Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 spe 14 Texcoco feb./mar. 2016
Articles
Survival and growth of black mangrove (Avicennia germinans L.) in reforested plantations and natural regeneration
1Colegio de Postgraduados-Campus Tabasco. Periférico Carlos A. Molina, km 3.5. Carretera, Cárdenas-Huimanguillo. H. Cárdenas, Tabasco. C. P. 86500. (lauro.gonzalez@colpos.mx; obradoro@colpos.mx).
2Colegio de Postgraduados- Campus Veracruz, km 26.5 carretera federal Veracruz-Xalapa. Predio Tepetates. (parturo@colpos.mx).
Abstract
The objective of this research was to quantify the survival and growth of black mangrove (Avicennia germinans L.) in reforested plantations in an area of 50 ha; the study was carried out from 2013 to 2014, in the mangrove ecosystem located in the Ejido “Las Coloradas” Cárdenas, Tabasco. Three environments were delimited (1: flooded zone Zi; low tide zone Zmb and 3: free-flowing zone Zlfa). For monitoring three sites of 500 m2 per environment were randomly located. The evaluated variables were survival, plant height (h), crown area (sc), stem diameter (dc), number and height of pneumatophores (nn, an). The evaluation found statistically significant differences in survival rate between environments, which has two groups (A and B) with alpha= 0.05. The group A is Zmb with 67.7% survival, and group B to Zi and Zlfa with 0% and 5.5% survival, respectively. Growth recorded no significant statistical differences. Zmb from reforested plantation grew to (h: 27 cm, sc1: 16.3 cm, sc2: 15.9 cm, dc: 0.5 cm, an: 2.8 cm and nn: 13). Zlfa grew to (h: 7.2 cm, sc1: 13.3 cm, sc2: 8.7 cm, dc: 0.2 cm, an: 4.3 and nn: 5.1). Natural regeneration in Zmb showed a growth of (h: 30.1 cm, sc1: 21.8 cm, sc2: 21.3 cm, dc: 0.5 cm, an: 2.2 cm and nn: 5.3 cm). Zmb promotes survival and growth of black mangrove in reforested plantations and natural regeneration of populations.
Keywords: environment; mortality; pneumatophores; sunlight; tide
El objetivo de esta investigación fue cuantificar la sobrevivencia, y crecimiento de mangle negro (Avicennia germinans L.), en plantaciones reforestadas en una superficie de 50 ha, el estudio se llevó cabo de 2013 a 2014, en el ecosistema de manglar ubicado en el Ejido “Las Coloradas”, Cárdenas, Tabasco. Se delimitó tres ambientes (1: ona inundada Zi; 2: zona de marea baja Zmb y 3: zona de libre fluidez del agua Zlfa). Para el monitoreo se ubicaron tres sitios al azar de 500 m2 por ambiente. Las variables evaluadas fueron sobrevivencia, altura de planta (h), el área de copa (sc), diámetro de cuello (dc), número y altura de neumatóforos (nn, an). La evaluación se encontraron diferencias estadísticas significativas al porcentaje de sobrevivencia entre ambientes, la cual presenta dos grupos (A y B) con un alpha= 0.05. El grupo A corresponde la Zmb con un 67.7% de sobrevivencia, y el grupo B a la Zi y Zlfa, con 0% y 5.5% de sobrevivencia, respectivamente. El crecimiento no se registró diferencias estadísticas significativas. La Zmb de plantación reforestada tuvo un crecimiento de (h: 27 cm, sc1: 16.3 cm, sc2: 15.9 cm, dc: 0.5 cm, an: 2.8 cm y nn: 13). La Zlfa se tuvo un crecimiento de (h: 7.2 cm, sc1: 13.3 cm, sc2: 8.7 cm, dc: 0.2 cm, an: 4.3 y nn: 5.1). La regeneración natural en la Zmb mostró un crecimiento de (h: 30.1 cm, sc1: 21.8 cm, sc2: 21.3 cm, dc: 0.5 cm, an: 2.2 cm y nn: 5.3 cm). La Zmb favorece la sobrevivencia y crecimiento del mangle negro en plantaciones reforestadas y poblaciones de regeneración natural.
Palabras clave: ambiente; marea; mortalidad; neumatóforos; radiación solar
Introduction
Mangroves are plant communities present throughout the tropical and subtropical coasts of the planet, and are located in the convergence zone between sea and land. These ecosystems are made up of plant and animals, have a number of physiological adaptations that allows them to develop in frequently flooded land with hypersaline waters (Carrillo et al., 2008). Globally there are a total of 73 mangrove species, mostly trees and shrubs (Chan et al., 2012). This type of ecosystem globally occupies 152 361 km2 (Spalding et al., 2010).
In Mexico four mangrove species predominate: red mangrove (Rhizophora mangle L.), white mangrove (Laguncularia racemosa (L.) C. F. Ga-erth), black mangrove (Avicennia germinans L.) and button mangrove (Conocarpus erectus L.) in an area of 582 415 ha (Rodríguez et al., 2013) and in the state of Tabasco there is a surface of 41 498.5 ha (Domínguez et al., 2011). The four species mentioned maintain the balance of mangrove ecosystem and provide environmental services of ecological and social importance (CONABIO, 2008).
The environmental services provided by mangroves are: capture and storage CO2 and releases O2 as a result of photosynthesis, respiration and degradation of dry matter; certainly mangroves release less carbon than other forest ecosystems (Sanjurjo and Welsh, 2005). Mangrove ecosystem stores between 12.8 and 39.9 kg cm2 during dry seasons and norths (Moreno et al., 2010). It also serves as protection of coastal areas against natural phenomena (hurricanes, storms, floods), and contributes to the conservation of wildlife and the production of water, food, medicine, genetic resources, and productive activities (Schuyt and Brander, 2004; Barba et al., 2010; Wood et al., 2013; Rodríguez et al., 2013).
However, mangrove ecosystem is one of the most subject to deterioration due to irrational exploitation of its resources, beyond their resilience (Basáñez et al., 2006; Flores et al., 2010). Besides polluting waste from industries affect feeding and reproduction of aquatic life, such as plants, insects and fish (Reeves, 2005; Olguín et al., 2007; Hernández-Menchor, 2013). Coupled to this, are also the effects from natural origin widespread and specific (Rodríguez et al., 2013). Among these mangrove degradation by the abrasive action of the sea on costs and rise of their tidal average level; disappearance of coastal lagoons by silting or natural closure of channels; accumulation of sand due to changes in coastal dynamics and migration of sediments, causing coating of mangrove roots and subsequently death; destructive effect by extreme weather events (cyclones and hurricanes) and variations in water regime (decreased rainfall).
Because of these impacts, mangrove ecosystem in 1980, Mexico counted with 1.12 million hectares, and by 2005, this area decreased to 820 000 ha; i.e. an annual loss of 12 000 ha (4%) for 25 years (FAO, 2007). Therefore, many institutions have been given the task of generating information through scientific research providing elements to reduce the effects and negative impacts towards these natural ecosystems. Among conservation strategies are the establishment of protected natural areas (PNA), parks and museums among others, in order to preserve, care for, maintain and study plant and animal communities through a management plan that favors its preservation (Carmona et al., 2004). Currently there are various programs: such as research, environmental education and conservation for sustainable management of mangroves (Carmona et al., 2004; Linares et al., 2004).
Therefore, maintaining the structure and function of mangrove ecosystem involves biotic and abiotic elements (temperature, precipitation, hydrology, soil) and contrition of each of the elements, allowing greater ecosystem productivity (Rodríguez et al., 2013). Hydrology is a fundamental part of the ecosystem as abiotic element, which causes changes in the structure and function, thus showing that each species is distributed according to hypersaline hydrology (tide) and adaptability of the species (Carrillo et al., 2008; Rodríguez et al., 2013). For example, red mangrove (R. mangle) grows in flooded areas on the banks of lagoons where water flow is constant (Ortiz and Méndez, 2000). For black mangrove (A. germinans), grows best within the mangrove away from the edges of estuaries or channels, at slightly higher elevations where the flow tide is less apparent with a short flooding time, due to it physiological state of adaptation in the environment. Although it is the species that has higher tolerance to high salinity conditions, as it is located in concentrations greater than 40 per thousand to 100 per thousand, due to the secretory glands of salt in their leaves (Cordero and Boshier, 2003). This shows that seawater level in growth areas of white mangrove (L. racemosa) generate a replacement by black mangrove (A. germinans) due to high salinities (Galmiche and Solana, 2011).
Regarding to soil groups as Histosols Solonchaks, Gleysols and Technosols are soils that can be found in mangroves, predominating the first two (Moreno et al., 2002; Dominguez et al., 2011). These mangrove soils are characterized for having a high water, salt and hydrogen sulfide and a low oxygen content and high proportion of organic matter (Lewis, 2005). Salinity in the sediment (interstitial salinity) of mangroves depends on the type of hydrology prevailing in them. The variations of depth, product of small scales slopes in mangrove land exerts physical pressure acting as restrictive for the expansion of mangrove (Ortiz and Mendez, 2000); also, the rainiest seasons stabilize salinity levels in the range between 49 -55% (Rodríguez et al., 2013).
The pH of the water in the mangrove ecosystem is variable due to the flooding period. The pH of interstitial water will be greater over long periods of flooding (Yañez et al., 2001), which can be from 4.7 to 5, classified as an acid soil (García, 2005). According to Kohen et al. (1995) plant growth is dependent of genetic variation and environmental conditions (soil-plant-atmosphere relationship). Therefore, the amount of radiation available influences physiological and morphogenetic and reproductive processes of the plants and significantly affects the overall functioning of the ecosystem (Kohen et al., 995). Also it triggers diverse evolutionary processes that come into play since adaptation to average radiation available to coevolution between animals and plants or parasites and hosts, passing through flexibility or plasticity to adapt to spatial and temporal changes of radiation (Valladares et al., 2004; Alcaraz, 2012).
Therefore, the need to consider biotic elements in the survival and growth of the species of interest, particularly when reforestation with black mangrove is performed; therefore, this study aimed to quantify the survival and growth of black mangrove (A. germinans) in reforested plantations and natural regeneration in the ejido "Las Coloradas" Cardenas, Tabasco. Area greatly affected by the caterpillar Anacamptodes spp., in 2010 (Sol et al., 2012).
Materials and methods
This work was conducted from 2013 to 2014, in the mangrove ecosystem located in the Ejido "Las Coloradas" Cardenas, Tabasco, located between UTM coordinates 2 026 900 and 441 000. This mangrove ecosystem is located in a lower plain of flood lagoon (Ortiz et al., 2005), in Solonchaks, Gleysols, Histosols, Technosol soils (Palma et al., 2006; Domínguez et al., 2011). The type of weather Am (w) is distributed in the great plain of the Gulf plain (SEDESPA, 2006). The rainfall varies from 1 500 mm annually in the far west to 2 000 mm in the estuary from Tonala (Palma et al., 2006). The experimental unit corresponded to an area of 50 hectares, an area that was reforested with the species A. germinans (Sol et al., 2012). This area was delimited in three environments: flooded area (Zi), low tide zone (Zmb) and free flow water zone (Zlfa) (Figure 1). The delimitation of these three environments was performed using the software Arc GIS 9 (ArcMap version 9.3), through the Spline interpolation method (Alvarez et al., 2011), and based on data of water depth of sampling sites.
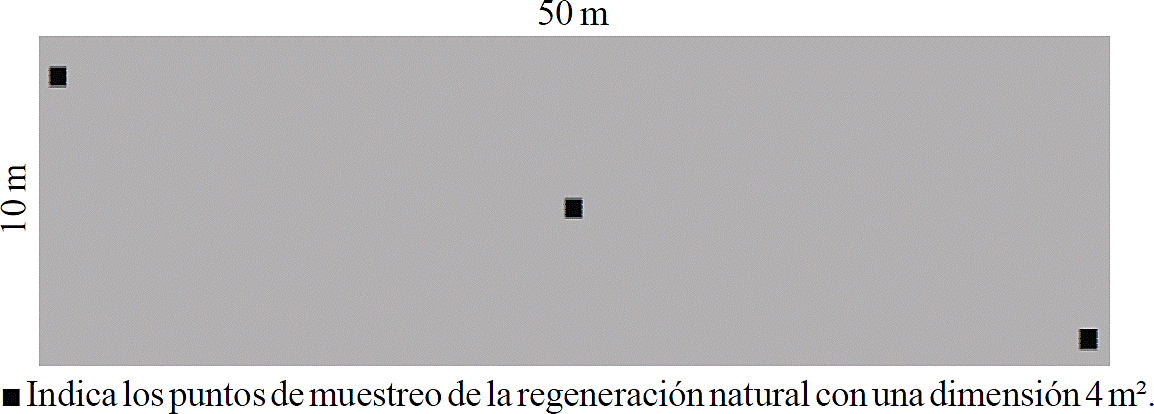
In each environment three permanent monitoring sites were established, giving a total of nine sites. These sites were established at a distance of approximately 200 meters of each other, under a completely randomized design (Herrera and Garcia, 2010). Each site has an area of 500 m2 (50 m long x 10 m wide) according to Melo and Vargas (2003). From this sites collected reforested plants (Prf) and natural regeneration (Prn) data.
In each sampling site, three permanent monitoring plots were established to record Prn data, with an area of 4 m2 (2 m x 2 m), considering all the plants within the quadrant (Figure 2).
Survival and mortality pattern was quantified based on the total planted in each sampling site. The mortality rate was grouped by mortality patterns, being: dead stand (mp), trunk broken (tp), fallen from root (cr), disappeared individuals (de), cut (cor) and unclassified (scla) (Londoño and Álvarez, 1997; Londoño and Jiménez, 1999).
For reforested plant growth and natural regeneration, height (h), crown area (sc1 and sc2), stem diameter (dc) and pneumatophores height (an) based on criteria from Barrera et al. (W. D) were measured. Variables were taken in three seasons: North (2013), dry (2014) and Rain (2014) according to Aceves et al. (2008). With data from growth variables, the dasometric relative growth rate was calculated (Carrillo et al., 2008; Sosa and Rodríguez, 2003).
Relative growth rate (TRC) in height:
Increased percentage (PI) in height
Relative growth rate (TRC) in diameter
Increased percentage (PI) in diameter
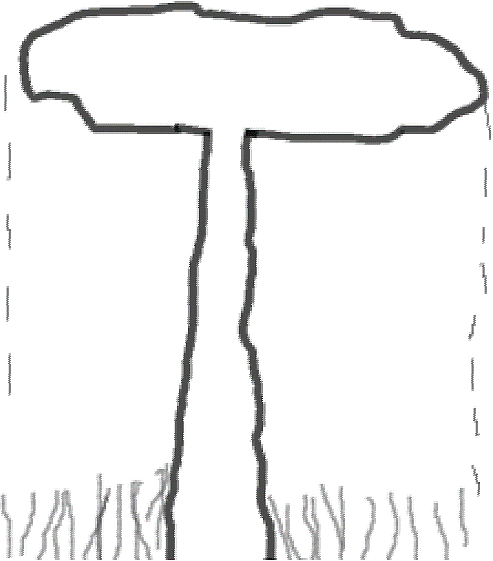
Also, the total number of pneumatophores per plant was counted taking as reference the plant and the surface of the farthest crown, forming a circumference around that integrates all the pneumatophores (Figure 3) (Vilmarie, 2008).
Solar radiation was measured from readings in 15 points of each sampling site in the three environments (Valladares, 2004). This was done using a LI-COR Terrestrial Radiation with a Quantum Q 40829, at 12:00 and 13:00 h, time of higher solar radiation. These data were compared with the growth of reforested plantations and natural regeneration. Soil samples were taken during the dry season according to the Official Mexican Standard (NOM-021-SEMARNAT-2000; Buduba, 2004) at two depths (0 ≥ 30 and 30 cm ≥ 60 cm) giving a total of six composite samples. The chemical and physical properties of these samples were analyzed (pH, EC, OM, Nt, P-Olsen, K, Ca, Mg, Na, CEC, Fe, Cu, Zn and Mn) and physical (texture), in the Laboratory for Chemical Analysis of Soil, Plants and Water (LASPA) from the Postgraduate College in Agricultural Sciences (Campus Tabasco).
In field water depth data was recorded during 2013-2014, historical precipitation data were considered based on the weather station Sánchez Magallanes, near the study area. The experimental design was a factorial 2 x 3, making a total of six treatments (Herrera and García, 2010). The six treatments were the combination of the three environments: flooded area (Zi); low tide zone (Zmb) and free zone (Zlfa), and the other factor was the type of plantation with two levels: reforested Plantations (Prf) and natural regeneration plants (Prn).
To analyze the data the Statistical Analysis System (SAS) version 9.1 (SAS, 1995) was used, through the procedure Linear Regression Model (GLM). Normal data on survival and growth in reforested plantations and regeneration were analyzed. When statistically significant differences between environment (treatment) were found, the Tukey test (alpha= 0.05) was performed to classify the best environment (treatment) regarding survival and growth. Natural regeneration data was analyzed descriptively due to the Zi and Zlfa environments showed no populations of natural regeneration. Growth variables were reported on average and mortality patterns in percentage.
Results
The survival of transplanted plants (Prf) from black mangrove showed significant differences between treatments. Tukey test separated two groups (with an alpha of 0.05). The first group corresponds to the treatment of seasonal low tide (Zmb) with 71.6% of survival and group two Zi and Zlfa treatments with 1.1% and 9.9% of survival, respectively. In the second sampling there was a decrease in the above percentages. The Zmb environment had 71.1% survival, in Zi and Zlfa was 0.5% and 6.1% respectively. In the last sampling in the Zmb environment recorded 67.7% survival, Zi reported no survival, and Zlfa was 5.5% Figure 4. That is, that total survival in Zmb was 40 plants per 500 m2 and in Zlfa with 3 surviving plants; with an age of two years and three months since its establishment.
Regarding mortality patterns there was no significant statistical difference between treatments was found. The highest percentage of missing individuals (d) and dead stand (mp) in the three environments was as follows: Zi a mortality of 73.3%, in Zmb 28.8%, in Zlfa 52.1%. Also Zi recorded 26.1% of dead stand plants (mp), in Zmb 1.6% and in Zlfa 41.6% (Figure 5).
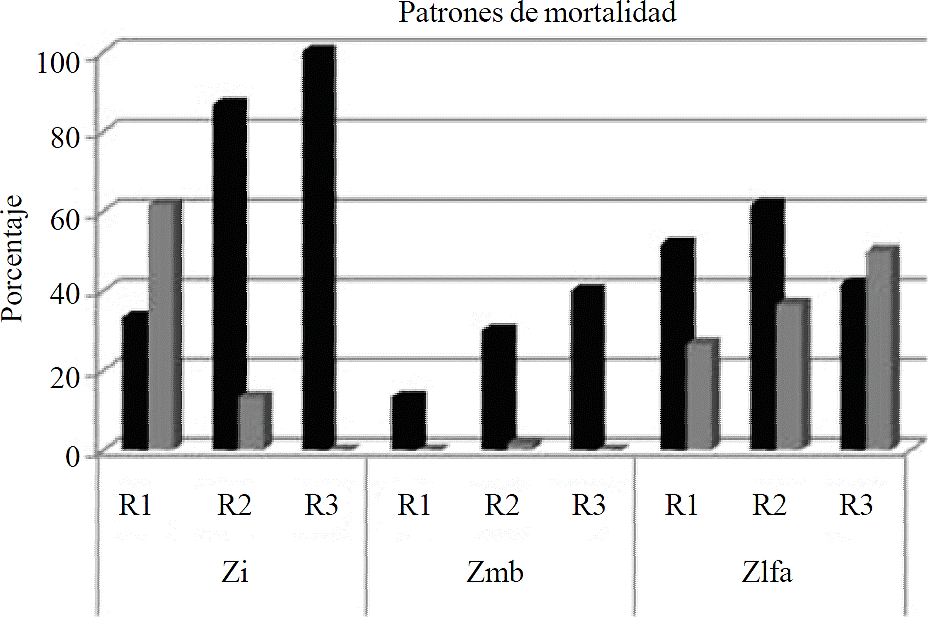
Figure 5 Mortality patterns in three types of environments in reforested plantations. Initials: disappeared (d), dead stand (mp).
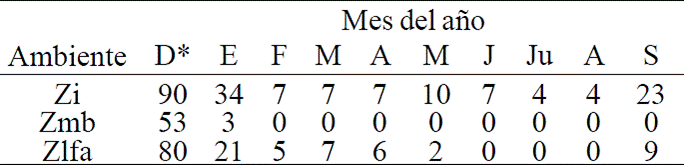
Natural regeneration recorded from five to thirteen plants per sampling site, mostly A. germinans. Also recorded from one to two plants of species L. racemosa and rarely of R. mangle. During the first two periods of evaluation there was no mortality pattern of the species mentioned. However, in the last evaluation recorded dead stands (mp) particularly the species L. racemosa, in its entirety. The growth of reforested plantations showed no statistically significant difference between environments. However, the greatest height growth was recorded in Zmb which was 16 cm, from 2013 to 2014 and grew 10.8 cm from April to September. This lower growth is due to long periods of drought in the area, which was reflected mainly in the last measuring period growing 10.8 cm. At the beginning recorded a total height of 146.2 cm and at 10 months a height of 173.8 cm (Table 1).
Table 1 Increases of plant variables.
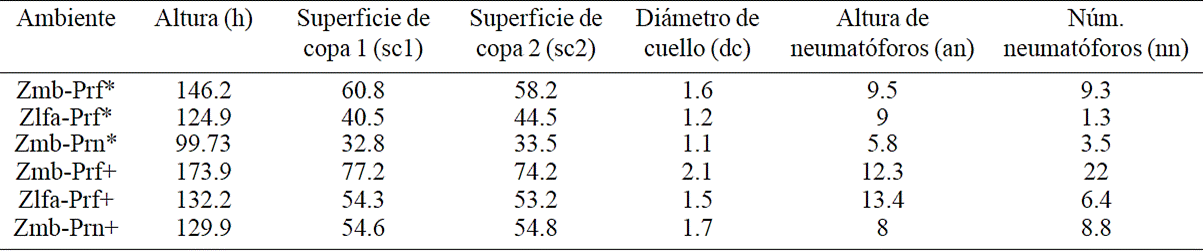
Siglas= Zmb, zona de marea baja; Zlfa= zona de libre fluidez del agua; Prf= plantación reforestada; Prn= población de regeneración natural; *, 2013; +, 2014.
In Zlfa the plants showed an increase in height of 3.5 cm in the first five months and 3.7 cm from april to september, being the initial height of 124.9 cm and at the end of the evaluation with a height of 132.2 cm. Crown surface in the Zmb environment showed an increase of 16.2 cm2 in 10 months. In Zlfa the area showed an increase of 11.2 cm2 from 2013 to 2014. The diameter of the stem in reforested plants in Zmb recorded a total growth of 0.5 cm. For Zlfa, the growth of stem diameter was 0.2 cm.
The physical and chemical analysis of soil from 0 to 30 cm depth was as follows: a pH slightly acid (5.58 to 6.57) and electrical conductivity (EC) of a highly saline soil (12.24 to 15.98 dS m-1). Zlfa showed high content of organic matter (5.7%) as well as Zi and Zmb (8.6 and 15.5%). In addition high and very high total nitrogen content in the three environments (Nt) (0.2 to 0.4%). Phosphorus (P Olsen) was high in the three environments (12.1 to 23.9 Mg kg -1). Exchangeable bases of K, Ca, Mg and Na were high in the three environments and high cation exchange capacity (CEC) in Zi and Zlfa (34.4 to 37.4 cmol (+) kg-1 soil) and very high in ZMB (42.9 cmol (+) kg-1 soil). The micronutrient iron (Fe) in Zlfa, Zi and ZMB was 86.9, 204.2 and 225.4, respectively. Copper (Cu) of 7.1, 7.7 and 4.0. Zn of 4.3, 7.0 and 7.1, manganese (Mn) was 34.1, 51.9 and 192.5. Both soil depths had a clay texture (Table 2).
Table 2 Chemical and physical properties of soil.
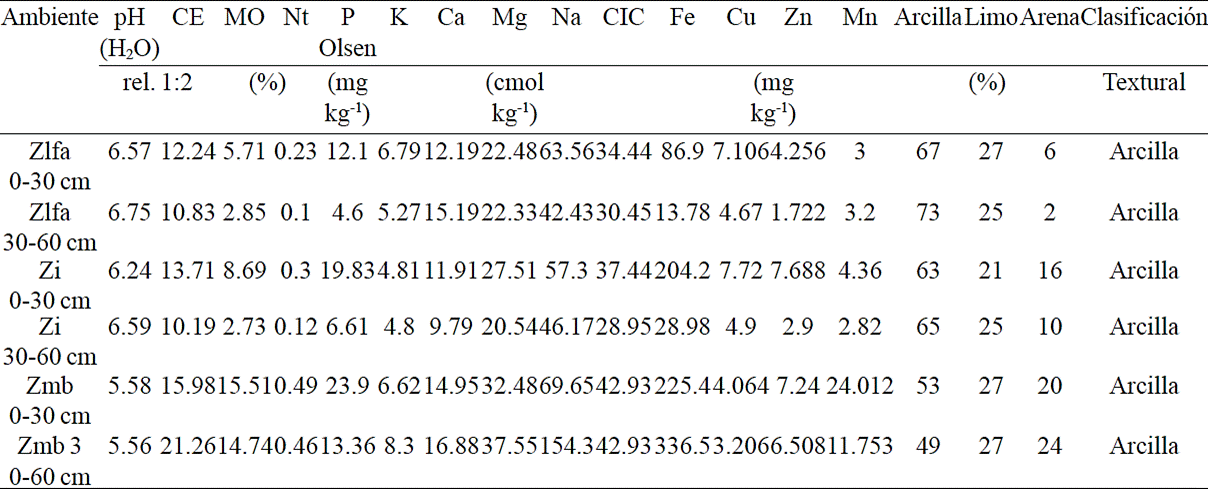
Siglas= CE conductividad eléctrica; MO= materia orgánica; P= Fósforo; Nt Nitrógeno total; K= Potasio; Ca= Calcio; Mg= Magnesio; Na= Sodio; CIC= capacidad de intercambio cationico; Fe= Fierro; Cu= Cobre; Zn= Zinc; Mn= Manganeso.
Soil analysis at depth of 30-60 cm in the three environments showed a moderately acidic pH (5.56 to 6.75) and a highly saline electrical conductivity (EC) in Zlfa and Zi (10.8 and 10.1 dS m-1) and strongly saline for Zmb (21.26 dS m-1). It also showed an average content of organic matter in Zlfa and Zi (2.8 and 2.7%) and high for Zmb (14.7%). Total nitrogen content (Nt) in Zlfa and and Zi was intermediate 0.10 and 0.12% and very high in Zmb (0.46%). Zlfa and Zi had an average content of phosphorus (P Olsen) (4.6 to 6.6 mg kg-1) and high for Zmb (13.3 mg kg -1). Exchangeable bases of K, Ca, Mg and Na showed high cation exchange capacity (CEC) in Zlfa and Zi (30.4 to 28.9 cmol (+) kg-1 soil) and very high in Zmb (42.9 cmol (+) kg-1 soil). The micronutrient iron (Fe) in Zlfa, Zi and ZMB was 13.7, 28.9 and 336.5 respectively; copper (Cu) of 4.6, 4.9 and 3.2; zinc (Zn) of 1.7, 2.9 and 6.5 and manganese (Mn) 38, 34.2 and 139.
Water depth in Zmb only showed flooding until January 2014. Zi presented flood during the 10 months of monitoring, and early February to August remained at a depth of 8 to 12 cm. Zlfa at the end of January to May had a depth of 2 to 6 cm, and in the months of July to August did not show floods (Table 3).
Discussion
Regarding the population of natural regeneration Hoyos et al. (2012) reported that the species A. germinans dominates sandbars, well-drained soils, close to mainland and the highest average values of sedimentation (5.10 cm/year). The statistical similarity between environments was due to high soil salinity limits the growth of this species. The growth of A. germinans and L. racemosa is affected by the amount of salt (Benfield et al., 2005 and López-Hoffman et al., 2006). Overall the excessive accumulation of salts in soils affects plant growth as it aggravates water stress by affecting water absorption. Similarly salts affect the ions balance of soil solution, since nutrients are proportionately less available IUSS et al. (2007). It is known that exist antagonistic effects between Na and K, between Na and Ca, and between Mg and K. In higher concentrations salts can be directly toxic to plants. With respect to Na and chloride ions, these are very harmful because disturb N metabolism.
The population of natural regeneration of black mangrove has little tolerance to shade of other trees and therefore requires open space to develop and lower flooding areas (Febles et al., 2009; Dominguez et al., 2011), this explains why it did not recorded plants with natural regeneration in Zi and Zlfa, but could also be considered that there were no fruits in 2010 and 2011 by the attack of the caterpillar Anacamtodes sp., to black mangrove.
It was evident Zmb had higher percentage of solar radiation, which allowed developing diverse physiological processes of the plant and therefore has greater growth and higher survival rate as described by Valladares et al. (2004) and Alcaraz (2012) that higher lighting percentage in one place is a niche for plant regeneration and higher growth. Proof of this is that there was no natural regeneration in Zi and Zlfa. Although Zi showed higher percentage of solar radiation, did not favor the survival of plants reforested due to long periods of flooding.
The total area of mangrove ecosystem in Tabasco covers about 56.4% of saline soils (Domínguez et al., 2011). These saline soils are located in a variety of reliefs, mainly in alluvial plains, because they have saline water intrusion due to the tide or by water table (Zavala et al., 1999, Palma-López et al., 2007). In a study from López et al. (2011) in five mangrove forests in the west central region of Venezuela; four located in the Paraguana peninsula and one on the mainland, in the estuary from the Ricoa River; reported the dominance of clayey soils in soil from estuarine mangrove tree and squat from hypersaline lagoon; in the rest of mangroves predominated sandy clay loam soils.
Conclusions
The information generated from the species A. germinans in this research, strengthens decision making in future reforestation of mangrove ecosystem. Due to the ecosystem is complex, because natural elements are present, of which does not have control over it. Therefore it is necessary to take them into account for the success of the reforestation project.
Black mangrove survival in reforested areas and populations of natural regeneration is favored in the short time of flooding. I.e. this species does not tolerate long periods of flooding, because it does not count with pneumatophores or are scarce and with small heights and are not sufficient to carry out the physiological processes of the plant specific respiration when it is exposed to flooding. Therefore the hypothesis is not accepted because the survival rates were lower than 85% in the three environments.
The growth of reforested plantations obviously has greater overall height in Zmb. Also in this environment it presented populations of natural regeneration of the species A. germinans, as Batis maritima as invasive species.
Solar radiation is important for many physiological processes in all plant species; especially for the process of photosynthesis, where the sugars which are used for growth and vital functions of the plant are produced. In particular the species A. germinans, solar radiation is vital for growth, however, involves other factors like high soil salinity that restricts growth also flooding level. Therefore, the hypothesis is not accepted because the growth of the species A. germinans intervenes on soil salinity.
Literatura citada
Aceves, N., L.A., J. F. Juárez L., D. J. Palma L., R. López L., B. Rivera H., J. A. Rincón R., R. Morales C., R. Hernández A., A. Martínez S. y J. L. Hernández S. 2008. Secretaría de Desarrollo Agropecuario Forestal y Pesca. TOMO 1. Estudios para determinar zonas de alta potencialidad de los cultivos en el estado de Tabasco (informe general). [ Links ]
Alcaraz, A. F.J. 2012. Temperatura, luz, atmosfera, viento. Geobotánica, Tema 20. Universidad de Marcia, España. 13 p. [ Links ]
Álvarez, O. D.S., J.C. Matiz L., y A. C. Cárdenas. 2011. Modelos digitales batimétricos generados por métodos de interpolación idw, kriging, Shepard y B-Spline en el archipiélago de Islas del Rosario. Revista Geomática UD. GEO No. 5:3-14. [ Links ]
Barba, E., J.F. Juárez y F.L. Estrada. 2010. Distribución y abundancia de crustáceos en humedales de Tabasco, México. Revista Mexicana de Biodiversidad 81:153-163. [ Links ]
Basáñez, M. A. de J., G. P. Olmedo y P.M. Rojas. 2006. Características estructurales y usos del manglar en el ejido Cerro de Tumilco, Tuxpan, Veracruz, México. Revista UDO Agrícola 6(1):114-120. [ Links ]
Benfield, S.L., Hector M. Guzman., James M. Mair. 2005. Temporal mangrove dynamics in relation to coastal development in Pacific Panana. Journal of Environmental Management. 76:263-276. [ Links ]
Buduba, C. 2004. Muestro de suelos. Criterios básicos. Ficha Técnica. Patagonia Forestal-Año X N° 1. 12 p. [ Links ]
Carmona, D. G., J.E. M. Morales y E.L. Rodríguez. 2004. Plan de manejo para el manglar de Sontecomapan, Catemaco, Veracruz, México: una estrategia para la conservación de sus recursos naturales. Madera y Bosques. 10(2):5-23. [ Links ]
Carrillo, E.G. 2008. Casos prácticos para muestreos e inventarios forestales. Edit. Universidad Autónoma de Chapingo. 172 p. [ Links ]
Carrillo, B. A., E.M. R. Elizalde, N.V. Torrescano y G.O. Flores. 2008. Adaptación ante disturbios naturales, manglar de Puerto Morelos, Quintana Roo, México. Foresta Veracruzana 10(1):31-38. [ Links ]
Cordero, J., y D. Boshier. 2003. Árboles de Centroamérica: un manual para extensionistas. Centro Agronómico de Investigación y Enseñanza - CATIE, Oxford, Reino Unido. 1079 p. [ Links ]
Chan, Hung Tuck, Mark Spalding, Shigeyuki Baba, Mami Kainuma, Alastair Sarre and Steve Johnson. 2012. The Tropical Forest Update is published by the International Tropical Timber Organization. 21(2):1-21. [ Links ]
CONABIO. 2008. Manglares de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. 35 p. [ Links ]
Domínguez, D. M., J.C. Zavala, P.Z. Martínez. 2011. Manejo forestal sustentable de los manglares de Tabasco. Secretaría de Recursos Naturales y Protección Ambiental. Colegio de Postgraduados. Villahermosa, Tabasco, México. 137 p. [ Links ]
FAO. 2007. The World´s mangroves. Roma Italia. 89 p. [ Links ]
Febles, P. JL., J.L. Novelo y E.S. Batllori. 2009. Pruebas de reforestación de mangle en una ciénaga costera semiárida de Yucatán, México. Madera y Bosques 15(3):65-86. [ Links ]
Flores, M. M.A., A.V. Aguirre, M.H. Flores y X.G. Guardado. 2010. El impacto que produce el sector turismo en los manglares de las costas mexicanas. 77:33-38. [ Links ]
García, C.S. 2005. Dinámicas del C y N en el suelo de manglar en Ventanilla, Oaxaca. Tesis de Licenciatura. Universidad del Mar. Biología Marina. Consultado en Julio 2014. http://www.umar.mx/tesis_PA/tesis_digitales/SANCHES-GARCIA-BIOMAR.pdf . [ Links ]
Hernández-Melchor, G.I. 2013. Legislación, cambio de uso de suelo y reforestación en manglares de Cardenas, Tabasco. Colegio de Posgraduados-Campus Veracruz. Tesis Doctoral. 108 p. [ Links ]
Herrera, H. J.G. y C. A. García. 2010. Bioestadística en Ciencias Veterinarias. Procedimientos de análisis de datos con SAS. Ed. Universidad Complutense de Madrid. 251 p. [ Links ]
Hoyos, G. R., L.E. G. Urrego y Á.T. Lema. 2013. Respuesta de la regeneración natural en manglares del Golfo de Urabá (Colombia) a la variabilidad ambiental y climática intraanual. Rev. Biol. Trop. (Int. J. Trop. Biol. ISSN-0034-7744). 61(3):1445-1461. [ Links ]
IUSS, ISRIC y FAO. 2007. Base referencial mundial del recurso suelo. Primera actualización traducida al español por Mabel Susana Pazos. Informes sobre recursos mundiales de suelos No. 103. FAO, Roma. 127 p. [ Links ]
Ke, L., Yu K.S.H., Wong Y.S., Tam N.F.Y. 2005. Spatial and vertical distribution of polycyclic aromatic hydrocarbons in mangrove sediments. Sci. Tot. Environ. 340, 177-187. [ Links ]
Kohen, E., R. Santus y J. G. Hirschberg. 1995. Photobiology. Academic Press, London. [ Links ]
Lewis, R.R. 2005. Ecological engineering for successful management and restoration of mangrove forests. Ecological Engineering. 24:403-418. [ Links ]
Londoño, A.C. y E. Álvarez. 1997. Mortalidad y crecimiento en bosques de tierra firme y várzea, Amazonía colombiana. TROPENBOS. Bogotá. 30 p. [ Links ]
Londoño, A.C. y E.M. Jiménez. 1999. Efecto del tiempo entre los censos sobre la estimación de las tasas anuales de mortalidad y de reclutamiento de árboles (períodos de 1, 4 y 5 años). En: Crónica Forestal y del Medio Ambiente. No. 14:41-58. [ Links ]
López, B.B., M.C. Beatriz y J. Eloy. 2011. Caracterización de los manglares de zonas semiáridas en el noroccidente de Venezuela. Asociación Interciencia Caracas, Venezuela. Interciencia. 36(12):888-893. [ Links ]
López, Hoffman. L., Niels P. R. A., M. Martinez R., David D. Ackerly. 2006. Salinity and light interactively affect mangrove seedlings at the leaf and whole plant levels. Oecologia. 150(4):545-556. [ Links ]
Melo, C. O. y R. R. Vargas. 2003. Evaluación ecológica y silvicultural de ecosistemas boscosos. Universidad del Tolima. Crq - Carder - Corpocaldas - Cortolima Ibagué. 1ra. Edición. 1-21 pp. [ Links ]
Moreno, M. G. de J., J.G B. Cerón, R.M. B. Cerón, J.J. S. Guerra, L.E. del A. Amador y E.H. Endañú. 2010. Estimación del potencial de captura de carbono en los suelos de manglar de Isla del Carmen. UNICAR TECNOCIENCIA. 4(1):24-39. [ Links ]
NOM-021-SEMARNAT-2000. Norma Oficial Mexicana. Secretaría de Medio Ambiente y Recursos Naturales. Publicado en el diario oficial el día 31 de diciembre 2002. [ Links ]
Olguín, J. E., M. E. Hernández y G.G. Sánchez. 2007. Contaminación de manglares por hidrocarburos y estrategias de biorremediación, fitorremediación y restauración. Rev. Int. Contam. Ambient. 23(3):139-154. [ Links ]
Ortiz P. M. A., C. Siebe, y S. Cram. 2005. Diferenciación ecogeografica de Tabasco. Cap. 14: 305-322. En Bueno J., F. Álvarez y S. Santiago (Eds.) Biodiversidad del estado de Tabasco, 356 p. Instituto de Biología, UNAM-CONABIO. México, 2005. ISBN 970-9000-26-8. [ Links ]
Ortiz, P. M. A. y A. P. L. Méndez. 2000. Componentes naturales y de uso del suelo vulnerables a las variaciones del nivel del mar en la costa Atlántica de México. Investigaciones Geográficas, Abril, numero 041. Universidad Nacional Autónoma de México Distrito Federal, México. 46-61 pp. [ Links ]
Palma, L. D. J., J. D. Cisneros, E.C. Moreno y J.A. R. Rincón. 2006. Plan de uso sustentable de los suelos de Tabasco. 3ª. Ed. ISPROTABFUNDACION PRODUCE TABASCO-COLEGIO DE POSTGRADUADOS. Villahermosa, Tabasco, México. 97 p. [ Links ]
Reeves, G. (2005). Understanding and monitoring hydrocarbons in water. Oakville, Ontario, Canada: Arjay Engineering LTD. [ Links ]
Rodríguez, Z. M. T., C. S. Troche, A. D. Vázquez, J. D. Márquez, B. B. Vázquez, L. L. Valderrama, S. S. Velázquez, M. I. Cruz, R. Ressl, A. M. Uribe, S. E. Cerdeira, J. Acosta, J. Díaz, R. Jiménez, L. Fueyo y C. Galindo. 2013. Manglares de México/ Extensión, distribución y monitoreo. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México D. F. 128 p. [ Links ]
Sanjurjo, R. E. y S. C. Welsh. 2005. Una descripción del valor de los bienes y servicios ambientales prestados por los manglares. Red de Revistas Científicas de América Latina, el Caribe, España y Portugal. Gaceta Ecológica, núm. 74, 55 68. [ Links ]
SAS Institute Inc. 1995. Logistic Regressión Examples Using the SAS System, Versión 6, First Edition, Cary, NC. 163 p. [ Links ]
Schuyt, K. y Brander, L. 2004. The economic values of the world’s wetlands. Gland, Switzerland: World Wide Fund for Nature (WWF). [ Links ]
SEDESPA. 2006. Secretaría de Desarrollo Social y Protección al Ambiente. Programa de Ordenamiento Ecológico del Estado de Tabasco. 99 p. [ Links ]
Sol, S. Á., G. I.M. Hernández y F.G. Sánchez. 2012. Volumen de madera de mangle negro (avicennia germinans l.) muerto defoliado por la oruga de Anacamptodes sp en el ejido Las Coloradas, Cárdenas, Tabasco, México. MEMORIAS DEL SEGUNDO CONGRESO MEXICANO DE ECOSISTEMAS DE MANGLAR. 22-26 de octubre de 2012. Ciudad del Carmen, Campeche, México. 33-34 pp. [ Links ]
Sosa, P., G. y D. A. T. Rodríguez. 2003. Efecto de la calidad de la planta en la supervivencia y crecimiento de Pinus patula en un área quemada. Vol. IX, NÚM. 1-2003. Revista Chapingo Serie Ciencias Forestales y del Ambiente 9(1):35-43. [ Links ]
Spalding, M, Kainuma M, Collins L. 2010. World Atlas of Mangroves. A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. London (UK): Earthscan, London. 319 p. [ Links ]
Valladares, F., I. Aranda. Y D.G. Sánchez. 2004. La luz como factor ecológico y evolutivo para las plantas y su interacción con el agua. Ecología del bosque mediterráneo en un mundo cambiante. Páginas 335-369. Ministerio de Medio Ambiente, EGRAF, S. A., Madrid. ISBN: 84-8014-552-8. [ Links ]
Vilmarie, R. 2008. Recursos acuáticos: el manglar. Programa de Educación en Recursos Acuáticos (PERA). Negociado de Pesca y Vida Silvestre. Departamento de Recursos Naturales y Ambientales (DRNA). [ Links ]
Wood, A.; Dixon, A.; McCartney, M.P. 2013. Conclusions: Transforming wetland livelihoods. In: Wetland management and sustainable livelihoods in Africa, eds., Wood, A. ; Dixon, A. ; McCartney, M.P. New York, USA: Routledge. 258-270 pp. [ Links ]
Yáñez, E. L., T. Terrazas y L. M. López. 2001. Effects of flooding on wood and bark anatomy of four species in a mangrove forest community. Trees 15:91-97. [ Links ]
Received: December 2015; Accepted: March 2016











 texto en
texto en