Rhizoctonia y Fusarium, pero para Zimatlán-Tacolula y Etla además se presentó Phytophthora y Pythium. Los resultados muestran la presencia de dos o más patógenos en el mismo sito, y consecuentemente mayor incidencia en campo de la enfermedad.
Palabras clave: Capsicum annuum; Phytophthora capsici; Rhizoctonia solani; hospedero-patógeno; patogenicidad
Introduction
It is recognized the incidence of phytopathogens and susceptibilities in the different variants of pepper, and in different production systems (greenhouse, biosphere, open sky, etc), so that diseases cause large losses in quality and yield, depending on favorable environmental conditions for its presence and crop management practices (Guigon-López and González-González, 2001). Among the major pathogens that attack the crop pepper are: Phytophthora capsici, Xanthomonas campestri sp. Vesicatoria, Alternaria spp., A. alternata, Oidiopsis taurica, Leveillula taurica, Fusarium spp., Rhizoctonia solani, Pythium spp., Sclerotinia sclerotium, Sclerotium rolfsii, various nematodes (Meloidogyne spp. and Heterodera spp.) and viral complexes tomato, cucumber, snuff, potatoes, peppers and others (Guigon-López and González-González, 2001; Velázquez et al., 2002; Velásquez-Valle and Amador-Ramírez, 2007; Chew et al., 2008).
The wilt of pepper or drier is one of the main diseases that cause losses in production from 26% to 90%, not only in Mexico but to worldwide. It is caused by a complex phytopathological of Fusarium spp., Phytophthora capsici, Verticillium spp., Macrophomina spp., Rhizoctonia solani, Pythium spp. and Sclerotium rolfsii, acting alone or in combination (Ristaino and Johnston, 1999; Velásquez et al., 2000 and 2001; González-Pérez et al., 2004; Rico-Guerrero et al., 2004; Vásquez et al., 2009; Montero-Tavera et al., 2013). As a result of the incidence of this disease, the acreage has declined or has moved into new areas (González-Pérez et al., 2004). The complex etiology or pathological complex makes it difficult to establish a specific epidemiological pattern of disease development, preventing establish a specific management program coupled with high genetic variability in pathogens involved (Montero-Tavera et al., 2013).
The symptoms of wilting of pepper or drier are varied but essentially reflected in leaf wilting without color change, chlorosis and then premature leaf fall, leaf ripening, early and irregular ripening of fruits, root rot or stem base rot and plant death (Avelar and Marban, 1989; Velásquez-Valle et al., 2001; Vásquez et al., 2002). However, it has improved pathogens identification techniques based on DNA extraction and PCR amplification (Rico-Guerrero et al., 2004).
Among the main management strategies of the pepper wilting, the use of rhizospheric fungi antagonistic to Fusarium spp, Rhizoctonia solani and Phytophthora capsici (Guillen-Cruz et al., 2006; Jiang et al., 2006; Robles-Yerena et al., 2010; Ramos-Sandoval et al., 2010); rhizobacteria antagonistic to P. capsici (Sang et al., 2008; Sang and Kim, 2012); combination of biofumigaciones and antagonistic microorganisms (Wang et al., 2014); inoculations with rhizospheric mycorrhizal fungi (Ozgonen and Erilic, 2007); combination of use of composts and grafting in resistant rootstocks (Gilardi et al., 2013); crop rotation (Lamour and Hausbeck, 2003), use of resistant germplasm to main complex Fusarium-Rhizoctonia-Phytophthora-Pythium (Koc et al., 2011; Babu et al., 2011); even when there is no total resistance to the complex; and fungicide application (Chew et al., 2008). However, they begin to identify expression profiles of specific DNA sequences (transcriptome) related phenotypic resistance to P. capsici from the creole collection Morelos-334 (Walker and Bosland, 1999; Richins et al., 2010). Also in native Mexican pepper varieties they have been identified sources of resistance to P. capsici (Moran-Bañuelos et al., 2010).
In general, it is necessary to continue documenting the presence and magnitude of damage caused by pepper wilt, the regional situation and associated pathogens, with the purpose of making decisions about management programs and duly justified. The regional diagnostics help detect changes and prevalence of the disease, relations associated pathogens, pathogen-host interactions and aspects of culture system that favor or stop the development of the disease, among other aspects (Velásquez-Valle et al., 2001a; Guigón-López and González-González, 2001; González-Pérez et al., 2004; Chew et al., 2008; Vásquez et al., 2009). In this context, the objective of the study was to perform a rapid diagnosis of the symptom and pathogens associated with chill wilt in the Central Valleys of Oaxaca, Mexico.
Materials and methods
Study area
The Central Valleys are located in the central region of the state of Oaxaca and is comprised of 121 municipalities, which in its orography define three valleys: Etla, Tlacolula and Zimatlán-Zaachila-Ocotlán where the main agricultural activities are made to (Arellanes et al., 2006). In the region there is a dry and semi-dry climate, warm to semi-dry with an annual average temperature of 18 to 22 °C, annual precipitation of 725 mm and evaporation of 1 862 mm. To cover the three valleys of the region, 27 communities from 18 municipalities were visited, which were grouped into four subregions: 1) Coatlan to Sola of Vega; 2) Ocotlan to Ejutla; 3) Zimatlán to Tlacolula; aAnd 4) Etla, which lie between 16° 13’ 2.17’’ to 17° 14’ 48.14’’ north latitude at 96° 28’ 27.59’’ to 96° 70’ 11.11’’ west longitude and altitudes of 1 412 at 1 760 m.
Interview with producers of pepper
From June 2013 To March 2014, 63 producers who had pepper crops were interviewed in Central Valleys of Oaxaca. During the visit and tour of the cultivated plots (open sky, greenhouse and shade mesh or biospaces), a survey was applied to the farmer with the objective of describing the management practices of his crop, main problems, evaluation of incidence of wilt Cultivation under a general scale (1-25% mild, 26-50% moderate, 51-75% severe and 76-100% very severe; Guigón-López and González-González, 2001) and a sample of diseased plants with symptoms of wilting pepper.
Phytopathological diagnosis in diseased plant samples
Isolation and identification of pathogens. After obtaining samples of plants with symptoms of wilting, they were rinsed for 30 min in running water and cut sections of 1 cm root and 0.5 cm stem. They were then disinfested with 3% sodium hypochlorite for 3 min, rinsed three times with sterile distilled water and the stem and root sections were deposited in petri dishes with potato-dextrose agar (PDA), V8- Agar (V8A) and agar-water (A-A) plus two lactic acid drops per 100 mL of prepared medium, the latter to inhibit the growth of bacteria. They were incubated for 48 hours at room temperature (25-35 °C) with daily monitoring to verify colony growth.
Those sections with colonies were transferred to boxes with new medium, in order to obtain pure colonies by the hyphae method or monozoospores culture. These colonies were the basis for making the preparations with methyl blue and glycerol and thus proceed to the identification of pathogens, using a composite microscope (10x, 40x and 100x). Complementarily, direct preparations of the infected material were made where colony growth was observed (wet chambers, method of taping). The phytopathological identification was based on the keys of Barnett and Hunter (1972) for Rhizoctonia and Fusarium, and Romero-Cova (1988) for Phytophthora and Pythium.
Pathogenicity tests of isolates of Rhizoctonia and Phytophthora
Preparation and quantification of the inoculum. Once identified the pathogens in the above mentioned isolates were cultivated in PDA medium for their massive increase and after 20 days of growth, the inoculate were prepared for the pathogenicity test. A total of 937 isolates were obtained. In each pathogen the preparation was as follows:
a) Phytophthora capsici. In petri dishes with 20 mL sterilized distilled water were placed 10 discs of culture medium containing mycelium and incubated for 8 days at room temperature (25-35 °C) for the formation of sporangia and subsequent release of their zoospores place the petri dishes under refrigeration at 6 °C for half an hour. Once generated zoospores and to determine the dose of the inoculum using a hemacytometer the zoospore suspension was adjusted to a concentration of 25 000 zoospores mL-1 and each pepper plant target were added to the stem base 10 mL of suspension of zoospores.
b) Rhizoctonia solani. In following the growth of the fungus in petri dishes, when it covered the total surface of the boxes, 20 mL of sterile distilled water was added and the mycelium was removed with a spatula to separate it from the agar. Subsequently, the resulting suspension was homogenized and milled to obtain minute fragments and counted with the hematocytometer. From the count, a suspension of R. solani fragment at a concentration of 95 000 mycelial fragments mL-1 was prepared and 10 mL of the suspension were added to the base of the stem of the plant pepper target.
Prior to inoculation pepper seeds of water were sown and performing 45 day old, seedlings were transplanted into polystyrene cups with sterile substrate (peat moss) and when the plants had 4 to 6 leaves no cotyledons were inoculated with the suspensions Rhizoctonia solani and Phytophthora capsici previously prepared. The application was made 2 cm below the neck of the pepper seedlings. The plants were maintained for 60 days in the greenhouse at a temperature of 25 ± 5 °C and a relative humidity of 80 ± 5%. The moisture of the substrate supporting the plants was kept constant for all treatments, 937 isolates.
In both inoculations, at 60 days of age the plants were evaluated for damage and reproduction of pepper wilt symptom. In the presence of Rhizoctonia stem base necrosis it was observed with detachment of epidermis to 15 days of inoculation and Phytophthora loss of turgor, yellowing and necrosis was observed in the stem base 10 days inoculation. In both cases the pathogenicity was quantified as number of dead plants or with symptoms of the disease.
Statistic analysis
With the farmers’ responses to their socio-demographic data and crop management, chi-square tests were performed to assess the homogeneity of response and independence classes among agroecological niches (subregions) and pathogens identified for isolation of plant samples with symptoms of wilting pepper. In addition, a correspondence analysis was carried out with the objective of identifying the variables with the highest descriptive value of the pepper crop. Finally, a chi-square test was applied to assess the independence of pathogenic isolates of Phytophthora and Rhizoctonia and subregions of the Central Valley where there were crops of pepper. All analyzes were done with the statistical packages SPSS (Visauta, 2002; SAS, 1999).
Results and discussion
As for the description of the pepper production system, it was found that the age of the producers who cultivated pepper went from 40 to 60 years of age (65.1%) and there were farmers who had from 10 to 30 years planting the crop. However, most of them sow half a hectare or less (88.9%) and regionally, water pepper is the most cultivated type (92.1%). Although there were plantations of habanero, jalapeño, tabiche, poblano and tusta. At the time of the field visit, the crops had 90 to 120 days of being transplanted and were in flowering and production, when the presence of wilting was most noticeable. They always fertilize either organically or inorganic-organic combinations, and usually perform chemical controls of diseases or pests.
The seed comes from its own previous cultivation or exchange with neighbors for both irrigation and temporary crops. Farmers point out that they always have problems with the cultivation, whether of a sanitary type (pests or diseases) or high costs of inputs and low sales in the regional markets that are the main destination of production. The main crop is the water pepper, and is practically sown all year round in the Central Valleys. However, when wilt was present the damage was evaluated from mild to moderate (90.5%) of sites visited; that is, the disease began to appear and the base of the stem had an advanced degree of necrosis. Combinations of root rot, weevil attack, or presence of viral infections were sometimes present. The results show that the management of the pepper crop is variable.
The most water pepper plant almost all year, this is verified with statistical significant differences (p< 0.01) between classes of responses. The results corroborate previous signs of Velasco et al. (1998) and López (2007), who stated that the production of water pepper in small areas, besides generating income for the producers, stimulates the local economy because, if the parcels are irrigated, they can grow pepper throughout year. On the other hand, the exchange of seed without health care can help to spread disease in the region, this coincides with the field observations of López (1989), noting that the rapid increase in area sown between 1970 and 1985, brought with it the appearance and development of problems and diseases, because there are no improved varieties or seed health checks.
In correspondence analysis, it was determined that the age of the farmer, area planted, type of pepper planted, planting season and transplant, phytosanitary problems, fertilizer use and seed origin, were determinant in the description of the pepper production system of 63 farmers in the Central Valleys of Oaxaca. The fourth main dimension explained 82.4% of total variability (Table 1).
Table 1 Values and eigenvectors of the correspondence analysis based on farmers’ responses to the management of the pepper crop in Central Valleys of Oaxaca.
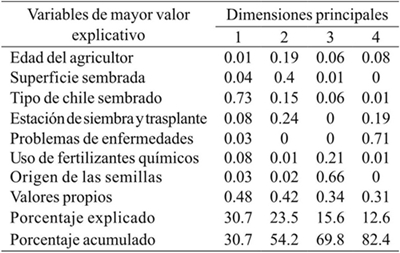
In the graphical representation of the correspondence analysis, the first two main dimensions (Figure 1) showed that the pepper production systems follow a pattern determined by the area sown, planting and transplanting, type of pepper and use of fertilizers. Thus, some plots in the Coatlán-Sola of Vega subregion follow a particular pattern because they are essentially planted with tusta pepper, and in the case of certain plantations established in the Tlacolula-Zimatlán subregion, mainly of water pepper, based on the planting season and transplant and use of agrochemicals (30.7%). It was also possible to find a pattern of differences of small, medium and large sown areas of 0.25 or less than 1 ha. In the case of farmers with an area of less than 0.25, they correspond to the Coatlán-Sola of Vega, Ocotlán-Ejutla and Etla subregions, with the largest area corresponding to the Etla subregion.
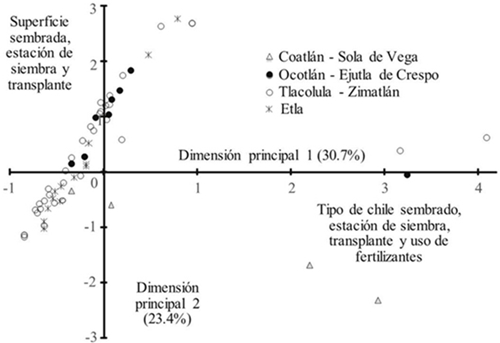
Figure 1 Description of management of pepper production units in three subregions of Central Valleys of Oaxaca, based on the first two main dimensions of correspondence analysis.
The 79.4% of the area sown corresponds to irrigation and irrigation-time, which means that when irrigation rolled flooding in the plot, coinciding with the rainy season and flooding. Thus, the water dispersed sporangia and motile zoospores then find a favorable environment to disperse released, and the waterlogged soil becomes focus of infection and means of survival of the pathogen, as indicated earlier reports to P. capsici (Erwin and Ribeiro, 1996; Ávila-Quezada et al., 2005 and Silva-Rojas et al., 2009).
As for incidence of pepper wilt caused by phytopathological complex it was observed in the entire region of the Central Valleys explored four diseases were presented: Rhizoctonia, Fusarium, Phytophthora and Pythium. The subdivision into four subregions made it possible to observe a relationship between pathogens and subregions. For example, for the regions of Coatlan-Sola of Vega and Ejutla-Ocotlán there was a higher incidence of Rhizoctonia and Fusarium, but also Zimatlán-Tlacolula Phytophthora and Pythium also presented. The presence of the four pathogens also became evident in the Etla region. It is thought that wilt damages occur more frequently if there is a prevalence of two or more pathogens in the soil, and specifically the regions of Etla and Zimatlán-Tlacolula presented with a higher incidence of the disease. This results are consistent with previous reports of Vásquez et al. (2009) in water pepper (Table 2).
Table 2 Observed frequency of pathogens identified in the samples (n= 937) isolates in pepper plants with wilt symptoms, in the Central Valleys of Oaxaca.
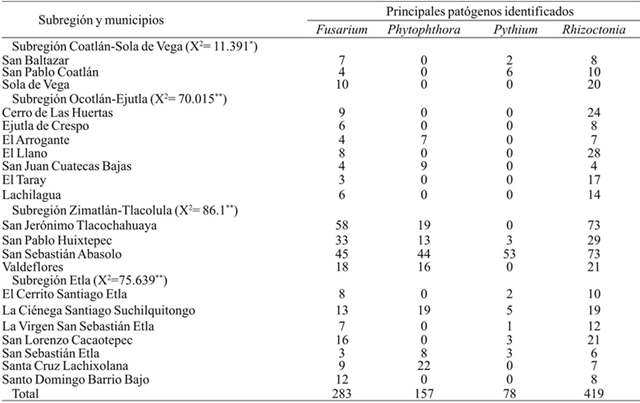
*= diferencias significativas a nivel p≤ 0.05; **= diferencias significativas a nivel p≤ 0.01 (prueba de χ2).
Diagnostic results on the association of phytopathological complex Fusarium Rhizoctonia-Fusarium-Phytophthora-Pythium, was observed and identified the presence of two or more of them and manifesting symptoms of wilting, which coincide with the work of Durán-Ortiz et al. (2001) of wilt in the States of Zacatecas and Aguascalientes. In Camargo, Delicias and South of Chihuahua was determined to Phytophthora capsici as the causal agent of wilting pepper (Silva-Rojas et al., 2009 and Guigon-López and González-González, 2001). Vásquez et al. (2009) study found that Phytophthora and Rhizoctonia are the causative agents of wilting for three communities in the state of Oaxaca. Other studies indicate that pathogens were more frequently associated with Rhizoctonia spp. and Fusarium spp., in Aguascalientes and Zacatecas (Rico-Guerrero, 2002).
When pathogenicity test was performed at 17 and 52 isolations of Phytophthora and Rhizoctonia, respectively; in both cases, a number of non-pathogenic isolates were detected rather than pathogenic. Of these, three in Phytophthora and seven in Rhizoctonia were determined. Even though in these pathogenicity tests there were isolates that independently did not reproduce the symptoms, it is not a reflection of loss of pathogenicity because in the field two or more pathogens regularly converge in the same site to reproduce the symptoms of wilt. It should also be noted that inoculate were only made in water pepper plants at 45 days of age, the first growth phase (Table 3).
Table 3 Results of tests of pathogenicity of 17 isolates of Phytophthora and 52 in Rhizoctonia, and their relation to the subregion of origin in Central Valleys of Oaxaca.
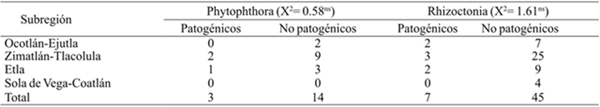
A inoculations 60 days, symptoms of Rhizoctonia plants were chlorosis, after necrosis the stem base with detachment of epidermis and finally death of the plant. In inoculations with Phytophthora, symptoms appeared 55 days of inoculation; among the most notable were loss of leaf turgor or wilting, defoliation, slight yellowing, necrosis at the base of the stem and plant death. Pathogenicity was quantified as the number of dead plants with symptoms of the disease.
Conclusions
The agro-systems of pepper production in Central Valleys of Oaxaca are diverse in infrastructure used, area planted, crop management, pests and diseases. In this sense, water pepper is highlighted as the most frequent and largest area planted throughout the year. Producers usually obtain their own seed, use chemical fertilizers, regularly present pest incidences such as fruit weevil and root rot associated with wilt symptoms, and 84.1% of them use agrochemicals for their control. In the correspondence analysis, it was determined that the age of the farmer, area planted, type of pepper planted, planting season and transplant, phytosanitary problems, fertilizer use and seed origin were the variables with the highest descriptive value of the production system of Pepper from 63 farmers in Central Valleys of Oaxaca.
The different symptoms were observed and listed as pepper wilting in several production sites in the Central Valleys and related pathogens were Rhizoctonia and Fusarium symptoms, and two oomycetes: Phytophthora and Pythium. A significant relationship is also determined (X2, p< 0.05) between subregions visited and frequency of distribution of pathogens: in the regions of Coatlán-Sola of Vega and Ejutla-Ocotlán had higher incidence of Rhizoctonia and Fusarium, but Zimatlán-Tlacolula and Etla also presented Phytophthora and Pythium. The results show the presence of two or more pathogens in the same site, and consequently higher incidence in the field of the disease.
In the pathogenicity tests one or more symptoms of wilt pepper reproduced by inoculating 3 and 7 pathogenic isolates of Phytophthora and Rhizoctonia respectively; in plants of 45 days old water pepper. These pathogenic isolates are feasible to be used in tests of tolerances or resistance of pepper germplasm.
Literatura citada
Arellanes, M. A.; de la Cruz, V.; Romero, M. A.; Sánchez, C.; Ruiz, F. J.; Martínez, V. R. y López, E. 2006. Historia y geografía de Oaxaca. Carteles Editores, Oaxaca, México. 159 p. [ Links ]
Avelar, M. J. y Marban, M. 1989. Intentos de control de la marchitez del chile ocasionada por el hongo Phytophthora capsici en la región de Valsequillo. Puebla. In: Memorias XVI Congreso Nacional de Fitopatología, Sociedad Mexicana de Fitopatología, A. C. Texcoco. México. 11 p. [ Links ]
Ávila-Quezada, G. D.; Gardea, A.; Pedroza-Sandoval, A.; Silva-Rojas, H. V. and Fernández-Pavía, S. 2005. Spatial dynamic of pepper wilt. Phytopathology. 95:149. [ Links ]
Babu, B. S.; Pandravada, S. R.; Prasada-Rao, R. D. V. J.; Chakrabarty, S. K. and Varaprasad, K. S. 2011. Global sources of pepper genetic resources against arthropods, nematodes and pathogens. Crop Protection. 30:389-400. [ Links ]
Barnett, H. L. and Hunter, B. B. 1972. Illustrated genera of imperfect fungi. Burgess publishing company. Minneapolis, MN, USA. 273 p. [ Links ]
Chew, M. Y. I.; Vega, P. A.; Palomo, R. M. y Jiménez, D. F. 2008. Principales enfermedades del chile (Capsicum annuum L.). INIFAP-Centro de Investigación Regional Norte Centro. Campo Experimental, La Laguna. México, D. F. Folleto Técnico Núm. 153. 2 p. [ Links ]
Durán-Ortiz, L.; Pérez-Moreno, J. L.; Sánchez-Pale, J. R. y Olalde-Portugal, V. 2001. Identificación de los hongos que ocasionan la “marchitez del chile” en la región del Bajío. In: Memorias. XXVIII Congreso Nacional de la Sociedad Mexicana de Fitopatología. Querétaro, México. 13 p. [ Links ]
Erwin, D. C. and Ribeiro, O. K. 1996. Phytophthora diseases worldwide. American Phytopathological Society Press. St. Paul, Minnesota. 562 p. [ Links ]
FAOSTAT. 2010. Food and Agriculture Organization of the United Nations. Exportaciones: país por producto. Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO). [ Links ]
Gilardi, G.; Baudino, M.; Moizio, M.; Pugliese, M.; Garibaldi, A. and Gullino, M. L. 2013. Integrated management of Phytophthora capsici on bell pepper by combining grafting and compost treatment. Crop Protection. 53:13-19. [ Links ]
González-Pérez, E.; Yáñez-Morales, M. J.; Santiago-Santiago, V. y Montero-Pineda, A. 2004. Biodiversidad fungosa en la marchitez del chile y algunos factores involucrados de José Mazo. El Verde, Puebla. Agrociencia. 38:653-661. [ Links ]
Guigón-López, C. y González-González, P. 2001. Estudio regional de las enfermedades del chile (Capsicum annuum L.) y su comportamiento temporal en el sur de Chihuahua, México. Rev. Mex. Fitopatol. 19:49-56. [ Links ]
Guillem-Cruz, R.; Hernández-Castillo, F. D.; Gallegos-Morales, G.; Rodríguez-Herrera, R.; Aguilar-González, C. N.; Padrón-Corral, E. y Reyes-Valdés, M. H. 2006. Bacillus spp. como biocontrol en un suelo infestado con Fusarium spp., Rhizoctonia solani Kühn y Phytophthora capsici Leonian y su efecto en el desarrollo y rendimiento del cultivo de chile (Capsicum annuum L.). Rev. Mex. Fitopatol. 24:105-114. [ Links ]
Jinag, Z-Q.; Guo, Y-H.; Li, S-M.; Qi, H-Y and Guo, J-H. 2006. Evaluation of biocontrol efficiency of different Bacillus preparations and field applications methods against phytophthora blight of bell pepper. Biological Control. 36:216-223. [ Links ]
Koc, E.; Üstün, A. S.; Islek, C. and Arici, Y. K. 2011. Defense responses in leaves of resistant and susceptible pepper (Capsicum annuum L.) cultivars infected with different inoculum concentrations of Phytophthora capsici Leon. Sci. Hortic. 128:434-442. [ Links ]
Lamour, K. H. and Hausbeck, M. K. 2003. Effect of crop rotation on the survival of Phytophthora capsici in Michigan. Plant Disease. 87:841-845. [ Links ]
López, P. 1989. El chile de agua (Capsicum annuum L.) en Valles Centrales de Oaxaca. Campo Experimental Valles Centrales de Oaxaca. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP). Oaxaca, México. 22 p. [ Links ]
López, P. 2007. El chile de agua: un chile típico de Valles Centrales de Oaxaca. Agroproduce. 16:8-12. [ Links ]
Montero-Tavera, V.; Guerrero-Aguilar, B. Z.; Anaya-López, J. L.; Martínez-Martínez, T. O.; Guevara-Olvera, L. y González-Chavira, M. M. 2013. Diversidad genética de aislados de Rhizoctonia solani (Kuhn) de chile e México. Rev. Mex. Cienc. Agríc. 4:1043-1054. [ Links ]
Moran-Bañuelos, S. H.; Aguilar-Rincón, V. H.; Corona-Torres, T. y Zavaleta-Mejía, E. 2010. Resistencia a Phytophthora capsici Leon de chiles nativos del sur de Puebla, México. Rev. Fitotec. Mex. 33:21-26. [ Links ]
Ozgonen, H. and Erkilic, A. 2007. Growth enhancement and phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper. Crop Protection. 26:1685-188. [ Links ]
Ramos-Sandoval, R.; Gutiérrez-Soto, J. G.; Rodríguez-Guerra, R.; Salcedo-Martínez, S. M.; Hernández-Luna, C. E.; Luna-Olvera, H. A.; Jiménez-Bremont, J. F.; Fraire-Velázquez, S. y Almeyda-León, I. H. 2010. Antagonismo de dos ascomicetos contra Phytophthora capsici Leonian, causante de la marchitez del chile (Capsicum annuum L.). Rev. Mex. Fitopatol. 28:75-86. [ Links ]
Rico, G. L; Guerrero, A. B.; López, V. A.; Guevara, G. L.; Torres, P. I. y González, C. M. 2001. Búsqueda de resistencia natural en plantas de chile (Capsicum spp.) contra aislados del complejo fúngico que causa pudrición de raíz. In: XXVIII Congreso Nacional de Fitopatología. [ Links ]
Rico-Guerrero, L.; Medina-Ramos, S.; Muñoz-Sánchez, C. I.; Guevara-Olivera, L.; Guevara-González, R. G.; Guerrero-Aguilar, B. Z.; Torres-Pacheco, I.; Rodríguez-Guerra, R. y González-Chavira, M. M. 2004. Detección de Phytophthora capsici Leonian en plantas de chile (Capsicum annuum L.) mediante PCR. Rev. Mex. Fitopatol. 22:1-6. [ Links ]
Richins, R. D.; Micheletto, S. and O’Connell, M. A. 2010. Gene expression profiles unique to chile (Capsicum annuum L.) resistant to phytophthora root rot. Plant Sci. 178:192-201. [ Links ]
Ristaino, J. B. and Johnston, S. B. 1999. Ecologically based approaches to management of phytophthora blight on bell pepper. Plant Des. 83:1080-1089. [ Links ]
Robles-Yerena, L.; Rodríguez-Villareal, R. A.; Ortega-Amaro, M. A.; Fraire-Velázquez, S.; Simpson, J.; Rodríguez-Guerra, R. and Jiménez-Bremont, J. F. 2010. Characterization of a new fungal antagonist of Phytophthora capsici. Sci. Hortic. 125:248-255. [ Links ]
Romero-Cova, S. 1988. Hongos fitopatógenos. Universidad Autónoma Chapingo (UACH). Dirección del Patronato Universitario. A. C. 347 p. [ Links ]
Sang, M. K. and. Kim, K. D. 2012. Plant growth-promoting rhizobacteria suppressive to phytophthora blight affect microbial activities and communities in the rhizosphere of pepper (Capsicum annuum L.) in the field. Appl. Soil Ecol. 62:88-97. [ Links ]
Sang, M. M.; Chun, S-C. and Kim, K. D. 2008. Biological control of phytophthora blight of pepper by antagonistic rhizobacteria selected from a sequential screening procedure. Biological Control. 46:424-433. [ Links ]
Silva-Rojas, H. V.; Fernández-Pavía, S. P.; Góngora-Canul, C.; Macías-López, B. C. y Ávila-Quezada, G. D. 2009. Distribución espacio temporal de la marchitez del chile (Capsicum annuum L.) en Chihuahua e identificación del agente causal Phytophthora capsici Leo. Rev. Mex. Fitopatol. 27(2):134-147. [ Links ]
SAS. 1999. SAS® Procedures guide, Version 8. SAS Institute Inc. Cary, NC, USA. 1 643 p. [ Links ]
Vásquez L. A.; Tlapal, B.B; Yáñez, M. M.; Pérez P. R. y Quintos, E. M. 2009. Etiología de la marchitez del ‘chile de agua’ (Capsicum annuum L.) en Oaxaca, México. Rev. Fitotec. Mex. 32:127-134. [ Links ]
Velásquez, V. R.; Rincón, V. J. F. y López, F. L. C. 2000. Guía para controlar la pudrición de la raíz de chile en Aguascalientes y Zacatecas. Folleto para Productores Num. 25. Campo Experimental Calera-INIFAP, SAGARPA. 16 p. [ Links ]
Velásquez-Valle, R.; Medina-Aguilar, M. M. y Luna-Ruiz, J. J. 2001. Sintomatología y géneros de patógenos asociados con las pudriciones de la raíz del chile (Capsicum annuum L.) en el norte-centro de México. Rev. Mex. Fitopatol. 19:175-181. [ Links ]
Velázquez, V.; Medina, A. y Mena, C. 2002. Guía para identificar y manejar las principales enfermedades parasitarias del chile en Aguascalientes y Zacatecas. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Folleto técnico Núm. 20. 41 p. [ Links ]
Velásquez-Valle, R. y Amador-Ramírez, M. D. 2007. Análisis sobre la investigación del chile seco (Capsicum annuum L.), realizadas por el Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias en los estados de Aguascalientes y Zacatecas, México. Rev. Mex. Fitopatol. 25:80-84. [ Links ]
Velasco, V. V.; Trinidad, S. A.; Tirado, T. J.; D. Téliz, O. D.; Martínez, G. A. y Cadena, H. M. 1998. Efecto de algunos nutrimentos en plantas de chile de agua infectadas con virus. Terra Latinoam. 16:317-324. [ Links ]
Visauta, V. B. 2002. Análisis estadístico con SPSS para Windows. McGraw-Hill, Madrid España. 304 p. [ Links ]
Walker, S. J. and Bosland, P. W. 1999. Inheritance of phytophthora root rot and foliar blight resistance in pepper. J. Am. Soc. Hortic. Sci. 124:14-18. [ Links ]
Wang, Q.; Y.,Ma. G. ; Wang, Z.; Gu, D.; Sun, X. and Chang, Z. 2014. Integration of biofumigation with antagonistic mrioorganism can control phytophthora blight of pepper plants by regulating soil bacterial community structure. Eur. J. Biol. 61:58-67. [ Links ]
Received: November 2016; Accepted: January 2017











 texto em
texto em 


