Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 spe 16 Texcoco May./Jun. 2016
Articles
In vitro fermentation and the correlation of the nutritional content of leucaena associated with star grass
1Posgrado en Producción Animal- Universidad Autónoma Chapingo. Carretera México-Texcoco km 38.5, C. P. 56230. Tel: 595 952 1621. (albertomiranda@correo. chapingo.mx; alarab_11@hotmail.com; maximino_h@hotmail.com; miguel2mtz@gmail.com).
The in vitro fermentation was determined by the technique of gas production of Leucaena leucocephala (Ll) and Cynodon nlemfuensis (Cn) 35, 42, 49, 56, 63 and 70 days of regrowth (DR) of an intensive silvopastoral system in San Luis Potosi, Mexico, in the windy season. A sample batch of fodder randomized, with three replications was taken by DR. The variables were: maximum volume (Vm; mL g-1), rate (S; h-1) and delay time (L; h) of gas production, and in vitro digestibility of dry matter (DIVMS): these they were correlated with mineral content and FDN, FDA, PC, EE, cellulose and lignin. The Vm, DIVMS and S were higher, and L lower for Ll with respect to Cn (0.039 vs 0.026 h-1; 258.9 vs 227.1 mL g-1; 63.6 vs 42.8%; and 1.93 vs 5.63 h). For both fodder Vm, DIVMS and S were higher (p< 0.05; 73.23 and 47.98%; 307 and 272 mL g-1; 0.044 and 0.29 h-1) at 35 DR. The DR affected the fermentation variable, the grass Cn greater than the Ll legume. The variables fermentation and DIVMS were negatively correlated (R= -0.681, p< 0.01) with FDA in both forages. To Leucaena, the EE was negative correlation (R= -0.68, p< 0.01); while for Cn it was positive correlation (R= 0.642, p< 0.01) with PC. Regarding minerals, the correlation was positive (p< 0.01) with P for both forages. In the legume, K, Fe and Cu was positively correlated (p< 0.01). It DIVMS only negatively correlated with Ca:P ratio for lecuaena. It was concluded that, according to the fermentation of Cn and Ll in a silvopastoral system, they should be grazed 35 DR, and increase in Cn content PC and P in Ll, which can be achieved by fertilizing with P and inoculating mycorrhizal.
Keywords: fiber; gas production kinetics; proximal analysis
Se determinó la fermentación in vitro, por la técnica de producción de gas, de Leucaena leucocephala (Ll) y Cynodon nlemfuensis (Cn) de 35, 42, 49, 56, 63 y 70 días de rebrote (DR) de un sistema silvopastoril intensivo en San Luis Potosí, México, en la época de nortes. Se tomó una muestra de forrajes de lotes distribuidos al azar, con tres repeticiones por DR. Las variables fueron: volumen máximo (Vm; mL g-1), tasa (S; h-1) y tiempo de retardo (L; h) de producción de gas, y digestibilidad in vitro de la materia seca (DIVMS): estas se correlacionaron con el contenido mineral y de FDN, FDA, PC, EE, celulosa y lignina. El Vm, DIVMS y S fueron mayores, y L menor para Ll con respecto a Cn (0.039 vs 0.026 h-1; 258.9 vs 227.1 mL g-1; 63.6 vs 42.8%; y 1.93 vs 5.63 h). Para ambos forrajes Vm, DIVMS y S fueron mayores (p< 0.05; 73.23 y 47.98%; 307 y 272 mL g-1; 0.044 y 0.29 h-1) a 35 DR. El DR afectó a la variable fermentación, al pasto Cn mayor que a la leguminosa Ll. Las variables de fermentación y DIVMS se correlacionaron negativamente (R= -0.681, p< 0.01) con FDA, en ambos forrajes. Para Leucaena, la correlación con EE fue negativa (R= -0.68, p< 0.01); mientras que para Cn la correlación fue positiva (R= 0.642, p< 0.01) con PC. Respecto a los minerales, la correlación fue positiva (p< 0.01) con P para ambos forrajes. En la leguminosa, el K, Fe y Cu se correlacionó positivamente (p< 0.01). La DIVMS solo se correlacionó negativamente con la relación Ca:P, para Lecuaena. Se concluyó que, acorde a la fermentación de Cn y Ll en un sistema silvopastoril, éstas deben pastorearse a 35 DR, e incrementar en Cn el contenido de PC y de P en Ll, lo cual puede lograrse fertilizando con P e inoculando con micorriza.
Palabras clave: análisis proximal; cinética de producción de gas; fibra
Introduction
The woody forage species in silvopastoral systems can be used for feeding ruminants are an alternative to reduce production costs because it reduces the use of external inputs (Gil et al., 2005). The association of shrubby legumes with pasture improves the nutritional quality for livestock in a sustainable way. The leucaena, for its high ability to fix nitrogen, protein content, nutritional value and potential dry matter production, has been used eff iciently for animal feed in the Mexican tropics because it improves the diet consumed by livestock, which is based pastures of low nutritional quality (Shelton et al., 1991). On the other hand, although fodder are the main source of minerals for the ruminant, mineral imbalance in soils and forages in the tropics causes a productive and poor reproductive performance of grazing ruminants (McDowell andArthington, 2005), but in silvopastoral systems, milk production and increase daily weight gain (Ibrahim et al., 2006).
However, in order to implement an intensive silvopastoral system based on the use of Leucaena leucocephala associated with Cynodon nlemfuensis rotational grazing by (Solorio et al., 2009), it is worth analyzing the ruminal assimilation of such fodder depending on the age of regrowth and nutrient content, so in this research was determined, as a first step, the in vitro fermentation technique gas production and its correlation with the mineral and nutrient content of de Leucaena leucocephala and Cynodon nlemfuensis six regrowth ages during the windy season, which were associated in a silvopastoral system in the Huasteca potosina region of Mexico.
Materials and methods
The experiment was performed in the silvopastoral module, whose total area is one hectare with 62 rows Leucaena leucocephala at a density of 52 000 plants ha-1 (distance between plants 12 cm and 1.6 m between rows) associated with Cynodon nlemfuensis between rows. The prairie was established in October 2010 in the Production Unit "The Gargaleote" University Autónoma Chapingo, located in Tamuin, San Luis Potosí, Mexico. The climate is warm sub-humid with summer rainfall, annual average temperature of 25 °C and the average annual rainfall is 990 mm (García, 1981). The rainy season runs from july to october, the windy season from november to february and dry period from March to June. The soils of the study area are fluvisols and vertisols, deep and well drained, textured silt or silty clay sandy, with alkaline pH above 7.5.
Experimental unit: the module was divided into 18 lots of 550 m2 each (11 x 50 m), with seven rows of leucaena per batch. The treatments (6) and repetitions (3) they were randomized. The treatments were 35, 42, 49, 56, 63 and 70 days of regrowth from the last pruning of leucaena and star grass during the windy season (November, 2011 to January, 2012). Fodder for sampling were selected batch, three sites of 3.2 m2 (2 x 1.6 m) on the rows 2, 3 and 5 of leucaena. The sampling was done by removing vegetative material leucaena (leaves and tender tips of shoots) and star grass (leaves and stems) (Jiménez and Ortiz, 2012).
Determination of forage yield: total material removed from each species was weighed fresh, dried to constant weight in a forced air oven at 65 °C, and ground with a sieve of 2 mm. With these data it was estimated kg of MH and MS per hectare of each species forage and total (sum of both).
Digestibility and gas production in vitro: fermentation kinetics of both forages investigated indirectly by the technique of gas production (Menke and Steigass, 1988), for which bottles amber 125 mL capacity were used to which he placed them 0.5 g of MS of each substrate (Leucaena leucocephala or Cynodon nlemfuensis) of the DR already indicated. Subsequently, under a continuous f low of carbon dioxide (CO2) to each flask they were added 90 mL of diluted ruminal inoculum (1:10) which was obtained two sheep Rambouillet with ruminal cannula, filtered through 8 layers of cheesecloth and was added at a ratio of 1: 9 to a mineral solution reduced consisting of K2HPO4 (0.45 g L-1), KH2PO4 (0.45 g L-1), NaCO3 (0.6 g L-1), (NH4)2SO4 (0.45 g L-1), NaCl (0.9 g L-1), MnSO4 (0.18 g L-1), CaCl2 (0.12 g L-1), L-cysteine (0.25 g L-1) y Na2S (0.25 g L-1).
The bottles, including four white bottles without substrate were sealed with a rubber stopper and aluminum ring. Excess CO2 each f lask was extracted with the equalize pressure gauge to zero and incubated in water bath at 39 °C. The gas pressure fermentation was measured with the pressure gauge (0 to 1 kg cm-2) at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 23, 26, 29, 33, 37, 43, 49, 55, 61 and 72 h incubation. At the end of the incubation period each bottle residue was filtered through pre-weighed paper filter. The papers with residue dried at 65°C for 48 hand weighed. By weight difference the MS residual was obtained to determine the DIVMS72 of 72 h of incubation.
Determination of mineral content and nutrients: ground subsamples of 2 g of each forage species and each DR were made separately and completely dried at 105 °C for 12 h. The samples were incinerated in a muff le furnace at 500 °C for 8 h, to quantify the proportion of ash and organic matter (MO) (AOAC, 1990). Quantification of Ca, Mg, Na, K, Cu, Zn, Fe and Mn was performed by atomic absorption spectrophotometry (Fick et al., 1979); P was determined using the colorimetric method (Clesceri et al., 1992); and by f luorometric method (Tamari et al., 1986). Ether extract (EE) was determined by the method Goldsfich (AOAC, 2000), total nitrogen by Kjeldahl (Harris, 1970), with which the percentage of crude protein (CP) was obtained by multiplying by 6.25, neutral detergent fiber (NDF), acid detergent fiber (FDA) and lignin in H2SO4. The hemicellulose and cellulose content was estimated by difference of FDN and FDA, and FDA and Lignin-permanganate (Robertson and Van Soest, 1981).
Variables analyzed: pressure values (kg cm-2) were converted to gas volume (mL g-1 substrate) with the regression equation (volume= pressure/0.019). On the one hand, the fractional volume of gas was analyzed, the graph where could find differences (p< 0.05) for Leucaena leucocephala fractional volume (Va) from 0 to 12 h was determined while for Cynodon nlemfuensis observed were (Va) from 0 to 14 h, (Vb) from 16 to 33 h and (Vc) of 37 to 61 h incubation. On the other hand, the accumulated volume of gas from 0 to 72 h of incubation was obtained, and the kinetic parameters of gas production were estimated: maximum volume (Vm; mL g-1), rate (S; h-1) and delay time (L; h), for the logistic model V=Vm/1+e (2-4S (T-L)) (Schofield and Pell, 1995) and using the statistical package.
Other variables were the digestibility in vitro of MS (DMSIV; %). Forage MS yield (kg MS ha-1) of vegetative material collected at sites and production efficiency of gas (mL g-1 substrate digested). In addition, organic matter digestibility and metabolizable energy with mathematical models were estimated:
DMO (%)= 14.88+0.889GP+0.45PC+0.0651CEN (R2= 0.92)
EM (MJ kg-1MS)= 2.20+0.136GP+0.057PC (R2= 0.94).
Where: GP= gas produced by 0.2 g of substrate in 24 h; PC= crude protein content of the substrate; CEN= amount of ash substrate (Sallam et al., 2007).
Experimental design and statistical analysis: was an analysis of variance with a completely randomized design for six treatments and three repetitions, using the statistical package and multiple comparison test of Tukey half (SAS, 2004).
Results
The values of Vm, L and S parameters are shown in Table 1.
Table 1 Parameters (S, L and Vm) of the kinetics of fermentation gas production, DIMS, DMO and EM of Leucaena leucocephala (LI) and Cynodon nlemfuensis (Cn) of six periods of regrowth.

ABC Valores con literal distinta en la misma columna son diferentes (p< 0.05). S= tasa de producción de gas; L= fase lac o tiempo de retardo; Vm= volumen máximo de gas; DIMS= digestibilidad in vitro de la materia seca a 72 h de incubación; DMO= digestibilidad de la materia orgánica; DMO= 14.88+0.889GP+0.45CP+0.651XA, R2= 0.92. Donde: GP= producción de gas a 24 h, CP= proteína cruda, XA= cenizas. EM= energía metabolizable; EM= (MJ/KgDM)= 2.2 + 0.136GP + 0.057CP, R2= 0.94; donde GP= producción de gas a 24 h; EM (Mcal kg-1)= EM (MJ)*0.238.
L. leucocephala ferments earliest (L= 1.92 h) and quickly (S= 0.039 h-1) that C. nlemfuensis (S= 0.026 h- 1; L= 5.63 h). Similarly, fermentation potential given the maximum volume of gas (Vm) was higher for L. leucocephala (Vm= 259.8 mL g-1) with respect to C C. nlemfuensis (Vm= 227.1 mL g-1). In contrast, production efficiency was higher for gas C. nlemfuensis that L. leucocephala (897.29 and 771.4 mL g-1 of MS digested). With respect to the in vitro dry matter digestibility (DIVMS) of fodder, the L. leucocephala had a higher digestibility than the C. nlemfuensis (63.61 vs 42.88%).
The estimated values of DMO and EM (Table 1) using mathematical models (Sallam et al., 2007) showed that the organic matter digestibility was similar to DIVMS for both forages, keeping the difference between them. The metabolizable energy was higher in L. leucocephala than C. nlemfuensis (2.21 and 1.4 Mcal kg-1 MS). On the other hand, regarding the age of regrowth, both forages 35 DR had better fermentation parameters (p< 0.05) greater Vm, S, DIVMS, DMO, MS and lower L (Table 1).
Based on Figure 1, it was determined that L. leucocephala reaches its highest fermentation 20 h before C. nlemfuensis.
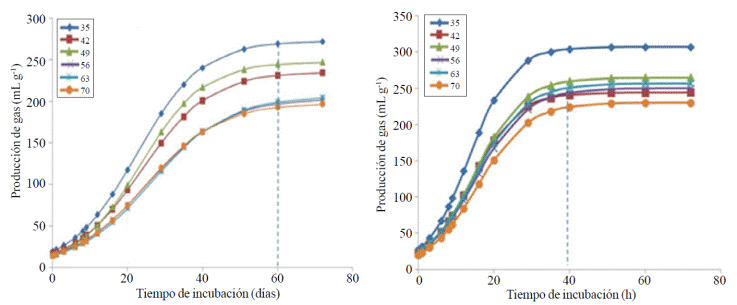
Figure 1 Production of gas during in vitro fermentation of (a) Cynodon nlemfuensis; and (b) Leucaena leucocephala 35, 42, 49, 56, 63 and 70 days regrowth, by ruminal bacteria.
The time to achieve the VM in L. leucocephala was 32 h and 51 h for C. nlemfuensis.
In L. leucocephala rate (S) of forages between 35 and 49 are similar (p> 0.05), but the 35 of DR is higher (p< 0.05) than fodder 56, 63 and 70 DR. The delay time (L) was not different (p> 0.05) between DR, and the potential of fermentation given by Vm and DIVMS was higher (p< 0.05) only for the L. leucocephala of 35 DR, regarding others DR (Table 1, Figure 1). A similar behavior was obtained for DMO and EM of L. leucocephala, which were higher (p< 0.05) for plants 35 DR.
Although, like L. leucocephala, plants C. nlemfuensis with 35 DR, had the best fermentation parameters and DIVMS, it was observed that the DR to 35 days older affects fermentative behavior of this grass than the legume, so that it could form three groups: high (35 DR), medium (42 and 59 DR) and low (56, 63 and 70 DR) fermentation to C. nlemfuensis (Figure 1A vs 1B).
This was reflected in the fractional volume of gas (Figure 2).

Figure 2 Fractional gas production in vitro fermentation of (A) Cynodon nlemfuensis; and (B) Leucaena leucocephala 35, 49 and 70 days of regrowth, by ruminal bacteria.
Usually have three fractions fodder fermentation: fast, medium and slow fermentation; sugars and oligosaccharides attributed to soluble reserve polysaccharides such as starch, dextran, pectin and cell wall polysaccharides (cellulose and hemicellulose linked to lignin), respectively. L. leucocephala showed a peak only high fractional gas production during the period of 0 to 12 h (Figure 2B and Table 2), which was attributed to sugars and polysaccharides reserve; while C. nlemfuensis showed three peaks fractional greater gas production: 0 to 14, 16 to 33 and 37 to 61 h incubation (Figure 2A and Table 2).
Table 2 Volume fractional cumulative gas produced during in vitro fermentation of C. nlemfuensis (PE) and L. leucocephala (LE) depending on the age of regrowth.

A, B y C = Valores con literal distinta en la misma columna son diferentes ( p< 0.05).
The fractional volume of 0 to 14 h, in C. nlemfuensis was higher (p< 0.05) in DR 35 d followed by 42 and 49 d and finally by 56, 63 and 70 d (Table 2). A similar trend was found for the other two fractions (16 to 33 and 37 to 61 h), where DR plants produced lower gas and therefore less fermented (Table 2). In L. leucocephala only in the DR of 35 days compared with the other DR distinguished analyzed.
The DMO estimated with the mathematical model showed a similar to DIVMS calculated (Table 1) behavior. For both forages, DMO was higher (p< 0.05) at 35 DR, in leucaena only 35 DR plants had the highest DMO than other DR (Table 1), while in C. nlemfuensis are more groups formed according to the DMO. The EM remained the same (p> 0.05) in leucaena independently the DR of 42 to 70 (p> 0.05), but decreased C. nlemfuensis the EM were higher as the DR (Table 1).
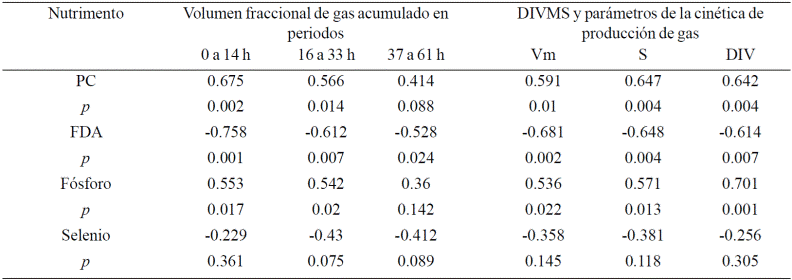
Significant correlations (p< 0.01) between variables fermentation kinetics (Vm, S and L), fractional and DIVMS, with organic nutrients and minerals to Cynodon nlemfuensis and Leucaena leucocephala shown in Tables 3 and 4, respectively.
Table 3 Significant correlations variables for fermentation and nutrient content of Cynodon nlemfuensis.
p= probabilidad: altamente significativa (<0.01), significativa (0.01< p< 0.1), no significativa (p> 0.1) S= tasa de producción de gas; L= fase lac ó tiempo de retardo; Vm=volumen máximo de gas; DIVMS= digestibilidad in vitro de la materia seca a 72 h de incubación.
Table 4 Significant correlations variables for fermentation and nutrient Leucaena leucocephala.

p= probabilidad: altamente significativa (<0.01), significativa (0.01< p< 0.1), no significativa (p> 0.1) S= tasa de producción de gas; L= fase lac ó tiempo de retardo; Vm=volumen máximo de gas; DIVMS= digestibilidad in vitro de la materia seca a 72 h de incubación.
The PC is the only nutritional component is positively correlated (R= 0.566 to 0.675, p< 0.02) with variables fermentation and DIVMS, but only in Cynodon nlemfuensis. The FDA, FDN, cellulose and lignin are negatively correlated (p< 0.03) with these variables. It is noteworthy that the FDA, in both forages, and lignin in leucaena are the highest correlation (R of -0528 to -0758; Tables 3 and 4). It is also noteworthy that only cellulose is negatively correlated with L. leucocephala and not C. nlemfuensis.
Regarding minerals, Ca:P ratio was negatively correlated (R= -0.524; p= 0.026) with the DIVMS of L. leucocephala, although there was a trend of negative correlation (R= 0.424; p= 0.080) with the gas volume (Vm and V0- 12). With C. nlemfuensis, selenium also showed a trend negative correlation (R= 0.430 and -0.412; p= 0.075 and 0.089) for the production of fractional gas 16 to 32 h, and 37to61h.
The phosphorus was positively correlated in both forages (R= 0.536 to 0.701; p< 0.03). Fermentation and DIVMS of L. leucocephala was more sensitive to the content of Fe, Na, K and Cu, because they showed a positive correlation (Table 4).
Discussion
Under the L. leucocephala samples were less fibrous and had a composition richer in chemicals, fermentation and DIVMS was higher than that of C. nlemfuensis. Among the reasons that L. leucocephala was more fermentable and digestible, it is that this feed comprises the upper stratum of silvopastures; while C. nlemfuensis develops in the lower stratum and the incidence of sunlight is less than the upper stratum. This fact increases as the DR are higher, and the incidence of light to the lower stratum is further reduced due to the height and thickness of the largest glass reaching L. leucocephala plants. It is for this reason that both the variables DIVMS gas production by fermentation, practically do not vary (p> 0.05) for L. leucocephala, but are reduced (p< 0.05) in C. nlemfuensis. This was corroborated by the fractional volume of gas (Figure 2) where it was observed that the greatest volume of gas in L. leucocephala is easily fermentable material (less than 20 h of incubation); while in C. nlemfuensis the fibrous fraction (over 40 h of fermentation) produces more gas than with leucaena (Figure 2).
The metabolizable energy estimated with mathematical models are compared to those calculated by other authors for these same fodder (Maya et al., 2005). Although we must continue evaluating these mathematical models to determine their reliability, data obtained in this research are suitable for working conditions.
As in other studies, the FDA was negatively correlated with fermentation and digestibility of forages, because this chemical component contains lignin and cellulose, the latter may contain greater proportion of the crystalline form of amorphous, which serve physical impediment to the penetration and activity of enzymes fibrolytic. It seems that the cellulose C. nlemfuensis is more fermentable than the L. leucocephala since in the latter was negatively correlated with variables fermentation and no correlation in C. nlemfuensis.
Many of the minerals involved in important cellular functions and nutrient transport through the membrane, the formation of structures and electron transport as cofactors in enzymatic activity. In this sense it is possible that the mineral content of forages might affect the fermentative activity of ruminal microorganisms, so that positive correlations (p< 0.05) with various minerals (P, K, Na, Fe and Cu) and there was only a trend (p< 0.1) negative correlation with selenium and Ca:P, in C. nlemfuensis (Table 3) and L. leucocephala (Table 4). In the case of selenium correlation was found with the volume fractions of medium and slow fermentation (V16-33, V37-61), for reserve polysaccharides and fibrous, but not with the DIVMS, S, Vm ni V0-14 (Table 3). It is indicating that the enzyme is not affect the activity of enzymes digestion or fermentation of soluble faction.
Conversely the Ca:P if it affects digestibility. There are reports of mid last century that indicate (Burroughs et al., 1951) show that elements such as Fe, Na, K, Ca affect the physiology of ruminal bacteria. Much research has been on the effect of minerals in the activity, physiology, cellulose digestion by rumen microorganisms, has come to estimate appropriate to optimize said activity concentrations, or toxic levels, very few studies have investigated the release of such minerals during digestion of forages and its effect on microbial activity, so this research provides evidence that the mineral content in forages may be related to the fermentative activity of rumen microorganisms; however in need for more research on the matter and determine its effect on in vivo and in situ systems, as this could be determined by the mineral composition of the rumen and this in turn by the origin or region where the crop was grown consuming and the formulation of diets in which mineral mixes are included.
Conclusion
Fermentation and in vitro digestibility of rumen microbial consortia, Cn and Ll grown in a silvopastoral system are determined by DR. Since fermentation C. nlemfuensis is more affected by DR older, it determines the DR forage that should grazed the system (35 to 42 DR), and to stimulate ruminal utilization management alternatives suggests looking to increase the content of PC in Cn and P in Ll, which can be achieved by fertilizing with P and inoculating mycorrhizal, given the high correlation found in this research, including fermentation and digestibility variables with the content of these nutrients.
Literatura citada
AOAC. 1990. Official Methods of Analysis of Association of Official Analytical Chemists. 15th ed. Method 930.04, 955.04, 930.05. USA: Association of Official Analytical Chemists. [ Links ]
Burroughs, W.; Latona, A.; DePaul, P.; Gerlaugh, P. and Bethke, R. M. 1951. Mineral influences upon urea utilization and cellulose digestion by rumen microorganisms using the artificial rumen technique. Journal of Animal Science. 10: 693-705 [ Links ]
Clesceri, S. L.; Greenberg, E. A. y Trusseli, R. R. 1992. Métodos normalizados para el análisis de aguas potables y residuales. Ed. Díaz Santos. España. 187-195 pp. [ Links ]
Fick, K. R.; Mcdowell, L. R.; Miles, P. H.; Wilkinson, N. S.; Funk, J. D.; Conrad, J. H. and Valdivia, R. 1979. Métodos de análisis de minerales para tejidos de plantas y animales. 2da ed. Universidad de Florida, Gainesville, Florida, 358 p. [ Links ]
García, E. 1981. Modificaciones al sistema de clasificación climática de Köppen. Tercera edición. Instituto de Geografía, UNAM. Distrito Federal, México. 252 p. [ Links ]
Gil, J. L.; Espinoza, Y. y Obispo, N. 2005. Relaciones suelo-planta-animal en sistemas silvopastoriles. Revista digital del Centro Nacional de Investigaciones Agropecuarias. 9: 20-26. [ Links ]
Harris, L.1970.Métodos para el análisis y evaluación biológica de los alimentos para animales. Universidad de Florida. Gainesville, Florida. [ Links ]
Ibrahim, M.; Villanueva, C.; Casasola, F. y Rojas, J. 2006. Sistemas silvopastoriles como una herramienta para el mejoramiento de la productividad y restauración de la integridad ecológica de paisajes ganaderos. Pastos y Forrajes. 29: 383-419. [ Links ]
Jiménez, R. J. D. y Ortiz T. M. 2013. Composición mineral de guaje (Leucaena leucocephala) y pasto estrella (Cynodon nlemfuensis) en un sistema silvopastoril intensivo. Tesis Profesional, Departamento de Zootecnia, Universidad Autónoma Chapingo. 40 p. [ Links ]
Maya, M. G. E.; Durán, C. C. V. y Ararat, J. E. 2005. Valor nutritivo del pasto estrella solo y en asociación con leucaena a diferentes edades de corte durante el año. Acta Agronómica. 54: 41-45. [ Links ]
McDowell, L. R. y Arthington, J. D. 2005. Minerales para rumiantes en pastoreo en regiones topicales. 4a Ed. Universidad de Florida, Gainesville, Florida.94 p. [ Links ]
Menke, K. H. and Steingass, H. 1988. Estimation of the energetic feed value obtained from chemical analyses and in vitro gas production using rumen fluid. Animal Research and Development. 28: 7-55 [ Links ]
Sallam, S. M. A.; Nasser, M. E. A.; El-Waziry, A. M.; Bueno, I. C. S. and Abdalla, A. L. 2007. Use of an in vitro rumen gas production technique to evaluate some ruminant feedstuffs. Journal of Applied Sciences Research. 3: 34-41. [ Links ]
SAS. 2004. SAS User ́s Guide: Statistics. Ver. 9.2. SAS Institute. Cary, N.C. 5180 p. [ Links ]
Schofield, P. and Pell, A. N. 1995. Measurement and kinetic analysis of the neutral detergent‐soluble carbohydrate fraction of legumes and grasses. Journal of Animal Science. 73: 3455-3463. [ Links ]
Shelton, H. M.; Lowry, J. V.; Gutteridgue, R. C.; Bray, R. A. and Wildin, J. H. 1991. Sustaining productive pastures in the tropics. Tree and shrub legumes in improved pastures. Tropical Grassland. 25: 119-128. [ Links ]
Solorio, S. F.; Bacab, H.; Castillo, J. B.; Ramírez, L. y Casanova, F. 2009. Potencial de los sistemas silvopastoriles en México. II Congreso sobre sistemas silvopastoriles intensivos. Yucatán, México. 10 p. [ Links ]
Tamari, Y.; Ohmori, S. and Hiraki, K. 1986. Fluorometry of nanogram amounts of selenium in biological samples. Clinical Chemistry. 32:1464-1467. [ Links ]
Received: April 2016; Accepted: June 2016











 text in
text in 


