Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.1 Texcoco Jan./Fev. 2017
https://doi.org/10.29312/remexca.v8i1.80
Investigation note
Cation exchange capacity: description of the silver thiourea method (AgTU + n )
1Colegio de Postgraduados. Carretera México-Texcoco km 36.5. Montecillo, Texcoco, Estado de México. CP. 56230. Tel. 01 595 9520200, ext. 1255. (galvispinola@gmail.com).
2Universidad Autónoma de Nayarit, Ciudad de la cultura. Amado Nervo s/n. Tepic, Nayarit, México. CP. 63155. Tel. 2118800, ext. 8951. (sip@uan.edu.mx).
3Universidad Autónoma Chapingo. Carretera México-Texcoco, km 38.5. CP. 56230. Chapingo, Estado de México. Tel. 01 595 9521649. (mariovazquez@coahuila.com).
4Colegio Mexicano de Especialistas en Recursos Naturales. Callejón de las flores núm. 8, s/n. San Luis Huexotla, Texcoco, Estado de México. CP. 56220. Tel. 01 595 9285235.
The cation exchange capacity (CIC) is an indirect indicator of soil damping capacity. The methods used to estimate the CIC are based on soil saturation with an index cation. The silver thiourea method (AgTU + n) is a technique for estimating CIC, since AgTU + n is not Found naturally in the soil; however, Ag + is sensitive to the visible spectrum and the alkaline pH of the solution, forming Ag0 and Ag2O. In the study soils, the amount of sample required for the estimation of CIC was 0.8 g. The protection of glassware with aluminum foil and the storage of extracts in the dark increased the shelf life of AgTU + n. the CICSI, as a function of CCICI of 166 soils, has a linear relationship, that is, that the variation in the CIC estimation is due to the adsorption of AgTU + n on the clays of the soils, and not by sources of variation generated by other ions. The AgTU + n method makes it possible to estimate the CIC quickly and inexpensively, taking into account the aforementioned considerations.
Keywords: : bentonite; flocs and adsorption
La capacidad de intercambio catiónico (CIC) es un indicador indirecto de la capacidad amortiguadora de los suelos. Los métodos empleados para estimar la CIC se basan en la saturación del suelo con un catión índice. El método de la tiourea de plata (AgTU + n ) es una técnica para la estimación de la CIC, puesto que el AgTU + n no se encuentra de forma natural en el suelo; sin embargo, el Ag + es sensible al espectro visible y al pH alcalino de la solución, formando Ag0 y Ag2O. En los suelos de estudio, la cantidad de muestra requerida para la estimación de la CIC fue 0.8 g. La protección de la cristalería con papel aluminio y el almacenamiento de los extractos en la oscuridad aumentó la vida útil del AgTU + n . La CICSI, como función de CICCI de 166 suelos tiene una relación lineal, es decir, que la variación en la estimación de la CIC es debida a la adsorción del AgTU + n en las arcillas de los suelos, y no por fuentes de variación generadas por otros iones. El método de la AgTU + n , permite estimar la CIC de manera rápida y a bajo costo, tomando en cuenta las consideraciones antes mencionadas.
Palabras clave: bentonita; flóculos y adsorción
Introduction
The cation exchange capacity (CIC) estimates the loading sites of the clays, both the permanent charges and the pH dependent charges. These exchange sites retain by electrostatic forces the cations. The CIC is an indirect indicator of the buffering capacity of soils and is a function of the quantity and type of clay (Yong et al., 1990). The methods used to estimate the CIC are classified as CIC, by sum of interchangeable cations, CIC at soil pH, CIC at buffered pH and CIC at zero point of loading, all of these are based on soil saturation with an index cation. (Jaremko ans Kalembasa, 2014) they estimate both positive and negative charges simultaneously; however, multiple washes and centrifugations, involve time, labor and sample loss.
Therefore, the AgTU+ n method is an efficient technique for estimating CIC, since no successive washes are performed and AgTU+ n is not found naturally in the soil. The TU forms stable complexes with silver (AgTU, AgTU+ 2, AgTU+ 3) due to the coordination between silver and sulfur. This method is based on the affinity of the complex AgTU+ n on the clay matrix. The CIC is determined by the difference between the initial concentration and the remaining of the saturating solution of AgTU+ n by atomic adsorption spectrometry (Cremers and Pleysier, 1973; Xing-Cun, 1998; Ahmad et al 2002; Oorts et al., 2004).
As an alternative to the use of atomic adsorption spectrometry, it has been proposed to use a suspension of bentonite, which because of its hydrophilic characteristics allows it to remain stable indefinitely. It is classified in sodium bentonite and calcium bentonite according to the dominant interchangeable ions. In the adosorption of the complex AgTU+ n in the fine fraction (<0.5 µm) of a bentonite suspension, the formation of flocs was observed, followed by a separation and sedimentation phase caused by the neutralization of the negative charges of the clay. This principle is used for the estimation of CIC, since the adsorption of AgTU+ n on the bentonite presents a linear behavior (200 ml of 1% suspension, adsorbing approximately of AgTU+ n) (Cremers and Pleysier, 1973; Pleysier et al., 1986, Ladoo, 1992; Pinzon, 2006 and Fu and Chung, 2011).
Conradie and Kotze (1989), evaluated the method of AgTU+ n where the results with Na22 are similar, whereas those obtained with ammonium chloride at soil pH are higher, conclude that the use of AgTU+ n is more practical with acceptable CIC values.
The silver is photosensitive to the visible spectrum (Prauss et al., 2008), being white or blue light, which initiates photoreduction of Ag+ to Ag0 (Oster and Oster 1959), due to photolysis of water where radicals are produced, e- aq, OHaq y Haq (Janata et al., 1994; Abid et al., 2002). In alkaline pH, precipitates of Ag2O (Kan-Sen et al., 2005) are formed, similarly AgTU+ n is unstable at pH> 8 (Cornell and Aksoyoglu, 1991). Some authors have modified this technique to prolong the stability of AgTU+ n, such as: Oorts et al. (2004), which increased the pH of the soil prior to the addition of AgTU+ n, with a 0.001 M NaOH solution, until a pH between 8 and 9 was reached. The results similar to AgTU+ n buffered with ammonium acetate. Dhorman (2006) proposes that the solution AgNO3 be added slowly, approximately for 2 min, while stirring the TU solution, then the ammonium acetate solution is added. This version is more stable with a duration of 48 to 72 hours. Given the sensitivity to photorefuction of Ag+, even as AgTU+ n, and the use of a bentonite slurry as an alternative to the atomic adsorption spectrophotometer to estimate the CIC of soils, the objective of this paper is to describe the methodological phase and the critical points of the CIC estimation with silver thiourea.
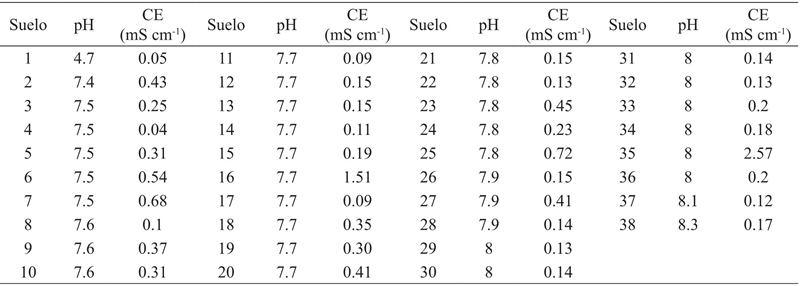
We used 204 air dried soils and sieved to two mm, which were estimated pH in water (2:1), electrical conductivity (CE) ratio 5:1 and CIC. In order to determine the CIC, the methodology proposed by Pleysier and Jou (1980) and Pleysier et al. (1986), with the following modifications: Weighed from 0.8 to 1 g of soil. During the preparation of the AgTU+ n extractant solution, the flasks where the TU and AgNO3 are prepared are protected from light with aluminum foil. The mixture of TU and AgNO3 is performed with a magnetic stirrer and the AgNO3 is slowly and gradually added by means of a 100 ml buret which is also protected. The final blend is placed in an amber container.
After centrifugation, the supernatant is filtered with Alstrhom num 90 or similar paper, and the extract is placed in light-protected containers. A blank is used to determine the initial concentration of AgTU+ n that is added. A 5 ml aliquot of the supernatant and five ml of EDTA is taken, the aliquot and EDTA are shaken in vortex test tubes for five seconds. It is titrated dropwise with a suspension of commercial bentonite, obtained from nonmetallic industrial minerals, which is called calcium bentonite. The particle size used was <37μm.
For the calibration solution of AgTU+ n, 100 ml of solution AgTU+ n (0.01M) are prepared, the materials are protected and stored in the same manner as in the preparation of the extractant solution. The final point of the titration is observed when in the supernatant when adding a few drops of bentonite, it goes from clear to slightly cloudy, at this point the adsorption decreases, this is a state indicating that the clay is close to saturation, it is necessary to continue adding a few drops of the bentonite suspension until that slight turbidity changes to a more intense hue, at which point the adsorption reaction ends (Figure 1).
The spent volume of the bentonite slurry in the calibration curve and the concentrations used are shown in Table 1. In Figure 2, the gradient of floc formation and sedimentation of the calibration solution AgTU+ n is shown.
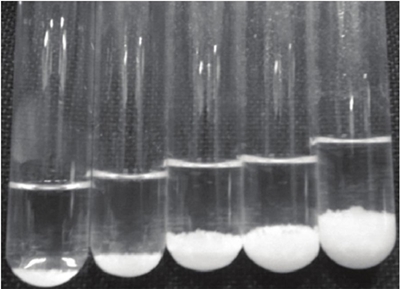
Figure 2 Visual state of the calibration points of the bentonite suspension and the solutions of AgTU+ n .
To quantify the amount of AgTU+ n adsorbed in the bentonite suspension in titration, the regression equation obtained on the calibration curve is used. The regression equations were determined with (CI) and without intercept (SI), Y= 0.0049X, Y= 0.0049X-3*10-5, whose correlation coefficients are: R² = 0.9961 and R² = 0.9962, respectively (Figure 3).
Thirty-eight soils of the 204 have the capacity to adsorb a concentration greater than 0.3 mmol of AgTU+ n; that is, they have a CIC>28 meq100 g-1 soil, which is the maximum concentration that is determined in these soils with the technique proposed by Pleysier and Jou (1980) Table 2. Since the holder with the first drops of the suspension of bentonite does not show the formation of flocs and the mixture becomes immediately cloudy, to estimate the soil CIC with this characteristic should be 0.8 g. With the original version of the method, Ag+ reduction occurs, due to the prolonged exposure to light and the lack of homogenization of the TU and AgNO3 mixture, this reaction is avoided using a magnetic stirrer and the AgNO3 by means of a 100 ml buret, together with the glassware used was protected from light with foil. When this procedure is not performed it is possible to see in the flask stains at the edges and an inhomogeneous mixture.
If the extract of AgTU+ n remains more than 12 hours exposed to light, the adsorption of this cation in the bentonite suspension decreases, since UV radiation produces hydrated electrons that reduce the (Janata et al., 1994; Kan-Sen et al, 2005), leading to an overestimation of the real value of the CIC.
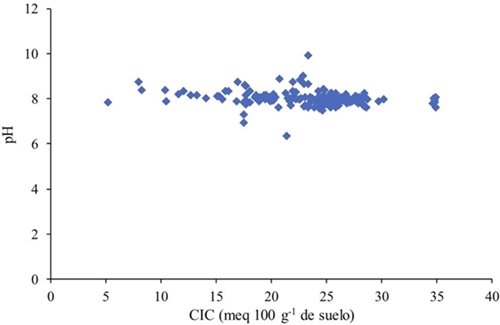
The damping capacity of soils is a function of clay type and quantity, since with similar values of CIC there are contrasting characteristics, being in this case pH and CE, both variables are dependent on the loading sites in the clays, however, these do not present a linear relation given to the formation processes that gave origin to the studied soils (Figure 4), thus with an equal amount of clay, the pH presents variations due to the type of clay present in the sample. The type of clay dominate in the soil determines the CIC, where the CIC of the clays of the type 2:1 is greater than the type 1:1, based on this, the CIC has a gradient according to the type of clays, Smectic> ilite> kaolinite (Yong et al., 1990).
The activity of the predominant ions in the soil (Na+, Ca2+, K+, Cl-, SO2- 4, y CO2- 3, HCO2- 3 ) (He et al., 2012), is determined by the type and amount of clay, since these ions depend on the exchange sites. Soils rich in clay have the ability to adsorb more ions than soils with a higher proportion of sand. The CE of the soils analyzed does not present a linear relation with the content of clay expressed as CIC. The CE of the soils studied is independent of the content and type of clay (Figure 5).
The CIC with intercept (CICCI) has a linear relationship with the CIC without intercept (CICSI); That is, that the estimation of the cation exchange capacity performed in the titration of AgTU+ n with the bentonite suspension, is due to the ability of soil clay to adsorb the AgTU+ n without others generating sources of variation that alter the final value of the CIC (Figure 6).
Literatura citada
CAbid, J. P.; Wark, A. W.; Brevet, P. F. and Girault H. H. 2002. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Comm. (7):792-793. [ Links ]
Ahmad, S.; Isab, A. A. and Perzanowski, H. P. 2002. Silver (I) complexes of thiourea. Trans. Metal Chem. 27:782-785. [ Links ]
Conradie, M. and Kotze, W. A. G. 1989 Comparison of methods for estimating cation exchange capacity of orchard soils, South African. J. Plant Soil. 6(2):136-137. [ Links ]
Cornell, R. M. and Aksoyoglu, E. S. 1991. Simultaneous determination of the cation exchange capacity and the exchangeable cations on marl. Clay Minerals. 26:567-570. [ Links ]
Cremers, A. and Pleysier, J. 1973. Adsorption of the silver-thiourea complex in montmorillonite. Nature Phys. Sci. 243(127):86-87. [ Links ]
Dohrmann, R., 2006. Cation exchange capacity methodology II: A modified silver-thiourea method. Appl. Clay Sci. 34:38-46. [ Links ]
Fu, Y. and Chung, D. D. L. 2011. Coagulation of oil in water using sawdust,bentonite and calcium hydroxide to form floating sheets. Appl.Clay Sci. 53:634-641. [ Links ]
He, Y.; DeSutter, T.; Prunty, L.; Hopkins, D.; Jia, X. and Wysocki, D.A. 2012. Evaluation of 1: 5 soil to water extract electrical conductivity methods. Geoderma. 185:12-17. [ Links ]
Janata, E.; Henglein, A. and Ershovt, B. G. 1994. First clusters of Ag+ ion reduction in aqueous solution. J. Phys. Chem. 98:10888-10890. [ Links ]
Jaremko, D. and Kalembasa, D. 2014. A comparison of methods for the determination of cation exchange capacity of soils. Ecol. Chem.Eng. S. 21(3):487-498. [ Links ]
Kan, S. C.; Yu, C. L and Hsien, H. L. 2005. Effect of alkaline ion on the mechanism and kinetics of chemical reduction of silver. Mat.Chem. Phys. 94:429-433. [ Links ]
Ladoo, R. B. 1922. Bentonite. J. Franklin Institute. 193(1):123-124. [ Links ]
Oorts, K.; Vanlauwe, B.; Pleysier, J. and Merckx, R. 2004. A new method for the simultaneous measurement of pH-dependent catión exchange capacity and pH buffering capacity. Soil Sci. Soc.Am. J. 68:1578-1585. [ Links ]
Oster, G. K. and Oster, G. 1959. Photoreduction of metal ions by visible light. J. Ame. Chem. Soc. 81(21):5543-5545. [ Links ]
Pleysier, J. L. and Juo, A. S. R. 1980. A single-extraction method using silver-thiourea for measuring exchangeable cations and effective CEC in soil with variable charges. Soil Sci. 129(4):205-211. [ Links ]
Pleysier, J.; Janssens, J. and Cremers, A. 1986. A clay suspension stability end point titration method for measuring cation Exchange capacity of soils. Soil Sci. Soc. Am. J. 50:887-891. [ Links ]
Praus, P.; Turicová, M. and Valášková, M. 2008. Study of silver adsorption on montmorillonite. J. Braz. Chem. Soc. 19(3):549-556. [ Links ]
Xing, C. H. 1998. Recovery of silver from thiourea solution by ion flotation. Separation Sci. Technol. 33(1):141-148. [ Links ]
Yong, R. N.; Warkentin, B. P.; Phadungchewit, Y. and Galvez, R. 1990.Buffer capacity and lead retention in some clay. Water, Air, and Soil Pollution. 53: 53-67. [ Links ]
Received: January 2017; Accepted: February 2017











 texto em
texto em