Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.1 Texcoco ene./feb. 2017
https://doi.org/10.29312/remexca.v8i1.75
Articles
Irradiation of tomato seeds with UV-B and UV-C: impact on germination, vigor and growth
1Universidad Autónoma Agraria Antonio Narro- Departamento de Horticultura- Centro de Capacitación y Desarrollo en Tecnología de Semillas. Calzada Antonio Narro 1923. Saltillo, Coahuila, México. CP. 25315.
3Departamento de Plásticos en la Agricultura- Centro de Investigación en Química Aplicada. Blvd. Ing. Enrique Reyna núm.140. Saltillo, Coahuila, México.
The UV-B and UV-C radiation modifies plant metabolism and morphology. Under the hypothesis that the irradiation of the seeds with UV-B and UV-C changes not only the germinative behavior but the morphology of the plants, this study was carried out with the objective of evaluating if the irradiation of the seeds induces changes in the germination, vigor and morphological variables of the leaf epidermis of tomato plants. In a first experimental stage, germination and vigor were evaluated using the following treatments: 0 (control), 1.8, 5.4, 10.8, 16.2, 21.6, 27 and 32.4 kJ m-2 of UV-B and 0 (control), 0.9, 2.7, 5.4, 8.1, 10.8, 13.5 and 16.2 kJ m-2 irradiation UV-C applied to the seed. The following variables were evaluated: (%) of germinated seeds and normal seedlings, root and hypocotyl length and dry weight. The irradiation with 5.4 kJ m-2 of UV-B favored the emergence and quantity of normal seedlings. Irradiation with UV-C caused negative effects on the emergence and quantity of normal seedlings. Based on the results of the first stage, irradiation doses were selected to be applied in the second stage, which were: 1.8, 5.4 and 10.8 kJ m-2 of UV-B and 0.9 and 2.7 kJ m-2 of UV-C. The irradiated seeds were sown in pots under greenhouse and the plants obtained were taken to flowering to evaluate the following variables: plant height, stem diameter, stomatal beam and back density, fresh and dry weight and SPAD units. The experimental design used was completely randomized. All treatments increased the stomatal density in the plants, in addition to the doses of 5.4 and 10.8 UV-B decreased SPAD units. It was concluded that seed irradiation is useful for seed pretreatment and phenotypic modification of plants.
Keywords: induction of tolerance; irradiance; phenotypic modification; signaling; stress
La radiación UV-B y UV-C modifica el metabolismo y morfología de las plantas. Bajo la hipótesis de que la irradiación de las semillas con UV-B y UV-C cambia no solamente el comportamiento germinativo sino la morfología de las plantas se realizó este estudio con el objetivo de evaluar si la irradiación de las semillas induce cambios en la germinación, vigor y variables morfológicas de la epidermis foliar de plantas de tomate. En una primera etapa experimental se evaluó la germinación y el vigor utilizando los siguientes tratamientos: 0 (testigo), 1.8, 5.4, 10.8, 16.2, 21.6, 27 y 32.4 kJ m-2 de UV-B y 0 (testigo), 0.9, 2.7, 5.4, 8.1, 10.8, 13.5 y 16.2 kJ m-2 de UV-C de irradiación aplicada a la semilla. Se evaluaron las siguientes variables: (%) de semillas germinadas y plántulas normales, longitud de raíz y de hipocótilo y peso seco. La irradiación con 5.4 kJ m-2 de UV-B favoreció la emergencia y cantidad de plántulas normales. La irradiación con UV-C causó efectos negativos en la emergencia y cantidad de plántulas normales. En base a los resultados de la primera etapa se seleccionaron las dosis de irradiación para aplicar en la segunda etapa, las cuales fueron: 1.8, 5.4 y 10.8 kJ m-2 de UV-B y 0.9 y 2.7 kJ m-2 de UV-C. Las semillas irradiadas se sembraron en macetas bajo invernadero y las plantas obtenidas se llevaron hasta floración para evaluar las siguientes variables: altura de planta, diámetro de tallo, densidad estomática del haz y envés, peso fresco y seco y unidades SPAD. El diseño experimental utilizado fue el completamente al azar. Todos los tratamientos incrementaron la densidad estomática en las plantas, además de que las dosis de 5.4 y 10.8 de UV-B disminuyeron las unidades SPAD. Se concluyó que la irradiación de la semilla es útil para el pretratamiento de semillas y la modificación fenotípica de las plantas.
Palabras clave: estrés; inducción de tolerancia; irradiancia; señalización; modificación fenotípica
Introduction
IThe solar radiation is a key factor for the functioning of terrestrial and aquatic ecosystems through the control of photobiological processes, environmental factors and natural cycles (Carrasco-Ríos, 2009; Bornman et al., 2015). According to Troy and Thennadil (2001), the electromagnetic spectrum reaching the earth’s surface includes UV radiation (200-400 nm), photosynthetically active (400-700 nm) and near infrared (800-2 500 nm).
Conventionally the UV radiation spectrum has been divided into three components. The so-called UV-A comprises wavelengths from 320 to 400 nm, the UV-B from 280 to 320 nm and UV-C from 200 to 280 nm (Carrasco-Ríos, 2009). The UV-A radiation penetrates the atmosphere and reaches the earth’s surface; UVB radiation is mostly absorbed by the atmospheric ozone layer, so only a small amount of it reaches the Earth’s surface. Of the total radiation on the Earth’s surface, UV-B accounted for only 1.5%. UV-C radiation is totally absorbed by atmospheric ozone, so it is not found naturally in ecosystems (Nawkar et al., 2013).
The plants use electromagnetic radiation as information to adjust their growth and development (Kami et al., 2010). To do this, they use sensorial proteins to create a connection between environmental stimulus and physiological responses such as dormancy breaking, germination rate and pos germinative responses of the embryo, allowing environmental signals to be collected and processed even by seeds Inactive species that are part of a seed or germplasm bank. This process of perception and signaling of electromagnetic radiation by a seed seems to be mediated by different receptors such as phytochromes (Magliano and Casal, 2004), and is very important from the point of view of the opportunity and survival capacity of the embryo once the seed germinates (Long et al., 2015). In the particular case of UV-B radiation the photoreceptor is called UVR8 (Kami et al., 2010; Heijde and Ulm, 2012).
Unlike UV-B radiation, so far no specific receptor for UV-C radiation has been found, perhaps because it naturally does not reach the Earth’s surface since it is completely blocked by the O3 molecules of the atmosphere. The UV-C radiation has been investigated mainly for the effects of post-harvest application on fruits, leaves and vegetables. Decreased protein synthesis, delayed maturation, increased flavonoid levels, accumulation of phytoalexins, and induction of resistance have also been found, with negative responses in cell processes, metabolism and growth (Promyou and Supapvanich, 2012).
The usefulness of the application in the irradiation of the seeds has been little studied for UV-B and UV-C radiation, despite the fact that, theoretically, the changes induced in the seed by irradiation could be seen in later stages of the Growth (Magliano and Casal, 2004; González-Aguilar et al., 2007) by counting seed coatings and underlying cell layers with a large number of receptors, proteins and signaling compounds (Moïse et al., 2005). It is known that in sunflower and soybean seeds irradiated with UV-C show positive responses to abiotic stress (Foroughbakhch et al., 2015), in the melon irradiation of the seeds with UV-B caused changes in the morphology and chemical composition of plants (Sosa-Flores et al., 2014), while in the cabbage seed irradiation caused tolerance to biotic stress in adult plants (Brown et al., 2001). It would be desirable to apply this same technique in horticultural species as important as the tomato; however, no information is available on the germinative response, vigor of seedlings and the growth of plants of this species when the seeds are subjected to irradiation.
The objective of this study was to test the feasibility of using UV-B and UV-C tomato seed irradiation as a biophysical tool to modify both germination and vigor responses as well as plant growth.
Materials and methods
The experiment was carried out at the Agraria Antonio Narro Autonomous University, in Buenavista Saltillo Coahuila, Mexico, located at 25° 21’ 19” north latitude and at 101° 01’ 48” west longitude at a height of 1 779 m. The plant material used was tomato (Solanum lycopersicum L.) hybrid Big Rio. The experiment consisted of two stages: in the first stage, the germination and vigor tests of the seedlings obtained from the seeds irradiated with several doses of UV-B and UV-C radiation. Based on the results obtained from the first stage the best doses were chosen to irradiate the seeds to be used in the second stage.
The first stage was initiated with the irradiation of the seeds on November 7, 2014 using an irradiation chamber with a lamp model 3UV-36, which emits radiation in different ranges: UV-A, UV-B and UV-C. The irradiance of the lamp in the UV-B range is 0.06 W m-2, while the UV-C is 0.03 W m-2. The applied treatments are shown in Table 1 (UV-B) and 2 (UV-C).
The energy doses in the UV-C range are lower because the lamp emits radiation with a lower photon flux density. Once irradiated, the seeds were placed in aluminized paper bags, to avoid that the surrounding radiation could modify the stimulation caused by UV radiation (Musil et al., 1998), and brought to seed the next day.
The irradiated seeds were seeded in rolls of anchor paper for germination, each roll corresponded to a replicate containing 25 seeds, and the rolls were distributed in a completely random design inside a LAB-LINE germination chamber at 25 °C with constant illumination. The paper moisture was checked and checked every day. The seeds were kept under these conditions for 15 days, after which the evaluations were done: percentage of germinated seeds, percentage of normal seedlings, length of hypocotyl (cm), length of radicle (cm) and dry weight (mg), the data were analyzed in the SAS program using an Anova and a means comparison test using Tukey (α≤ 0.05).
The second experimental stage was carried out from march to july 2015 in a greenhouse type chapel with rigid polycarbonate cover located at the Autonomous Agrarian Antonio Narro University in Saltillo, Coahuila, Mexico. The treatments applied in the second stage are shown in Table 3.
The irradiated seeds were seeded directly into polystyrene containers of 1L containing perlite substrate and acid peat in a 1:1 ratio and distributed under a completely random design. The containers were placed on plastic tables of 0.85 m height to facilitate handling and measurements. The irrigation of the containers was carried out initially with running water, the emergence of the seedlings occurred at 10 days and 10 days later the fertilization with Steiner solution (Steiner, 1961) was started at 25%, which was applied by through of tubines and droppers with a cost of 1.2 L h-1.
After 50 days after sowing, SPAD unit evaluations were performed on leaf blades with a SPAD 502 plus Minolta brand. The height of the plants was measured with a tape measure and the diameter of the stem with a digital vernier one centimeter away from the base, both variables were measured at 52 days after sowing. At 65 days after seeding, epidermal impressions were made on the third fully expanded leaf to determine the stomatal density of the beam and underside (stomas mm-2) using a 40x objective of a composite (Carl Zeiss) chambered microscope Digital (Pixera Winder Pro) and measurement software (Axion Vision Rel. 4.8). The fresh and dry weights of the plants were evaluated using 5 plants randomly chosen from each treatment 65 days after planting.
Data analysis was performed using the SAS version 9.1 program using ANOVA and Tukey’s mean comparison test (α≤ 0.05).
Results and discussion
Germination and vigor responses to UV-B irradiation
The UV-B radiation caused differences in the variables germinated seeds (%), normal seedlings (%) and dry weight (g), on the other hand there were no differences in the variables length of hypocotyl (cm) and length of root (cm).In the variables where differences between the treatments and the control (p≤ 0.05) were observed, a tendency to increase with a maximum in the treatment with 5.4 kJ m-2 is observed, then a decrease that reaches the lowest values with 16.2 kJ m-2, then again a positive trend reaching another peak in the treatment with 27 kJ m-2 (Figure 1).

Figure 1 Different doses of UV-B radiation applied to Big Rio hybrid tomato seeds and their effect on germination and vigor of seedlings. The bars in each graph indicate mean values for each variable and different literals on each bar indicate significant differences according to a Tukey test (p≤ 0.05).
In the germinated seeds variable (Figure 1a), the treatment with 5.4 kJ m-2 of UV-B radiation is statistically superior (p≤ 0.05) to the control, this kind of response has been explained by Luckey (1980), who Mentions that the sublethal application of an agent capable of inducing physical or chemical stress can generate positive responses. Apparently at 5.4 kJ m-2, a response threshold was reached in the seed in which certain receptors that trigger a response aimed at accelerating germination could act. Doses greater than 5.4 kJ m-2 resulted in responses not different from the control, but with the treatment of 27 kJ m-2 a value 21% higher than the control was reached again. Possibly with this higher amount of energy a second response threshold could be reached in the seed, in that threshold could be involved different receptors.
These kinds of responses with several maxima, and perhaps dependent on different receptors, are very valuable for the seeds from an adaptive point of view, since they allow a greater flexibility in the response to different environmental contexts (examples: different depths under the soil in a seed bank or different types or canopy coverings on a seed bank) and ensure that it is not only a certain threshold value or individual stimulus that triggers the germination process and the growth of the embryo (Long et al., 2015).
Similar results were found in the percentage of normal seedlings (Figure 1b), treatments with 1.8 and 5.4 kJ m-2 were superior to the control, perhaps as a consequence of a hormone-induced radiation effect mentioned by Luckey (1980). Normally these responses are positive since the formation of a normal seedling ensures optimum growth and better productivity (Delibaltova and Ivanova, 2006). The treatment with 27 kJ m-2 showed a new response peak as in germinated seeds variable, which could indicate the action of different signaling receptors or cascades activated with different energy thresholds.
In the hypocotyl length variable (Figure 1c) as well as in the root length (Figure 1d) there was no significant difference (p≥ 0.05). Other authors found response to irradiation in these variables, Kacharava et al. (2009) irradiated bean seeds for 30 minutes and found an increase in plant height, but with 90 min the height decreased.
Similarly, Hidema et al. (2006) found that supplementary UV-B radiation decreased plant growth and grain yield in rice. It is not available until an explanation of why stimulus against UV-B occur different, positive or negative, or neutral responses, between species even in the same family (Kossuth and Biggs, 1981). The same happens with the different responses exhibited by different variables in the same species, as shown in this study.
In the dry weight (Figure 1e), the same response already described for the percentage of germinated seedlings and normal seedlings, with maxima at 5.4 and 27 kJ m-2, is repeated. An analogous result was described by Kacharava et al. (2009), who reported an increase in dry matter by applying 60 minutes of ultraviolet radiation to bean seed.
Germination and vigor responses to UV-C irradiation
In contrast to UV-B irradiation in the case of UV-C, no positive responses were reported. This difference probably arises from the fact that, unlike UV-B, UV-C radiation is not a natural component in ecosystems (Nawkar et al., 2013). In agreement with Promyou and Supapvanich, (2012) it is possible that the observed responses in germinated seeds and seedlings are the result of the oxidative damage caused by the exposure even to the smaller doses of UV-C. This explains in part why the negative responses were evident in the processes most sensitive to oxidative damage on germination and normal seedling production after germination (Figure 2a and 2b). While the subsequent processes that gave rise to hypocotyl length, root length and dry weight, which are variables evidenced only in normal seedlings, they were not affected (Figures 2c, 2d and 2e). The latter results coincide with those reported by Foroughbakhch-Pournavab et al. (2015) in wheat and sunflower.
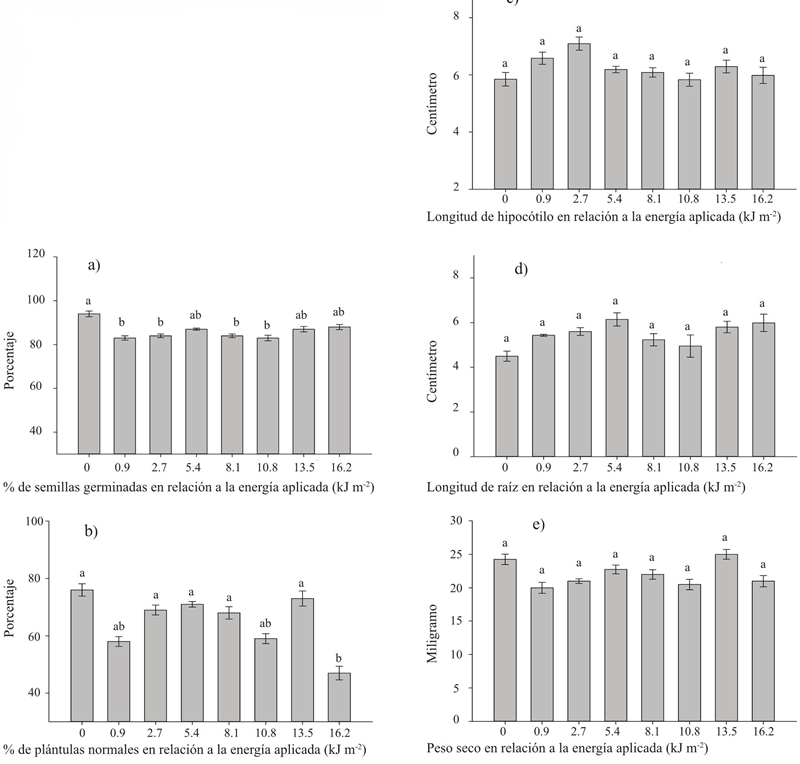
Figure 2 Different doses of UV-C radiation applied to Big Rio hybrid tomato seeds and their effect on germination and vigor of seedlings. The bars in each graph indicate mean values for each variable and different literals on each bar indicate significant differences according to a Tukey test (p≤ 0.05).
In relation to germinated seeds (Figure 2a) the response to radiation was practically the same with all doses of UV-C, showing lower values to the control with the doses of 0.9, 2.7, 8.1 and 10.8 kJ m-2, whereas with the doses of 5.4, 13.5 and 16.2 there were no significant differences (p≥ 0.05), these results indicate the great sensitivity of the germinative response to the UV-C stimulus and are very similar to those reported by Brown et al. (2001), who irradiated lettuce seeds and found responses of this type, depending on the dose, in the incidence variables of Xanthomonas campestris pv. campestris and in that of colony forming units.
In the percentage of normal seedlings (Figure 2b) only the highest dose of 16.2 kJ m-2 showed a lower value than the control, again supporting the explanation that unlike the UV-B stimulus, which resembles a response to a photomorphogenic stimulus, the response to UV-C is due to oxidative or other damage caused by the large amount of energy carried by UV-C photons. These results coincide with those of Brown et al. (2001), who reports that the diameter of the head of lettuce plants obtained from seeds treated with low doses of UV-C radiation was not different from the control, whereas in seeds treated with higher doses the diameter was reduced considerably. Other negative responses to UV-C radiation applied to plant tissues are low protein content, dysfunctions in chloroplasts, DNA damage, and in various cellular processes (Danon and Gallois, 1998; Rastogi et al., 2010; Ruiz-López et al., 2010).
Plant responses from seeds irradiated with UV-B and UV-C
In the second stage, the SPAD and stomatal density variables of the beam and underside were different to the control, indicating that the radiation applied to the seed is capable of generating changes that become visible in later stages (Figure 3), such as described by Musil et al. (1998) and Magliano and Casal (2004) for environmental signals perceived in pre-germination by phytochromes.

Figure 3 Response of the plants obtained from Big Rio hybrid seeds irradiated with UV-B and UV-C and planted in pots under greenhouse. The bars in each graph indicate mean values for each variable and different literals on each bar indicate significant differences according to a Tukey test (p≤ 0.05).
For the variables plant height (Figure 3a) and stem diameter (Figure 3d) no treatment was different from the control. This behavior of tomato plants was different from that reported for other species such as cabbage, which showed higher growth when the seeds were irradiated with 3.6 kJ m-2 of UV-C (Brown et al., 2001), Arabidopsis with decreased petiole vs. UV-B radiation (Gruber et al., 2009) or soybean showed lower seedling length when seeds were irradiated with UV-C (Foroughbakhch-Pournavab et al., 2015). Again, the different response of each species seems to apply (Kossuth and Biggs, 1981), considering factors such as seed size, seed thickness, chemical composition or other intrinsic seed properties of each species.
In the stomatal beam density (Figure 3c) and the underside (Figure 3d), changes induced by irradiation of the seeds with UV-B and UV-C were found determining that the response in the plants was to raise the density stomatal in the beam, whereas for the stomatal density in the back an inverse trend was found against the higher dose of energy applied with UV-B, but an opposite response when applying UV-C. Wargent et al. (2009) indicated that these adaptive responses to UV radiation impact on plant performance, modifying the interaction with different environmental factors through changes in form, physiology and biochemistry.
Similar results were obtained by Benavides-Mendoza et al. (2003) when irradiating wheat seeds with a low-intensity laser (AsAlGa), observing that at a dose of 6 kJ m-2 the seedlings obtained from the irradiated seeds showed higher values of stomatal density in the bundle and at the back to grow in an environment conducive to growth, while germinating in a medium with different levels of salinity irradiated seedlings presented a clear adaptive response decreasing the stomatal density, a response that was not observed in the control. It is probable that the tomato plants obtained from the seeds irradiated with UV show responses with some background of adaptation to an environment where a greater amount of radiant energy is detected, which would imply the necessity of adjustments in the characters of the epidermis.
In the variables of fresh weight (Figure 3e) and dry weight (Figure 3-F) no difference was found in any of the treatments with the control. Again these results seem to indicate a specific response for tomato plants, since Kacharava et al. (2009) observed increases in the fresh weight of some varieties of Phaseolus vulgaris with applications of 60 and 90 min of UV-B; as well as an increase in the weight of white beet with 60 min of UV-B applied to the seed. In the lettuce irradiation of the seeds with a low intensity laser caused increase in the radical biomass but did not change the weight of the leaves and the stem (Benavides-Mendoza et al., 2003).
The SPAD units (Figure 3g) decreased with UV-B treatments 5.8 and 10.5 kJ m-2. The response can be explained by the fact that SPAD units indirectly measure chlorophyll concentration in foliar sheets (Rodríguez-Mendoza et al., 1998) and that this pigment responds inversely to the amount of radiant energy detected in the environment (Vu et al., 1984; Strid et al., 1990). The results obtained clearly indicate that such signaling occurs from the seed. In contrast to UV-B in the case of UV-C irradiation there were no differences with the control, but Figure 3g shows a positive trend in data different from that exhibited by UV-B.
Conclussion
The irradiation of tomato seeds with UV-B and UV-C modified both the germination and vigor responses as well as the growth of the plants obtained from the irradiated seeds. Irradiation with UV-B at 5.4 kJ m-2 increased germination and the amount of normal seedlings obtained. In contrast, irradiation of tomato seeds with UV-C had no positive effect on germination and vigor.
The effects of irradiation of seeds with UV-B and UV-C were extended to the later stages of plant growth. UV-B irradiation with 10.8 decreased the readings of SPAD units in the leaves and decreased stomatal density in the underside. The stomatal beam density was increased in all seed plants irradiated with UV-B, regardless of the dose. On the other hand, plants obtained from seeds irradiated with UV-C showed an increase in the stomatal density in the beam and a decrease in the density of the underside.
Literatura citada
Benavides, M. A.; Garnica, S. J.; Michtchenko, A.; Hernández, A. C.;Ramírez, R. H.; Hernández, D. J. y Robledo, T. V. 2003.Respuesta al estrés y crecimiento de plántulas cuyas semillas fueron irradiadas con láser de baja intensidad. Agrofaz.3(1):269-272. [ Links ]
Bornman, J. F.; Barnes, P. W.; Robinson, S. A.; Ballare, C. L.; Flint, S.D. and Caldwell, M. M. 2015. Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems. Photochem. Photobiol. Sci. 14(1): 88-107. [ Links ]
Brown, J. E.; Lu, T. Y.; Stevens, C.; Khan, V. A.; Lu, J.; Wilson, C. L.and Droby, S. 2001. The effect of low dose ultraviolet light-C seed treatment on induced resistance in cabbage to black rot (Xanthomonas campestris pv. campestris). Crop Prot.20(10):873-883. [ Links ]
Carrasco, R. L. 2009. Efecto de la radiación ultravioleta-B en plantas.Idesia. 27(3):59-76. [ Links ]
Danon, A. and Gallois, P. 1998. UV‐C radiation induces apoptotic‐like changes in Arabidopsis thaliana. FEBS Lett. 437(1-2):131-136. [ Links ]
Delibaltova, V. and Ivanova, R. 2006. Impact of the pre-sowing irradiation of seeds by helium-neon laser on the dynamics of development of some cotton varieties J. Environ. Protec. Ecol. 7(4):909-917. [ Links ]
Foroughbakhch, P. R.; Bacópulos, M. E. y Benavides, M. A. 2015. Efecto de la irradiación con UV-C en la germinación y vigor de tres especies vegetales. Eco. Rec. Agrop. 2(5):129-137. [ Links ]
González, A. G. A.; Zavaleta, G. R. and Tiznado, H. M. E. 2007. Improving postharvest quality of mango ‘Haden’ by UV-C treatment.Postharv. Biol. Tech. 45(1):108-116. [ Links ]
Gruber, H.; Ulm, R. and Heijde, M. 2009. Regulation of UV-B-induced photomorphogenesis in Arabidopsis. Comp. Biochem. Phys.A. 153(2):S200. [ Links ]
Heijde, M. and Ulm, R. 2012. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 17(4):230-237. [ Links ]
Hidema, J. and Kumagai, T. 2006. Sensitivity of rice to ultraviolet-B radiation. Ann. Bot. 97(6):933-942. [ Links ]
Kacharava, N.; Chanishvili, Sh.; Badridze, G.; Chkhubianishvili, E. and Janukashvili, N. 2009. Effect of seed irradiation on the content of antioxidants in leaves of Kidney bean, Cabbage and Beet cultivars. Aust. J. Crop Sci. 3(3):137-145. [ Links ]
Kami, C.; Lorrain, S.; Hornitschek, P. and Fankhauser, C. 2010. Lightregulated plant growth and development. Curr. Top. Dev Biol.91:29-66. [ Links ]
Kossuth, S. V. and Biggs, R. H. 1981. Ultraviolet-B radiation effects on early seedling growth of Pinacea species. Can. J. For. Res.11(2):244-249. [ Links ]
Long, R. L.; Gorecki, M. J.; Renton, M.; Scott, J. K.; Colville, L.; Goggin, D. E.; Commander, L.E.; Westcott, D. A.; Cherry, H. and Finch, S. W. E. 2015. The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise.Biol. Rev. 90(1):31-59. [ Links ]
Luckey, T. D. 1980. Hormesis with ionizing radiation. Boca Raton, FL:CRC Press. 1-122 pp. [ Links ]
Magliano, T. A. and J. J., Casal. 2004. Pre-germination seed-phytochrome signals control stem extension in dark-grown Arabidopsis seedlings. Photochem. Photobiol. Sci. 3(6):612-616. [ Links ]
Moïse, J. A.; Han, S.; Gudynaitę, S. L.; Johnson, D. A. and Miki, B. L.2005. Seed coats: structure, development, composition, and biotechnology. In Vitro Cell. Dev. Biol. Plant. 41(5):620-644. [ Links ]
Musil, C. F.; Newton, R. J. and Farrant, J. M. 1998. Ultraviolet irradiation effects on serotinous shape Leucadendron laureolum seeds:altered seed physiology and ultrastructure, and seedling performance. Plant Ecol. 139(1):25-34. [ Links ]
Nawkar, G. M.; Maibam, P.; Park, J. H.; Sahi, V. P.; Lee, S. Y. and Kang, C. H. 2013. UV-induced cell death in plants. Int. J. Mol. Sci. 14(1):1608-1628. [ Links ]
Promyou, S. and Supapvanich, S. 2012. Effect of the ultraviolet-C (UV-C) illimination on postharvest quality and bioactive compounds in yellow bell pepper fruit (Capsicum annum L.) during storage.Afr. J. Agric. Res. 7(28):4084-4096. [ Links ]
Rastogi, R. P.; Richa; Kumar, A.; Tyagi, M.B. and Sinha, R. P. 2010. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucl. Acids. 2010(592980):1-32. [ Links ]
Rodríguez, M. M. N.; Alcántar, G. G.; Aguilar, S. A.; Etchevers, B. J. D. and Santizó, R. J. A. 1998. Estimación de la concentración de nitrógeno y clorofila en tomate mediante un medidor portátil de clorofila. Terra. 16(2):135-141. [ Links ]
Ruiz, L. G. A.; Qüesta, A. G. y Rodríguez, S. D. C. 2010. Efecto de luz UV-C sobre las propiedades antioxidantes y calidad sensorial de repollo mínimamente procesado. Rev. Iber. Tecnol. Poscosecha.11(1):101-108. [ Links ]
Sosa, F. V. P.; Ramírez, G. F.; Benavides, M. A. and H. Ramírez. 2014.Study of morphological and histological changes in melonM plants grown from seeds irradiated with UV-B. J. Appl. Hort.16(3):199-204 [ Links ]
Steiner, A. A. 1961. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 15(2):134-154. [ Links ]
Strid, Å.; Chow, W.S. and Anderson, J. M. 1990. Effects of supplementary ultraviolet-B radiation on photosynthesis in Pisum sativum.BBA-Bioenergetics. 1020(3):260-268. [ Links ]
Troy, T. L. and Thennadil, S. N. 2001. Optical properties of human skin in the near infrared wavelength range of 1 000 to 2 200 nm. J.Biomed. Opt. 6(2):167-176. [ Links ]
Vu, C. V.; Allen, L. H. and Garrard, L. A. 1984. Effects of enhanced UV-Bradiation (280-320 nm) on ribulose-1,5-bisphosphate carboxylase in pea and soybean. Environ. Exp. Bot. 24(2):131-143. [ Links ]
Wargent, J. J.; Gegas, V. C.; Jenkins, G. I.; Doonan, J. H. and Paul, N. D. 2009. UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 183(2):315-326. [ Links ]
Received: November 2016; Accepted: January 2017











 texto en
texto en